Optimization of the Decolorization of the Reactive Black 5 by a Laccase-like Active Cell-Free Supernatant from Coriolopsis gallica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Culture Conditions
2.2. Laccase-like Activity Assays of the Cell-Free Supernatant from Coriolopsis gallica
2.3. Experimental Design and Data Analysis
2.4. Plackett–Burman Design
2.5. Response Surface Methodology Using a Box–Behnken Design
2.6. Design and Statistical Analysis
2.7. Decolorization Assay in 500 mL Volume
2.8. RB5 Spectrum Analysis
3. Results
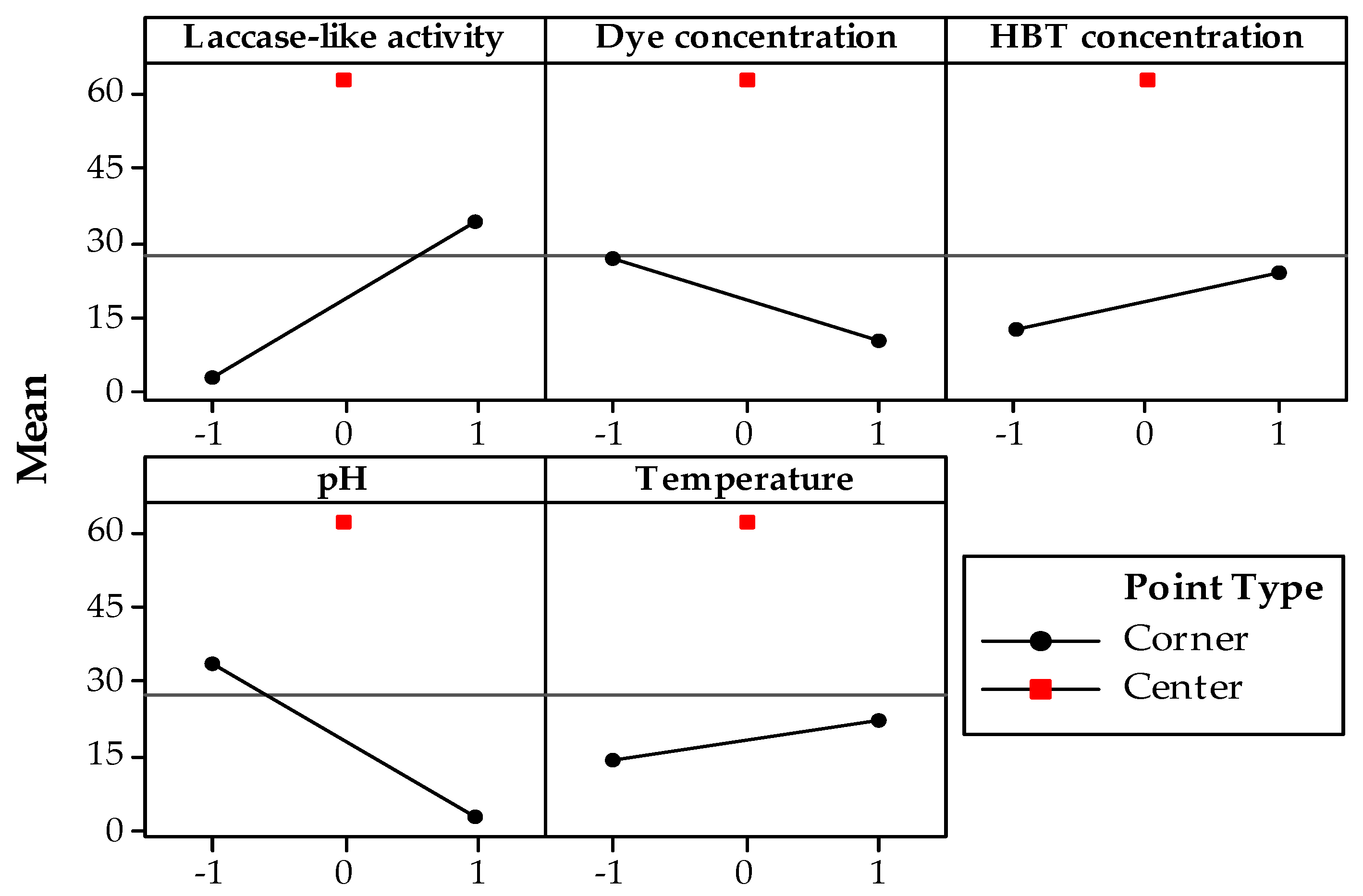
3.1. Screening Design
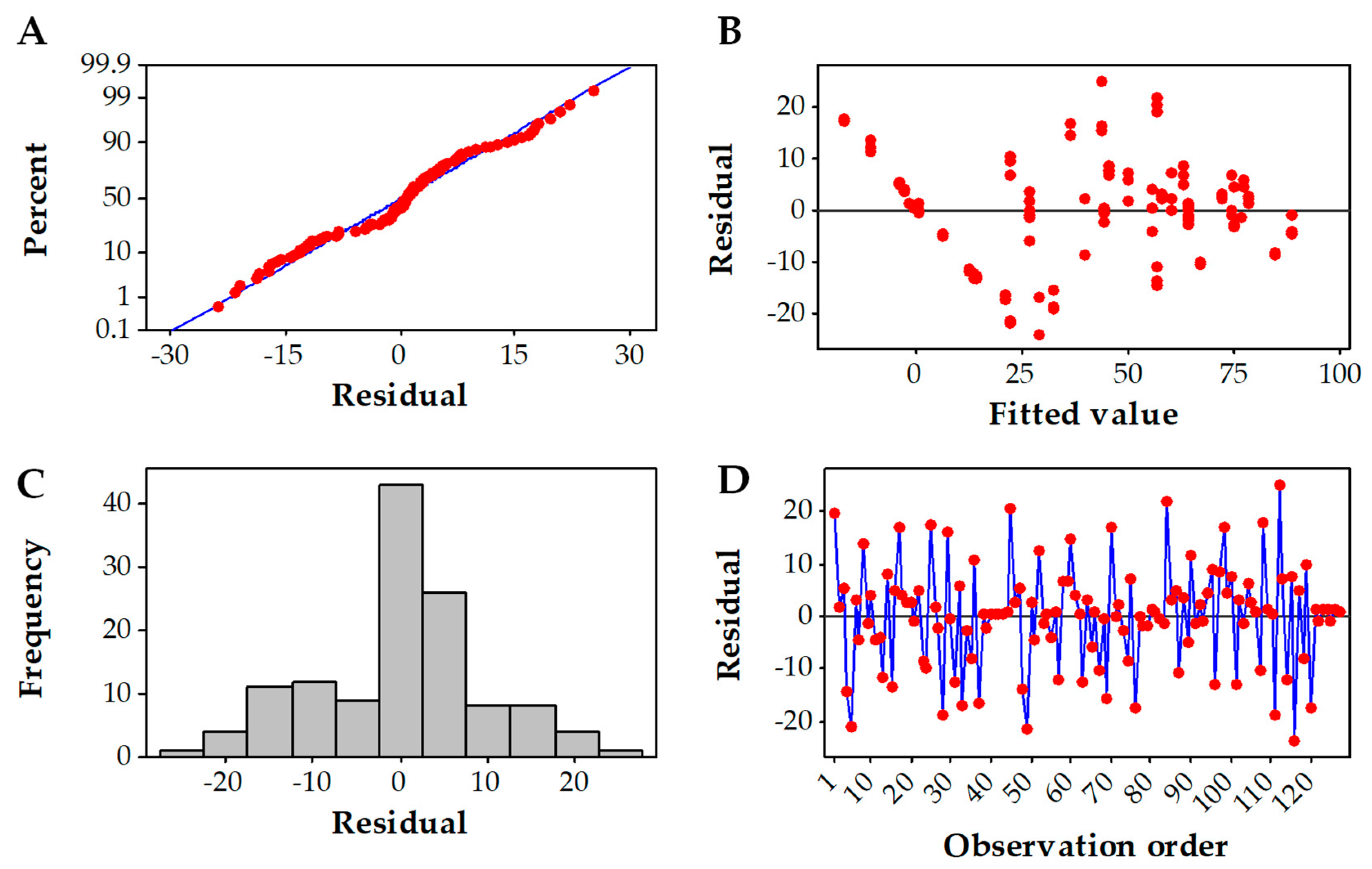
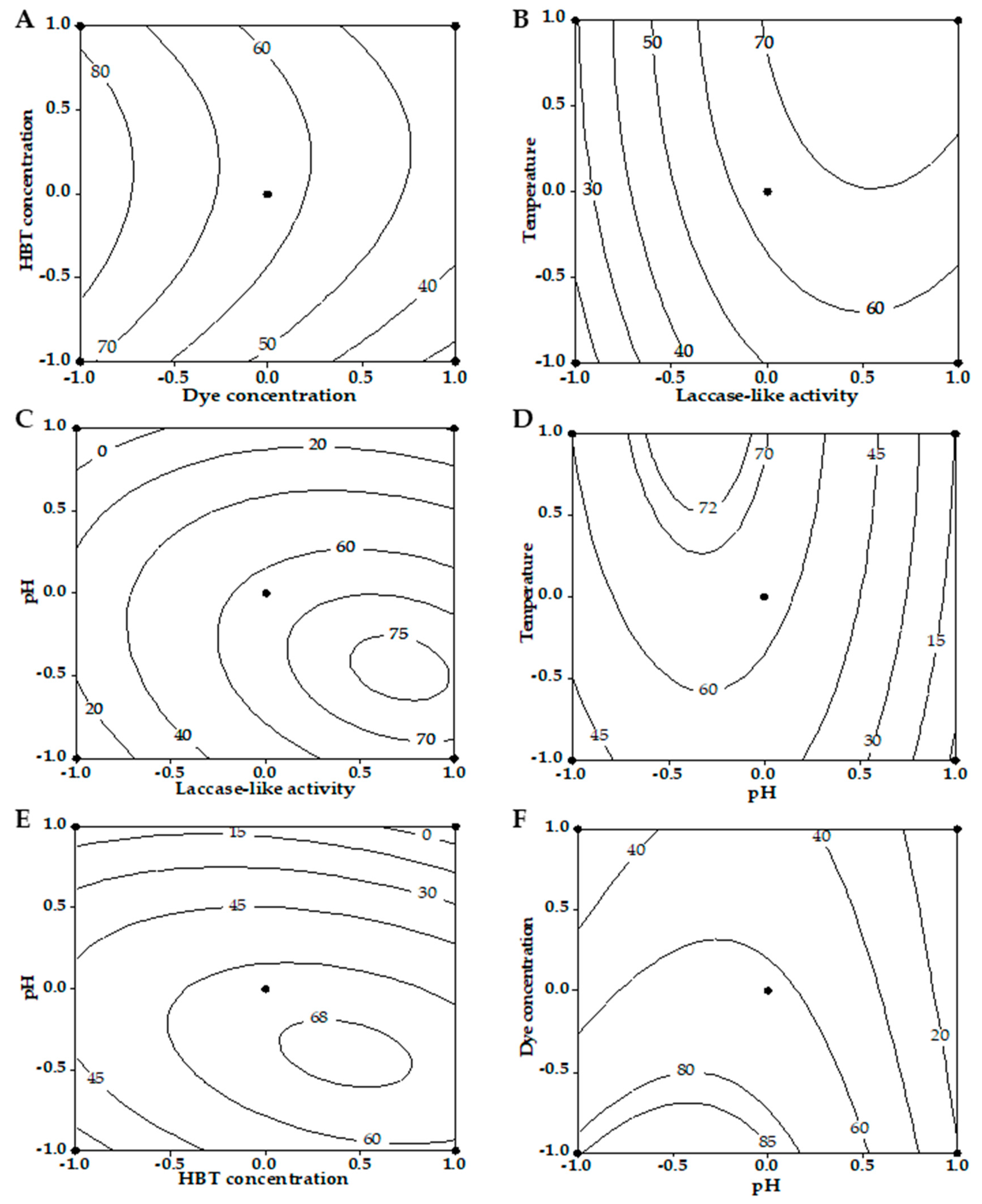
3.2. Box–Behnken Design
33.40⋅x4² − 3.60 x5² + 9.78⋅x1⋅x2 + 4.04⋅x1⋅x3 − 12.88⋅x1⋅x4 + 2.45⋅x1⋅x5 + 1.94⋅x2⋅x3 + 10.41⋅x2⋅x4 +
