Dietary Cysteamine Supplementation Remarkably Increased Feed Efficiency and Shifted Rumen Fermentation toward Glucogenic Propionate Production via Enrichment of Prevotella in Feedlot Lambs
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CS Product
2.2. Animals, Diet Preparation, and Experiment Design
2.3. Sample Collection
2.4. DNA Extraction and Sequencing
2.5. Chemical Analyses
2.6. Calculation
2.7. Statistical Analysis
3. Results
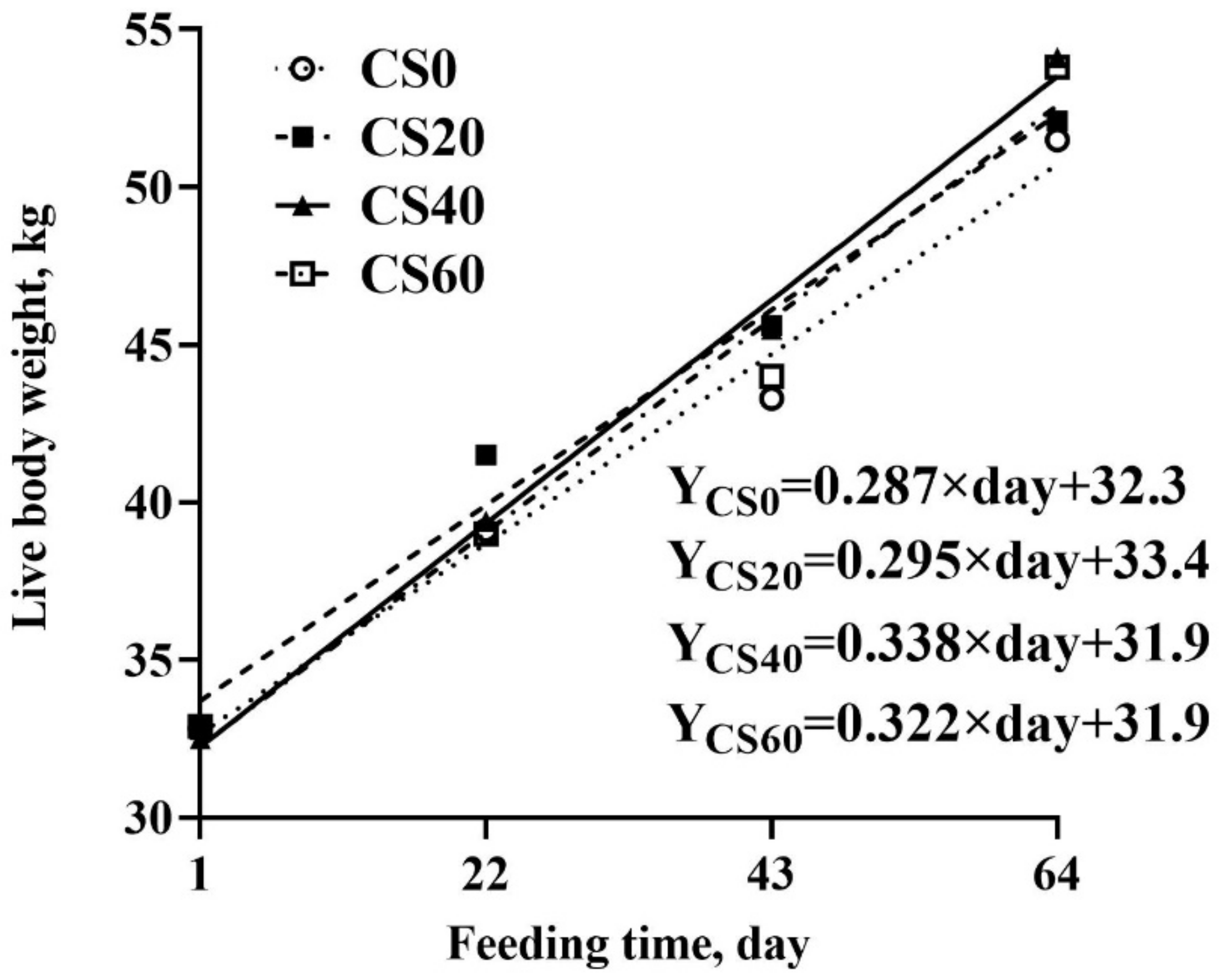
3.1. Effect of Increasing CS Addition on the Growth Performance in Feedlot Lambs
3.2. Effect of Increasing CS Addition on Blood Serum Concentrations along the Growth Hormone Axis
3.3. Effect of Increasing CS Addition on Rumen Fermentation Characteristics and Methane Production in Feedlot Lambs
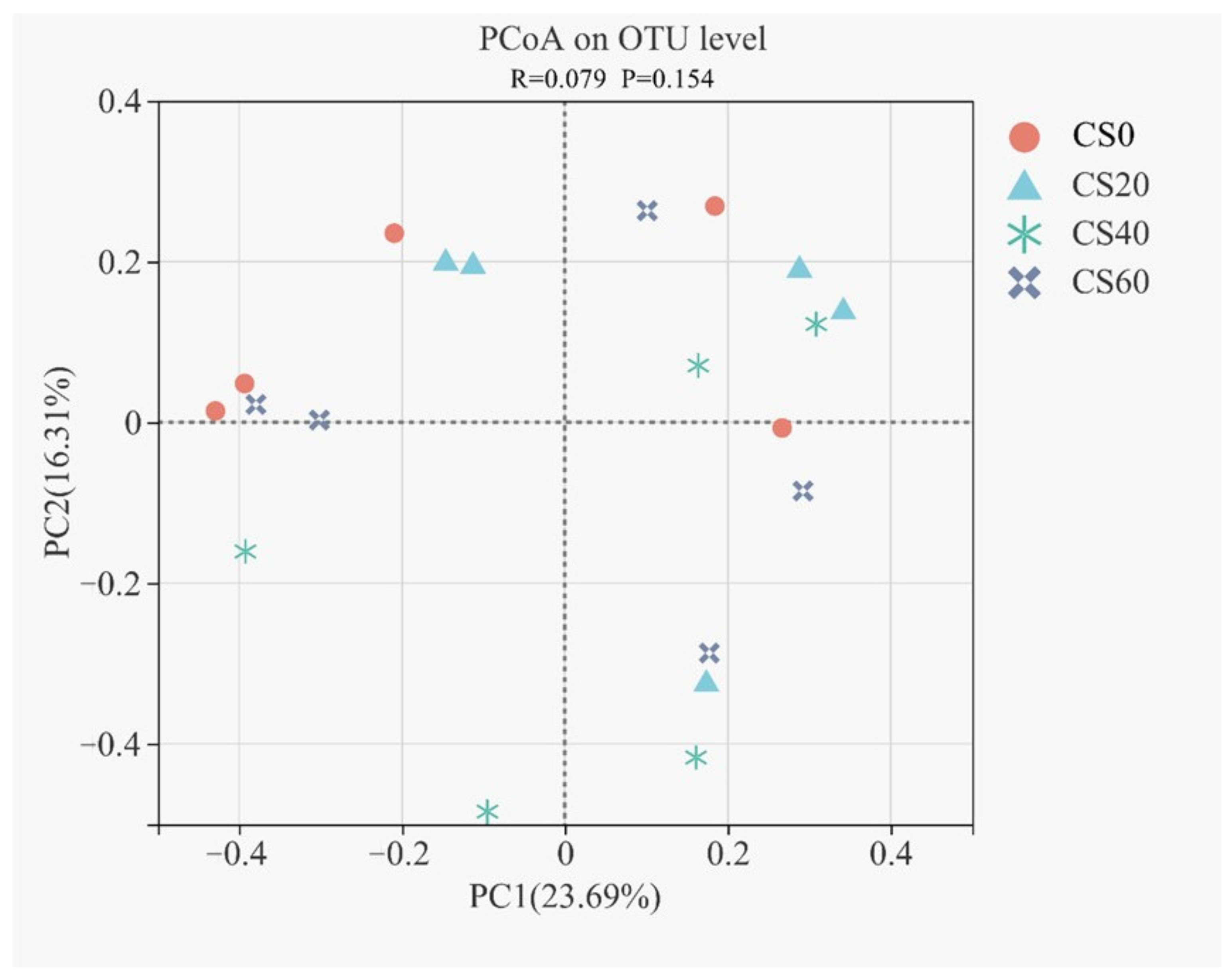
3.4. Effect of Bacterial Diversity and Community in Response to the CS Addition
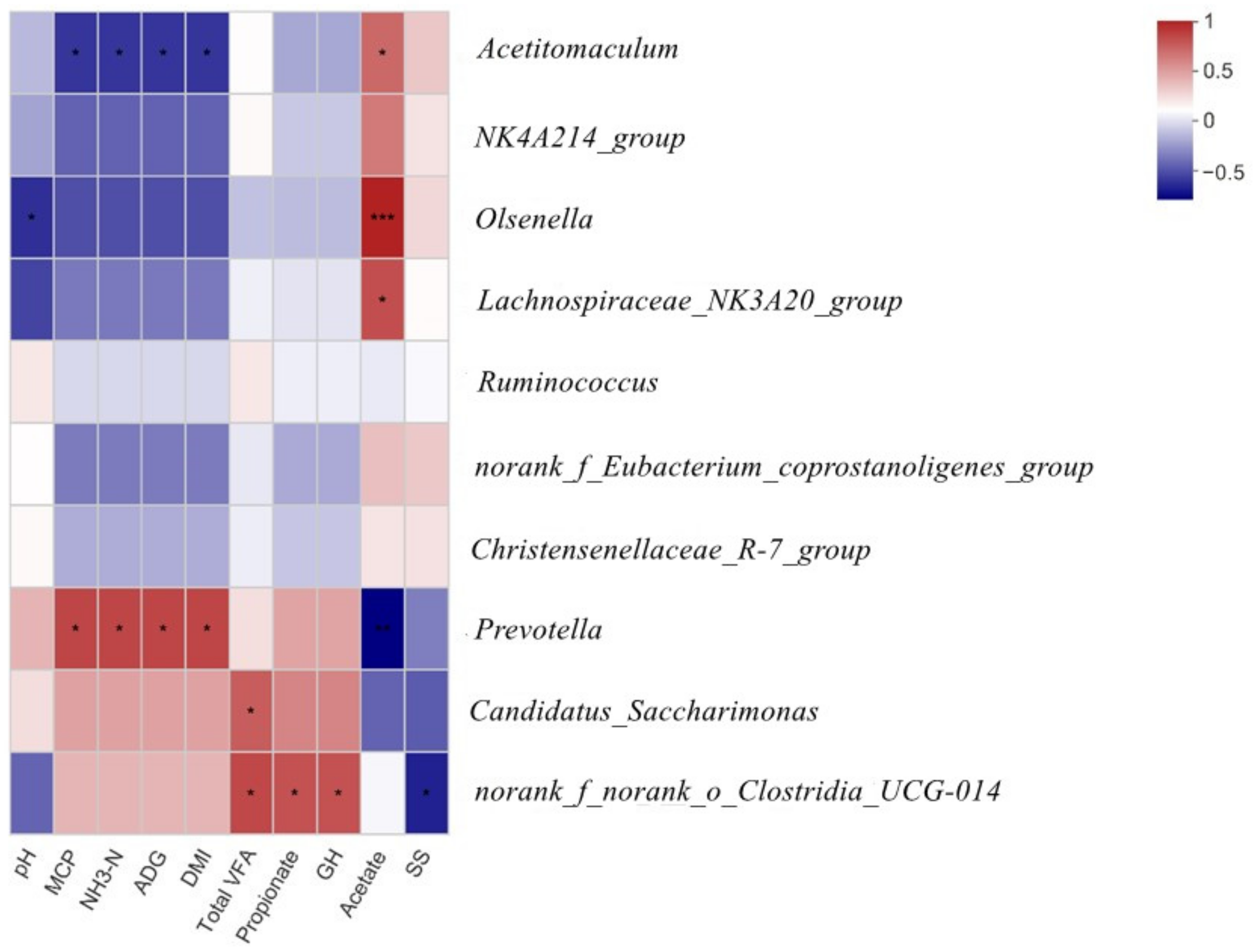
3.5. Correlations among the Top 10 Bacterial Genera and the Parameters of Rumen Fermentation, Blood Serum Index, and Growth Performance
4. Discussion
4.1. Growth Performance in Response to Dietary CS Addition and Its Possible Action Mechanism
4.2. Rumen Fermentation Characteristics
4.3. Bacterial Diversity and Community in Response to CS Addition
4.4. Association among Growth Performance, Serum Hormones, and Change in Bacterial Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isaacson, O.G.P.; Eden, S.; Jansson, J.O. Mode of action of pituitary growth hormone on target cells. Annu. Rev. Physiol. 1985, 47, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Argente, J.; Pozo, J.; Chowen, J.A. The growth hormone axis: Control and effects. Horm. Res. 1996, 45, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Perks, C.M.; Davis, A.J.; Denning-Kendall, P.A. Regulation of insulin-like growth factor-I and progesterone synthesis by insulin and growth hormone in the ovine ovary. Biol. Reprod. 1985, 53, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Walton, P.E.; Etherton, T.D. Stimulation of lipogenesis by insulin in swine adipose tissue: Antagonism by porcine growth hormone. J. Anim. Sci. 1986, 62, 1584–1595. [Google Scholar] [CrossRef]
- Galbraith, H. Hormones in international meat production: Biological, sociological and consumer issues. Nutr. Res. Rev. 2002, 15, 293–314. [Google Scholar] [CrossRef][Green Version]
