The Outer Membrane Proteins and Their Synergy Triggered the Protective Effects against Pathogenic Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
2.2. Characterization of E. coli O78
2.3. Homological Analysis of the OmpA, OmpC and BamA
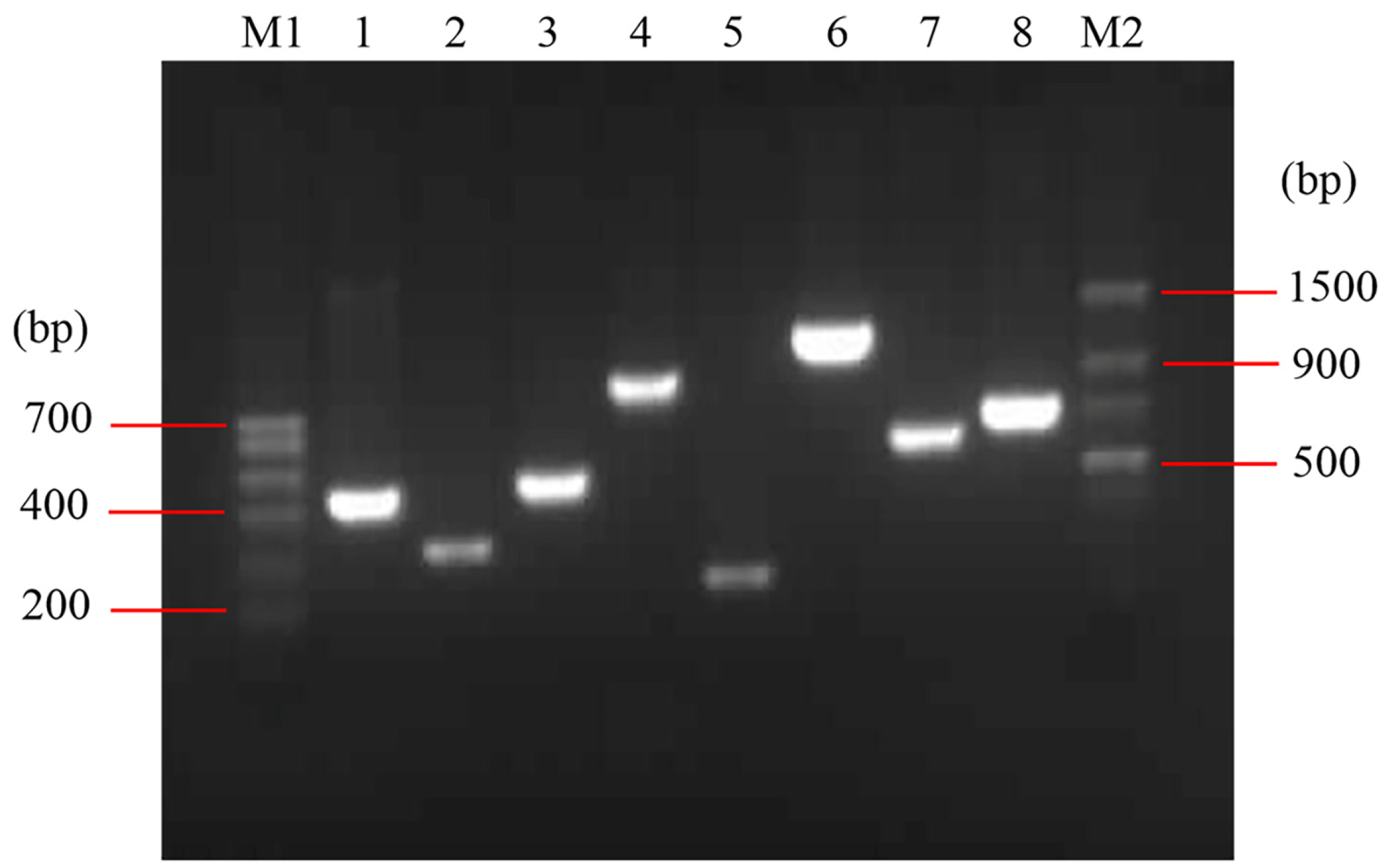
2.4. Cloning of the ompA, ompC and bamA Gene
2.5. Expression and Purification of the rOmpA, rOmpC and rBamA Protein
2.6. Mouse Immunization
2.7. Antibody Titer and Antibody Isotypes Detection by iELISA
2.8. Cross-Protection Property Detected by iELISA
2.9. Double-Immunodiffusion Assay
2.10. Opsonophagocytosis Assay
2.11. Challenge Assay
3. Result
3.1. Characterization of E. coli O78
3.2. Homological Analysis of the OmpA, OmpC and BamA
3.3. Cloning, Expression, and Purification of the Recombinant Proteins
3.4. Immunogenic Property of Recombinant Proteins in Mice
3.5. Double-Immunodiffusion Assay
3.6. Opsonophagocytosis Assay
3.7. Protection Efficacy of Recombinant Proteins against E. coli In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, J.R.; Russo, T.A. Extraintestinal pathogenic Escherichia coli: “The other bad E coli”. J. Lab. Clin. Med. 2002, 139, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Sáez-López, E.; Johnson, J.R.; Römling, U.; Dobrindt, U.; Cantón, R.; Giske, C.G.; Naas, T.; Carattoli, A.; Martínez-Medina, M.; et al. Escherichia coli: An old friend with new tidings. FEMS Microbiol. Rev. 2016, 40, 437–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, L.W. Distinguishing pathovars from nonpathovars: Escherichia coli. Microbiol. Spectr. 2020, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tapader, R.; Basu, S.; Pal, A. Secreted proteases: A new insight in the pathogenesis of extraintestinal pathogenic Escherichia coli. Int. J. Med. Microbiol. IJMM 2019, 309, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Cointe, A.; Mariani Kurkdjian, P.; Rafat, C.; Hertig, A. Shiga toxin-associated hemolytic uremic syndrome: A narrative review. Toxins 2020, 12, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwidder, M.; Heinisch, L.; Schmidt, H. Genetics, toxicity, and distribution of enterohemorrhagic Escherichia coli hemolysin. Toxins 2019, 11, 502. [Google Scholar] [CrossRef] [Green Version]
- Biran, D.; Ron, E.Z. Extraintestinal pathogenic Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 149–161. [Google Scholar] [CrossRef]
- Vandekerchove, D.; De Herdt, P.; Laevens, H.; Pasmans, F. Colibacillosis in caged layer hens: Characteristics of the disease and the aetiological agent. Avian Pathol. 2004, 33, 117–125. [Google Scholar] [CrossRef]
- Guabiraba, R.; Schouler, C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef]
- Dziva, F.; Stevens, M.P. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008, 37, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Guiral, E.; Bosch, J.; Vila, J.; Soto, S.M. Antimicrobial resistance of Escherichia coli strains causing neonatal sepsis between 1998 and 2008. Chemotherapy 2012, 58, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Orskov, F.; Orskov, I. Escherichia coli serotyping and disease in man and animals. Can. J. Microbiol. 1992, 38, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Meuskens, I.; Michalik, M.; Chauhan, N.; Linke, D.; Leo, J.C. A new strain collection for improved expression of outer membrane proteins. Front. Cell. Infect. Microbiol. 