4.33⋅x2⋅x5 − 13.17⋅x3⋅x4 + 10.57⋅x3⋅x5 − 1.71⋅x4⋅x5
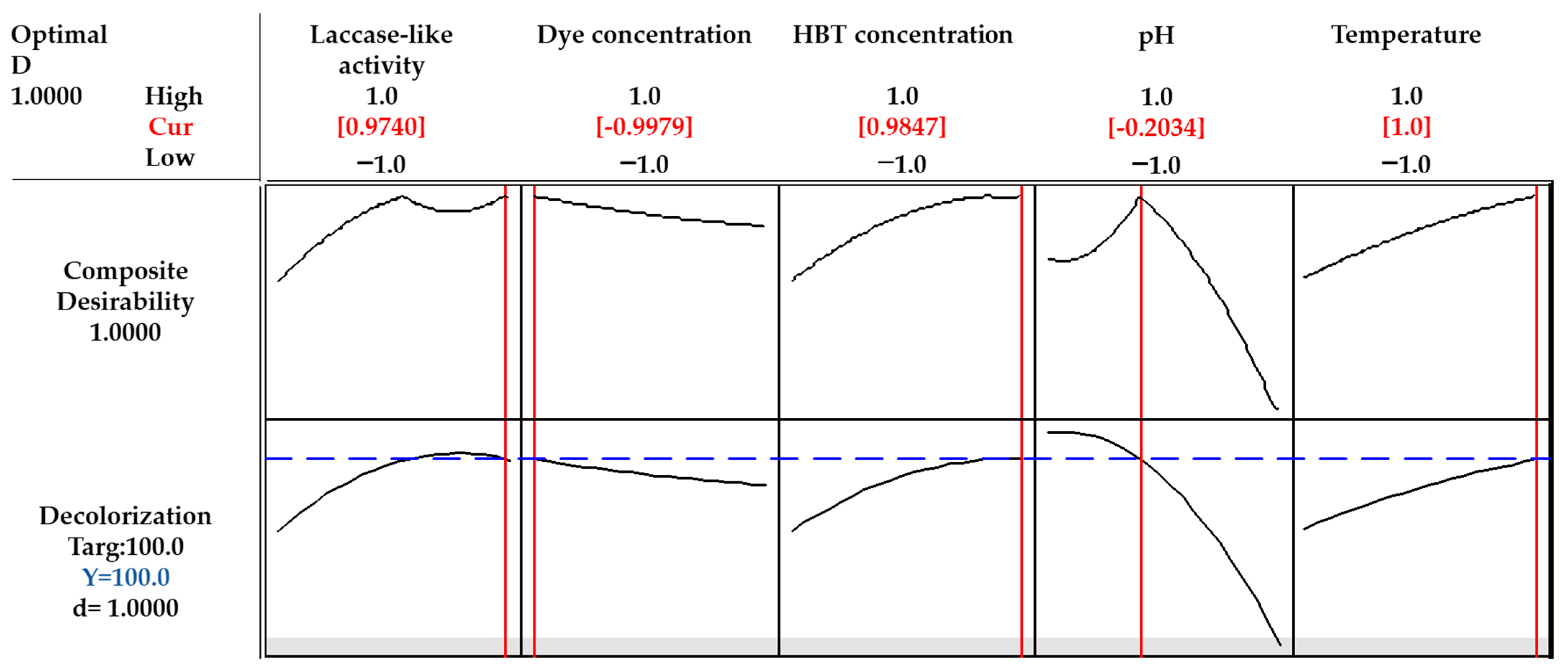
3.3. Conditions Optimization
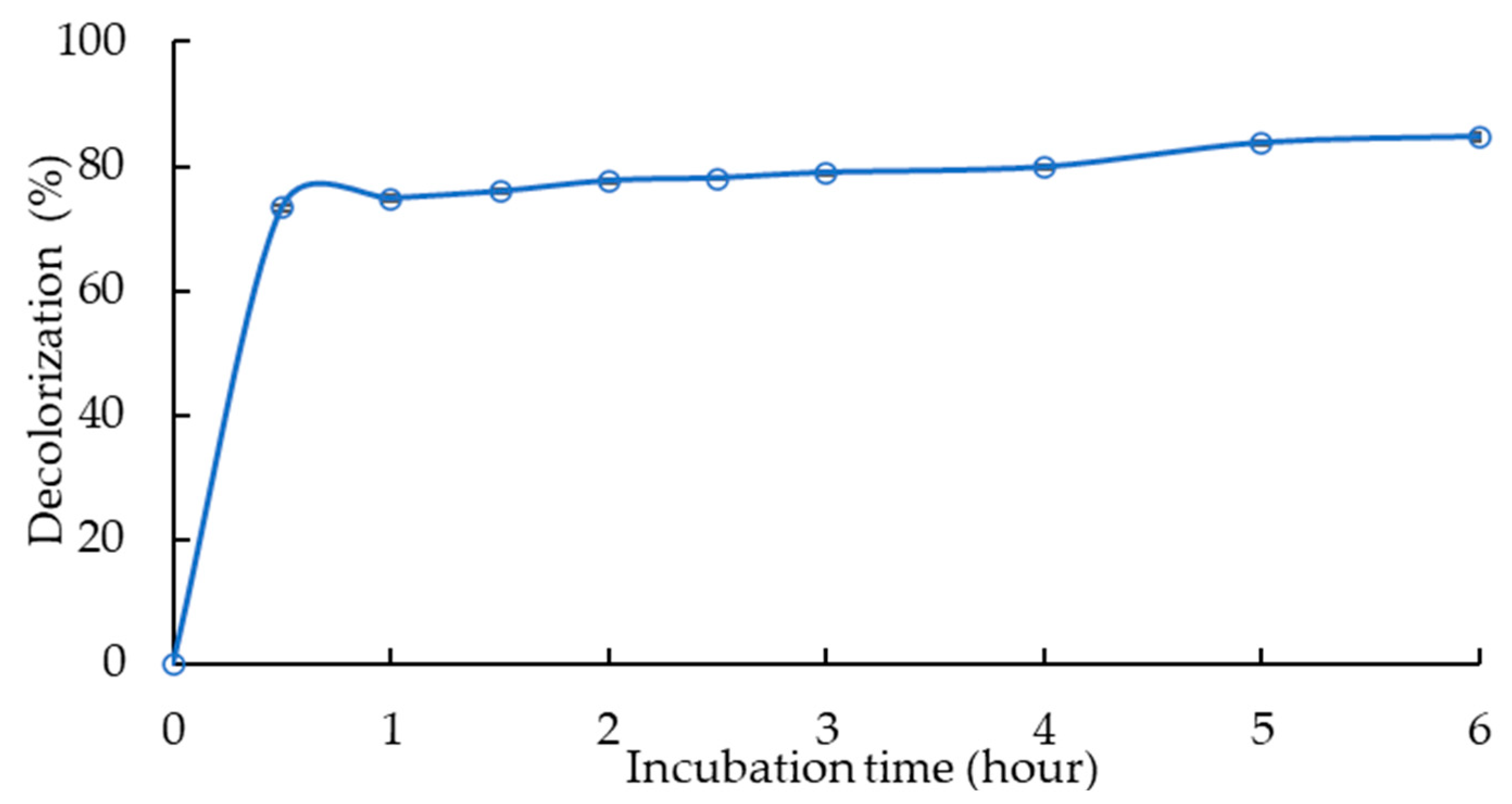
3.4. Decolorization Process in 500 mL Volume
3.5. UV-VIS Spectrum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganoulis, J. Risk Analysis of Water Pollution; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wu, C.; Maurer, C.; Wang, Y.; Xue, S.; Davis, D.L. Water pollution and human health in China. Environ. Health Perspect. 1999, 107, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.S.; Pandey, S.; Bhadauria, S. Status of water pollution about industrialization in Rajasthan. Rev. Environ. Health 2017, 32, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Chatha, S.A.S.; Asgher, M.; Iqbal, H.M. Enzyme-based solutions for textile processing and dye contaminant biodegradation—A review. Environ. Sci. Pollut. Res. 2017, 24, 14005–14018. [Google Scholar] [CrossRef]
- de Oliveira Neto, G.C.; Correia, J.M.F.; Silva, P.C.; de Oliveira Sanches, A.G.; Lucato, W.C. Cleaner Production in the textile industry and its relationship to sustainable development goals. J. Clean. Prod. 2019, 228, 1514–1525. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L. A review of energy use and energy efficiency technologies for the textile industry. Renew. Sustain. Energy Rev. 2012, 16, 3648–3665. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Zhao, J.; Geng, Y.; Tian, Y.; Jiang, P. Energy-related GHG emissions of the textile industry in China. Resour. Conserv. Recycl. 2017, 119, 69–77. [Google Scholar] [CrossRef]
- Holme, I. Recent developments in colorants for textile applications. Surf. Coat. Int. Part B Coat. Trans. 2002, 85, 243–264. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of dye and pigments. In Dyes and Pigments; Springer: Cham, Switzerland, 2016; pp. 31–45. [Google Scholar] [CrossRef]
- Mansour, H.; Boughzala, O.; Barillier, D.; Chekir-Ghedira, L.; Mosrati, R. Les colorants textiles sources de contamination de l’eau: Criblage de la toxicité et des méthodes de traitement. Revue des sciences de l’eau. J. Water Sci. 2011, 24, 209–238. [Google Scholar] [CrossRef] [Green Version]