- Niamh, S.; Laurence, D.; Skinner, D.C. Somatostatin-14 neurons in the ovine hypothalamus: Colocalization with estrogen receptor alpha and somatostatin-28 (1–12) immunoreactivity, and activation in response to estradiol. Biol. Reprod. 2003, 69, 1318–1324. [Google Scholar] [CrossRef]
- Szabo, S.; Reichlin, S. Somatostatin in rat tissues is depleted by cysteamine administration. Endocrinology 1981, 109, 2255–2257. [Google Scholar] [CrossRef]
- Hu, R.; Wang, Z.; Peng, Q.; Zou, H.; Wang, H.; Yu, X.; Jing, X.; Wang, Y.; Cao, B.; Bao, S.; et al. Effects of GHRP-2 and Cysteamine Administration on Growth Performance, Somatotropic Axis Hormone and Muscle Protein Deposition in Yaks (Bos grunniens) with Growth Retardation. PLoS ONE 2016, 11, e0149461. [Google Scholar] [CrossRef]
- Vasin, M.V. Comments to the Mechanism of Protective and Pharmacological Action of Radioprotectors from the Family of Aminothiols. Radiat. Prot. Dosim. 2014, 2, 15–36. [Google Scholar] [CrossRef]
- Horoupian, D.S.; Kress, Y.; Yen, S.H.; Gaskin, F. Nickel-induced changes and reappraisal of Rosenthal fibers in focal CNS lesions. J. Neuropathol. Exp. Neurol. 1982, 41, 664–675. [Google Scholar] [CrossRef]
- Dunshea, F.R. Porcine somatotropin and cysteamine hydrochloride improve growth performance and reduce back fat in finisher gilts. Anim. Prod. Sci. 2007, 47, 796–800. [Google Scholar] [CrossRef]
- Barnett, M.C.; Hegarty, R.S. Cysteamine hydrochloride increases bodyweight and wool fibre length, improves feed conversion ratio and reduces methane yield in sheep. Anim. Prod. Sci. 2014, 54, 1288–1293. [Google Scholar] [CrossRef]
- Wang, C.; Dong, C.J.; Wang, Z.Q.; Yang, F.; Mao, H.L.; Wu, Z.; Zhoua, Q.; Wanga, H.F. Effect of cysteamine hydrochloride supplementation on the milk performance of dairy cow. Livest. Sci. 2015, 178, 94–99. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Sheep; National Academies Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Geishauser, T. An instrument for the collection and transfer of ruminal fluid and for the administration of water soluble drugs in adult cattle. Bov. Pract. 1993, 27, 38–42. [Google Scholar] [CrossRef]