2017, 7, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Guan, Q.; Wang, X.; Teng, D.; Mao, R.; Yao, J.; Wang, J. Paving the way to construct a new vaccine against Escherichia coli from its recombinant outer membrane protein C via a murine model. Process Biochem. 2015, 50, 1194–1201. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, X.; Wang, X.; Teng, D.; Mao, R.; Zhang, Y.; Wang, J. Recombinant outer membrane protein A induces a protective immune response against Escherichia coli infection in mice. Appl. Microbiol. Biotechnol. 2015, 99, 5451–5460. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, X.; Wang, X.; Teng, D.; Wang, J. In silico analysis and recombinant expression of BamA protein as a universal vaccine against Escherichia coli in mice. Appl. Microbiol. Biotechnol. 2016, 100, 5089–5098. [Google Scholar] [CrossRef]
- Pore, D.; Chakrabarti, M.K. Outer membrane protein A (OmpA) from Shigella flexneri 2a: A promising subunit vaccine candidate. Vaccine 2013, 31, 3644–3650. [Google Scholar] [CrossRef]
- Li, Q.; Ren, J.; Xian, H.; Yin, C.; Yuan, Y.; Li, Y.; Ji, R.; Chu, C.; Qiao, Z.; Jiao, X. rOmpF and OMVs as efficient subunit vaccines against Salmonella enterica serovar Enteritidis infections in poultry farms. Vaccine 2020, 38, 7094–7099. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zhao, X.; Yang, Y.; Li, B.; Zhu, F.; Zhu, R. Immune enhancement of Taishan Robinia pseudoacacia polysaccharide on recombinant Proteus mirabilis OmpA in chickens. Int. Immunopharmacol. 2014, 22, 236–241. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Hu, D.; Dou, Y.; Xiong, L.; Wang, X.; Hu, J.; Xing, S.Z.; Li, W.; Cai, J.P.; Jin, M.; et al. Identification and evaluation of recombinant outer membrane proteins as vaccine candidates against Klebsiella pneumoniae. Front. Immunol. 2021, 12, 730116. [Google Scholar] [CrossRef]
- Duan, L.; Feng, J.; Peng, L.; Guo, S.; He, L.; Xiao, Y. Evaluation of an outer membrane protein as a vaccine against Edwardsiella anguillarum in Japanese eels (Anguilla japonica). Aquaculture 2019, 498, 143–150. [Google Scholar]
- Diao, J.; Li, L.; Fan, Y.; Wang, S.; Gai, C.; Wang, Y.; Yu, X.; Wang, X.; Xu, L.; Liu, H.; et al. Recombinant outer membrane protein C of Aeromonas salmonicida subsp. masoucida, a potential vaccine candidate for rainbow trout (Oncorhynchus mykiss). Microb. Pathog. 2020, 145, 104211. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Janssen, T.; Kiessling, S.; Philipp, H.C.; Wieler, L.H. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005, 49, 269–273. [Google Scholar] [CrossRef]
- Maciel, J.F.; Matter, L.B.; Trindade, M.M.; Camillo, G.; Lovato, M.; de Ávila Botton, S.; Castagna de Vargas, A. Virulence factors and antimicrobial susceptibility profile of extraintestinal Escherichia coli isolated from an avian colisepticemia outbreak. Microb. Pathog. 2017, 103, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Nayeem, M.M.H.; Sobur, M.A.; Ievy, S.; Islam, M.A.; Rahman, S.; Kafi, M.A.; Ashour, H.M.; Rahman, M.T. Virulence determinants and multidrug resistance of Escherichia coli isolated from migratory birds. Antibiotics 2021, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, Z.; Xia, Y.; Li, H.; Kou, Y.; Bao, Y.; Dai, J.; Lu, C. IbeB is involved in the invasion and pathogenicity of avian pathogenic Escherichia coli. Vet. Microbiol. 2012, 159, 411–419. [Google Scholar] [CrossRef] [PubMed]
- UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [CrossRef]
- Wang, X.; Teng, D.; Guan, Q.; Mao, R.; Hao, Y.; Wang, X.; Yao, J.; Wang, J. Escherichia coli outer membrane protein F (OmpF): An immunogenic protein induces cross-reactive antibodies against Escherichia coli and Shigella. AMB Express 2017, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Ramos, C.R.; Abreu, P.A.; Nascimento, A.L.; Ho, P.L. A high-copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Braz. J. Med. Biol. Res. Rev. Bras. De Pesqui. Med. E Biol. 2004, 37, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Moore, J.; Derrick, J.P. Expression, purification, and crystallization of neisserial outer membrane proteins. Methods Mol. Biol. 2012, 799, 91–106. [Google Scholar] [CrossRef]
- Chang, P.; Huang, L.; Huang, M.; Tian, S.; Yang, Z. Improvement and optimization of a T-cell-dependent antibody response (TDAR) method for BALB/c mice using keyhole limpet hemocyanin (KLH) as specific antigen. J. Immunotoxicol. 2019, 16, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Gong, R.; Guo, A.; Chen, H. Protective effect of ligand-binding domain of fibronectin-binding protein on mastitis induced by Staphylococcus aureus in mice. Vaccine 2010, 28, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P. Double-immunodiffusion assay for detecting specific antibodies. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Swamydas, M.; Luo, Y.; Dorf, M.E.; Lionakis, M.S. Isolation of mouse neutrophils. Curr. Protoc. Immunol. 2015, 110, 3.20.21–3.20.15. [Google Scholar] [CrossRef] [Green Version]
- Grund, M.E.; Soo, J.C.; Cote, C.K.; Berisio, R.; Lukomski, S. Thinking outside the bug: Targeting outer membrane proteins for Burkholderia vaccines. Cells 2021, 10, 495. [Google Scholar] [CrossRef]
- Schweizer, M.; Henning, U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J. Bacteriol. 1977, 129, 1651–1652. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, D.W.; Ricker, N.; Barbieri, N.L.; Allen, H.K.; Nolan, L.K.; Logue, C.M. Outer membrane protein A (OmpA) of extraintestinal pathogenic Escherichia coli. BMC Res. Notes 2020, 13, 51. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, C.; Zhou, L.; Zhang, Y.; Ge, Y.; Chen, W.; Pan, J. Evaluation of the β-barrel outer membrane protein VP1243 as a candidate antigen for a cross-protective vaccine against Vibrio infections. Microb. Pathog. 2020, 147, 104419. [Google Scholar] [CrossRef]
- Peng, B.; Ye, J.Z.; Han, Y.; Zeng, L.; Zhang, J.Y.; Li, H. Identification of polyvalent protective immunogens from outer membrane proteins in Vibrio parahaemolyticus to protect fish against bacterial infection. Fish Shellfish. Immunol. 2016, 54, 204–210. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Rodríguez, E.G. Vaccine adjuvants revisited. Vaccine 2007, 25, 3752–3762. [Google Scholar] [CrossRef] [PubMed]
- Fontes, J.A.; Barin, J.G.; Talor, M.V.; Stickel, N.; Schaub, J.; Rose, N.R.; Čiháková, D. Complete Freund’s adjuvant induces experimental autoimmune myocarditis by enhancing IL-6 production during initiation of the immune response. Immun. Inflamm. Dis. 2017, 5, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Gangaplara, A.; Massilamany, C.; Lasrado, N.; Steffen, D.; Reddy, J. Evidence for anti-viral effects of complete Freund’s adjuvant in the mouse model of Enterovirus infection. Vaccines 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Hajizade, A.; Salmanian, A.H.; Amani, J.; Ebrahimi, F.; Arpanaei, A. EspA-loaded mesoporous silica nanoparticles can efficiently protect animal model against enterohaemorrhagic E. coli O157:H7. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1067–S1075. [Google Scholar] [CrossRef] [Green Version]