- Lodge-Ivey, S.L.; Browne-Silva, J.; Horvath, M.B. Technical note: Bacterial diversity and fermentation end products in rumen fluid samples collected via oral lavage or rumen cannula. J. Anim. Sci. 2009, 87, 2333–2337. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Bradford, M.M.; Williams, W.L. New, rapid, sensitive method for protein determination. Fed. Proc. 1976, 35, 274–284. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Barnett, A.R.; Jouany, J.P.; Newbold, J.; Agabriel, J.; Givens, I. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Patra, A.K.; Lalhriatpuii, M.; Debnath, B.C. Predicting enteric methane emission in sheep using linear and non-linear statistical models from dietary variables. Anim. Prod. Sci. 2016, 56, 574–584. [Google Scholar] [CrossRef]
- Sun, Y.K.; Yan, X.G.; Ban, Z.B.; Yang, H.M.; Hegarty, R.S.; Zhao, Y.M. The effect of cysteamine hydrochloride and nitrate supplementation on in-vitro and in-vivo methane production and productivity of cattle. Anim. Feed Sci. Technol. 2017, 232, 49–56. [Google Scholar] [CrossRef]
- Du, G.; Shi, Z.; Xia, D.; Wei, X.; Zhang, L.; Parvizi, N.; Zhao, R. Cysteamine improves growth performance and gastric ghrelin expression in preweaning piglets. Domest. Anim. Endocrinol. 2012, 42, 203–209. [Google Scholar] [CrossRef]
- Ueno, H.; Yamaguchi, H.; Kangawa, K.; Nakazato, M. Ghrelin: A gastric peptide that regulates food intake and energy homeostasis. Regul. Pept. 2005, 126, 11–19. [Google Scholar] [CrossRef]
- Barnett, M.C.; Hegarty, R.S. Cysteamine: A human health dietary additive with potential to improve livestock growth rate and efficiency. Anim. Prod. Sci. 2016, 56, 1330–1338. [Google Scholar] [CrossRef]
- McMahon, C.D.; Radcliff, R.P.; Lookingland, K.J.; Tucker, H.A. Neuroregulation of growth hormone secretion in domestic animals. Domest. Anim. Endocrinol. 2001, 20, 65–87. [Google Scholar] [CrossRef]
- Haenen, G.R.; Vermeulen, N.P.; Timmerman, H.; Bast, A. Effect of thiols on lipid peroxidation in rat liver microsomes. Chem. Biol. Interact. 1989, 71, 201–212. [Google Scholar] [CrossRef]
- Le Roith, D.; Bondy, C.; Yakar, S.; Jun-Li, L.; Andrew, B. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Berger, L.L.; van der Aar, P.J.; Fahey, G.C., Jr. Effects of lasalocid and monensin on nutrient digestion, metabolism and rumen characteristics of sheep. J. Anim. Sci. 1984, 58, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.A. Hormonal growth promotant use in the Australian beef industry. Anim. Prod. Sci. 2010, 50, 637–659. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, fat mass and immune system: Role for leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef]