- Samo, M.; Choudhary, N.R.; Riebe, K.J.; Shterev, I.; Staats, H.F.; Sempowski, G.D.; Leduc, I. Immunization with the Haemophilus ducreyi trimeric autotransporter adhesin DsrA with alum, CpG or imiquimod generates a persistent humoral immune response that recognizes the bacterial surface. Vaccine 2016, 34, 1193–1200. [Google Scholar] [CrossRef]
- Ronco, T.; Stegger, M.; Olsen, R.H.; Sekse, C.; Nordstoga, A.B.; Pohjanvirta, T.; Lilje, B.; Lyhs, U.; Andersen, P.S.; Pedersen, K. Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. BMC Genom. 2017, 18, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Maurer, J.J.; Hubert, S.; De Villena, J.F.; McDermott, P.F.; Meng, J.; Ayers, S.; English, L.; White, D.G. Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates. Vet. Microbiol. 2005, 107, 215–224. [Google Scholar] [CrossRef]
- Moulin-Schouleur, M.; Répérant, M.; Laurent, S.; Brée, A.; Mignon-Grasteau, S.; Germon, P.; Rasschaert, D.; Schouler, C. Extraintestinal pathogenic Escherichia coli strains of avian and human origin: Link between phylogenetic relationships and common virulence patterns. J. Clin. Microbiol. 2007, 45, 3366–3376. [Google Scholar] [CrossRef] [Green Version]
- Pyrski, M.; Rugowska, A.; Wierzbiński, K.R.; Kasprzyk, A.; Bogusiewicz, M.; Bociąg, P.; Samardakiewicz, S.; Czyż, M.; Kurpisz, M.; Pniewski, T. HBcAg produced in transgenic tobacco triggers Th1 and Th2 response when intramuscularly delivered. Vaccine 2017, 35, 5714–5721. [Google Scholar] [CrossRef]
- Hussain, R.; Dawood, G.; Abrar, N.; Toossi, Z.; Minai, A.; Dojki, M.; Ellner, J.J. Selective increases in antibody isotypes and immunoglobulin G subclass responses to secreted antigens in tuberculosis patients and healthy household contacts of the patients. Clin. Diagn. Lab. Immunol. 1995, 2, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, function, and therapeutic use of IgM antibodies. Antibodies 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pen, G.; Yang, N.; Teng, D.; Hao, Y.; Mao, R.; Wang, J. The Outer Membrane Proteins and Their Synergy Triggered the Protective Effects against Pathogenic Escherichia coli. Microorganisms 2022, 10, 982. https://doi.org/10.3390/microorganisms10050982
Pen G, Yang N, Teng D, Hao Y, Mao R, Wang J. The Outer Membrane Proteins and Their Synergy Triggered the Protective Effects against Pathogenic Escherichia coli. Microorganisms. 2022; 10(5):982. https://doi.org/10.3390/microorganisms10050982
Chicago/Turabian StylePen, Guihong, Na Yang, Da Teng, Ya Hao, Ruoyu Mao, and Jianhua Wang. 2022. "The Outer Membrane Proteins and Their Synergy Triggered the Protective Effects against Pathogenic Escherichia coli" Microorganisms 10, no. 5: 982. https://doi.org/10.3390/microorganisms10050982
APA StylePen, G., Yang, N., Teng, D., Hao, Y., Mao, R., & Wang, J. (2022). The Outer Membrane Proteins and Their Synergy Triggered the Protective Effects against Pathogenic Escherichia coli. Microorganisms, 10(5), 982. https://doi.org/10.3390/microorganisms10050982