- Pandit, R.; Beerens, S.; Adan, R.A. Role of leptin in energy expenditure: The hypothalamic perspective. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R938–R947. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Boisclair, Y.R. Leptin and the regulation of food intake, energy homeostasis and immunity with special focus on periparturient ruminants. Domest. Anim. Endocrinol. 2001, 21, 215–250. [Google Scholar] [CrossRef]
- Heath, T. Dukes’ Physiology of Domestic Animals. Aust. Vet. J. 2010, 71, 187. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Song, T.Z.; Feng, J.; Xia, C.Y.; Yuhong, B.; Ke, F.; Guoqing, S. Effects of Cysteamine on pH, Total Dehydrogenase and TVFA of Rumen in Weaned Lambs of Tibetan Sheep. Anim. Feed Sci. Technol. 2014, 35, 4–5. [Google Scholar]
- Quanjun, W.; Shengyong, M.; Hongxia, Z.; Weiyun, Z. Effects of cysteamine on in vitro fermentation by rumen microbes from goats. J. Huazhong Agric. 2002, 21, 535–539. [Google Scholar]
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Broucek, J. Production of Methane Emissions from Ruminant Husbandry: A Review. J. Environ. 2014, 5, 11. [Google Scholar] [CrossRef]
- Hegarty, R.S. Reducing rumen methane emissions through elimination of rumen protozoa. Crop Pasture Sci. 1999, 50, 1321–1328. [Google Scholar] [CrossRef]
- Min-Oo, G.; Ayi, K.; Bongfen, S.E.; Tam, M.; Radovanovic, I.; Gauthier, S.; Santiago, H.; Rothfuchs, A.G.; Roffê, E.; Sherf, A.; et al. Cysteamine, the natural metabolite of pantetheinase, shows specific activity against Plasmodium. Exp. Parasitol. 2010, 125, 315–324. [Google Scholar] [CrossRef]
- Shibata, M.; Terada, F.; Kurihara, M.; Nishida, T.; Iwasaki, K. Estimation of methane production in ruminants. Anim. Feed Sci. Technol. 1993, 64, 790–796. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W.; Carvalho, S.B.; Davies, T.J.; Ferrier, S.; Fritz, S.A.; Grenyer, R.; Helmus, M.R.; Jin, L.S.; Mooers, A.O.; et al. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 2017, 92, 698–715. [Google Scholar] [CrossRef]
- Pereira, D.H.; Pereira, O.G.; da Silva, B.C.; Leao, M.I.; Valadares, S.D.; Chizzotti, F.H.M.; Garcia, R. Intake and total and partial digestibility of nutrients, ruminal pH and ammonia concentration and microbial efficiency in beef cattle fed with diets containing sorghum (Sorghum bicolor (L.) Moench) silage and concentrate in different ratios. Livest. Sci. 2007, 107, 53–61. [Google Scholar] [CrossRef]
- Chen, H.; Guo, B.; Yang, M.; Luo, J.; Hu, Y.; Qu, M.; Song, X. Response of Growth Performance, Blood Biochemistry Indices, and Rumen Bacterial Diversity in Lambs to Diets Containing Supplemental Probiotics and Chinese Medicine Polysaccharides. Front. Vet. Sci. 2021, 8, 681389. [Google Scholar] [CrossRef]
- McCann, J.C.; Wiley, L.M.; Forbes, T.D.; Rouquette, F.M.; Tedeschi, L.O.; Zilberstein, D. Relationship between the rumen microbiome and residual feed intake-efficiency of brahman bulls stocked on bermudagrass pastures. PLoS ONE 2014, 9, e091864. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Maekawa, M.; Beauchemin, K.A.; Christensen, D.A. Effect of concentrate level and feeding management on chewing activities, saliva production, and ruminal pH of lactating dairy cows. J. Dairy Sci. 2002, 85, 1165–1175. [Google Scholar] [CrossRef]
- Kim, J.N.; Méndez-García, C.; Geier, R.R.; Iakiviak, M.; Chang, J.; Cann, I.; Mackie, R.I. Metabolic networks for nitrogen utilization in Prevotella ruminicola 23. Sci. Rep. 2017, 7, 7851. [Google Scholar] [CrossRef]
- Wen, Z.; Morrison, M. Glutamate dehydrogenase activity profiles for type strains of ruminal Prevotella spp. Appl. Environ. Microbiol. 1997, 63, 3314–3317. [Google Scholar] [CrossRef]
- Xue, F.; Pan, X.; Jiang, L.; Guo, Y.; Xiong, B. GC–MS analysis of the ruminal metabolome response to thiamine supplementation during high grain feeding in dairy cows. Metabolomics 2018, 14, 67. [Google Scholar] [CrossRef]
- Zhang, T.; Mu, Y.Y.; Zhang, R.Y.; Xue, Y.F.; Guo, C.Z.; Qi, W.P.; Zhanga, J.; Maoac, S. Responsive changes of rumen microbiome and metabolome in dairy cows with different susceptibility to subacute ruminal acidosis. Anim. Nutr. 2022, 8, 331–340. [Google Scholar] [CrossRef]
- Liang, D.; Li, N.; Dai, N.L.; Zhang, H.; Hu, H. Effects of different types of potato resistant starches on intestinal microbiota and short-chain fatty acids under in vitro fermentation. Int. J. Food Sci. Technol. 2020, 56, 2432–2442. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 6, 971–982. [Google Scholar] [CrossRef]
- Yost, W.M.; Young, J.W.; Schmidt, S.P.; McGilliard, A.D. Gluconeogenesis in Ruminants: Propionic Acid Production from a High-Grain Diet Fed to Cattle2. J. Nutr. 1977, 107, 2036–2043. [Google Scholar] [CrossRef]
- Hu, R.; Zou, H.; Wang, Z.; Cao, B.; Peng, Q.; Jing, X.; Wang, Y.; Shao, Y.; Pei, Z.; Zhang, X.; et al. Nutritional Interventions Improved Rumen Functions and Promoted Compensatory Growth of Growth-Retarded Yaks as Revealed by Integrated Transcripts and Microbiome Analyses. Front. Microbiol. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Sandra, K.; Pinares-Patiño, C.S.; Henning, S.; Seedorf, H.; Kirk, M.R.; Ganesh, S.; Janssen, P.H. Two Different Bacterial Community Types Are Linked with the Low-Methane Emission Trait in Sheep. PLoS ONE 2014, 9, e103171. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic Alterations in Yak Rumen Bacteria Community and Metabolome Characteristics in Response to Feed Type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Hamar, D.; Borchers, R. Glycolytic pathway in rumen microorganisms. J. Anim. Sci. 1967, 26, 654–657. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Liu, X.; Zhao, X.; Liu, S.; Li, Y.; Zhang, Y. Growth, health, rumen fermentation, and bacterial community of Holstein calves fed Lactobacillus rhamnosus GG during the preweaning stage1. J. Anim. Sci. 2019, 97, 2598–2608. [Google Scholar] [CrossRef]
- Kraatz, M.; Wallace, R.J.; Svensson, L. Olsenella umbonata sp. nov., a microaerotolerant anaerobic lactic acid bacterium from the sheep rumen and pig jejunum, and emended descriptions of Olsenella, Olsenella uli and Olsenella profusa. Int. J. Syst. Evol. Microbiol. 2011, 61, 795–803. [Google Scholar] [CrossRef]
| Item | Content |
|---|---|
| Ingredient, g/kg DM | |
| Foxtail millet silage | 100 |
| Corn stover | 100 |
| Corn meal | 500 |
| Soybean meal | 170 |
| Rapeseed meal | 80 |
| Vitamin and mineral premix 1 | 50 |
| Chemical composition, g/kg DM | |
| Crude protein | 153 |
| Ether extract | 33 |
| Neutral detergent fiber | 203 |
| Acid detergent fiber | 102 |
| Calcium | 9.1 |
| Phosphorus | 3.6 |
| Gross energy, MJ/kg | 15.8 |
| Item 1 | Supplementary Level (mg/kg BW) 2 | SEM | p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| CS0 | CS20 | CS40 | CS60 | Linear | Quadratic | ||
| ADG (g/d) | 256 b | 276 ab | 313 a | 307 ab | 16.7 | 0.02 | 0.46 |
| DMI (kg/d) | 1.33 c | 1.34 c | 1.49 a | 1.38 b | 0.02 | <0.01 | <0.01 |
| F:G | 5.18 a | 4.83 b | 4.77 b | 4.48 c | 0.02 | <0.01 | 0.12 |
| Item 1 | Supplementary Level (mg/kg BW) 2 | SEM | p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| CS0 | CS20 | CS40 | CS60 | Linear | Quadratic | ||
| Somatostatin | 89.3 a | 82.7 b | 81.2 b | 76.9 b | 1.88 | <0.01 | 0.53 |
| GH | 1.55 c | 1.99 b | 2.18 b | 2.44 a | 0.08 | <0.01 | 0.29 |
| IGF-1 | 36.6 c | 41.2 b | 42.1 b | 45.3 a | 0.93 | <0.01 | 0.42 |
| Leptin | 2.97 a | 2.49 b | 2.42 b | 2.07 c | 0.08 | <0.01 | 0.46 |
| Item 1 | Supplementary Level (mg/kg BW) 2 | SEM | p-Value 3 | ||||
|---|---|---|---|---|---|---|---|
| CS0 | CS20 | CS40 | CS60 | Linear | Quadratic | ||
| pH | 6.26 | 6.29 | 6.28 | 6.24 | 0.11 | 0.89 | 0.77 |
| Ammonia N, mg/mL | 22.8 | 25.0 | 26.8 | 26.4 | 2.43 | 0.24 | 0.59 |
| MCP, mg/mL | 0.377 b | 0.396 ab | 0.413 a | 0.405 a | 0.01 | <0.01 | 0.07 |
| Total VFA, mmol/L | 118.3 b | 129.9 ab | 127.5 ab | 136.8a | 4.16 | 0.01 | 0.79 |
| VFA patterns, % molar | |||||||
| Acetate | 50.2 a | 47.8 ab | 46.8 b | 47.8 ab | 2.76 | 0.03 | 0.04 |
| Propionate | 22.9 c | 24.3 b | 25.6 a | 25.7 a | 2.40 | <0.01 | 0.12 |
| Butyrate | 6.52 | 7.63 | 5.51 | 6.63 | 1.30 | 0.23 | 0.98 |
| Acetate-to-Propionate | 2.15 a | 1.98 ab | 1.83 b | 1.86 b | 0.06 | <0.01 | 0.15 |
| CH4e, mmol/L | 19.1 a | 17.6 b | 16.2 c | 16.7 bc | 0.43 | <0.01 | 0.04 |
| Methane, L/day | 15.8 b | 15.9 b | 17.5 a | 17.5 a | 1.62 | <0.01 | 0.69 |
| Methane, L/kg ADG | 61.9 a | 57.7 b | 55.9 c | 57.1 b | 0.23 | <0.01 | <0.01 |
| Item | Supplementary Level (mg/kg BW) 1 | SEM | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|
| CS0 | CS20 | CS40 | CS60 | Linear | Quadratic | ||
| Coverage | 0.99 | 0.99 | 0.99 | 0.99 | <0.01 | 0.77 | 0.02 |
| Chao | 446 | 401 | 444 | 504 | 46.65 | 0.34 | 0.29 |
| Ace | 461 | 396 | 445 | 508 | 45.99 | 0.37 | 0.17 |
| Shannon | 3.47 | 3.25 | 3.37 | 3.84 | 0.21 | 0.21 | 0.13 |
| Simpson | 0.07 | 0.09 | 0.11 | 0.05 | 0.01 | 0.64 | 0.08 |
| Item | Supplementary Level (mg/kg BW) 1 | SEM | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|
| CS0 | CS20 | CS40 | CS60 | Linear | Quadratic | ||
| Phylum, % | |||||||
| Firmicutes | 58.1 ab | 68.5 a | 28.3 b | 63.7 ab | 11.4 | 0.64 | 0.28 |
| Bacteroidota | 4.08 a | 14.37 b | 60.92 b | 19.25 b | 8.35 | 0.03 | 0.01 |
| Actinobacteriota | 36.42 a | 3.40 b | 1.36 b | 3.21 b | 7.17 | <0.01 | 0.03 |
| Patescibacteria | 0.60 | 3.74 | 3.93 | 2.32 | 1.80 | 0.50 | 0.21 |
| Proteobacteria | 0.08 | 8.62 | 2.17 | 0.87 | 3.97 | 0.81 | 0.23 |
| Synergistota | 0.07 | 0.71 | 1.22 | 0.15 | 0.54 | 0.74 | 0.13 |
| Spirochaetota | 0.10 | 0.19 | 1.34 | 0.11 | 0.61 | 0.66 | 0.29 |
| Genus, % | |||||||
| Norank_f_Eubacterium_coprostanoligenes_group | 14.19 | 19.23 | 5.33 | 9.38 | 5.62 | 0.26 | 0.93 |
| Prevotella | 1.54 b | 8.54 b | 44.58 a | 9.45 b | 9.57 | 0.17 | 0.04 |
| Olsenella | 33.81 a | 1.04 b | 0.76 b | 11.20 ab | 7.98 | 0.07 | 0.02 |
| Lachnospiraceae_NK3A20_group | 12.83 | 4.20 | 3.60 | 9.35 | 3.10 | 0.42 | 0.04 |
| Christensenellaceae_R-7_group | 6.92 | 12.05 | 4.71 | 2.89 | 5.75 | 0.44 | 0.55 |
| Acetitomaculum | 5.02 | 5.29 | 0.41 | 3.92 | 2.14 | 0.39 | 0.46 |
| Ruminococcus | 2.71 | 7.13 | 1.83 | 3.53 | 3.25 | 0.84 | 0.68 |
| Norank_f_F082 | 0.52 | 3.09 | 2.35 | 2.04 | 1.23 | 0.48 | 0.26 |
| NK4A214_group | 3.15 | 3.04 | 0.64 | 4.58 | 2.02 | 0.83 | 0.33 |
| Candidatus_Saccharimonas | 0.60 | 3.74 | 3.92 | 1.85 | 1.78 | 0.61 | 0.17 |
| norank_f__norank_o__Clostridia_UCG-014 | 0.62 | 1.31 | 1.20 | 4.18 | 1.26 | 0.08 | 0.37 |
| Rikenellaceae_RC9_gut_group | 1.44 | 0.56 | 2.74 | 0.72 | 1.31 | 0.99 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.-C.; Wang, W.-K.; Zhang, F.; Li, W.-J.; Wang, Y.-L.; Lv, L.-K.; Yang, H.-J. Dietary Cysteamine Supplementation Remarkably Increased Feed Efficiency and Shifted Rumen Fermentation toward Glucogenic Propionate Production via Enrichment of Prevotella in Feedlot Lambs. Microorganisms 2022, 10, 1105. https://doi.org/10.3390/microorganisms10061105
Wu Q-C, Wang W-K, Zhang F, Li W-J, Wang Y-L, Lv L-K, Yang H-J. Dietary Cysteamine Supplementation Remarkably Increased Feed Efficiency and Shifted Rumen Fermentation toward Glucogenic Propionate Production via Enrichment of Prevotella in Feedlot Lambs. Microorganisms. 2022; 10(6):1105. https://doi.org/10.3390/microorganisms10061105
Chicago/Turabian StyleWu, Qi-Chao, Wei-Kang Wang, Fan Zhang, Wen-Juan Li, Yan-Lu Wang, Liang-Kang Lv, and Hong-Jian Yang. 2022. "Dietary Cysteamine Supplementation Remarkably Increased Feed Efficiency and Shifted Rumen Fermentation toward Glucogenic Propionate Production via Enrichment of Prevotella in Feedlot Lambs" Microorganisms 10, no. 6: 1105. https://doi.org/10.3390/microorganisms10061105
APA StyleWu, Q.-C., Wang, W.-K., Zhang, F., Li, W.-J., Wang, Y.-L., Lv, L.-K., & Yang, H.-J. (2022). Dietary Cysteamine Supplementation Remarkably Increased Feed Efficiency and Shifted Rumen Fermentation toward Glucogenic Propionate Production via Enrichment of Prevotella in Feedlot Lambs. Microorganisms, 10(6), 1105. https://doi.org/10.3390/microorganisms10061105