- Mohorčič, M.; Friedrich, J.; Pavkob, A. Decoloration of the diazo dye Reactive Black 5 by immobilized Bjerkandera adusta in a stirred tank bioreactor. Acta Chim. Slov. 2004, 51, 619–628. [Google Scholar]
- Iscen, C.F.; Kiran, I.; Ilhan, S. Biosorption of Reactive Black 5 dye by Penicillium restrictum: The kinetic study. J. Hazard. Mater. 2007, 143, 335–340. [Google Scholar] [CrossRef]
- Rodriguez-Couto, S.; Toca-Herrera, J.L. Laccases in the textile industry. Biotechnol. Mol. Biol. Rev. 2006, 1, 115–120. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of synthetic azo dyes of textile industry: A sustainable approach using microbial enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Kusvuran, E.; Irmak, S.; Yavuz, H.I.; Samil, A.; Erbatur, O. Comparison of the treatment methods efficiency for decolorization and mineralization of Reactive Black 5 azo dye. J. Hazard. Mater. 2005, 119, 109–116. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A. Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dyes Pigments 2006, 71, 236–244. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Rajkumar, K.; Mathkumar, M. Statistical optimization of electro oxidation process for removal of textile dye CI Reactive Blue 198. Int. J. Environ. Sci. Nat. Resour. 2017, 1, 555570. [Google Scholar]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, R.; Nordlinder, R.; Wass, U. Asthma, rhinitis and dermatitis in workers exposed to reactive dyes. Occup. Environ. Med. 1993, 50, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Murugesan, K.; Kalaichelvan, P.T. Synthetic dye decolorization by white rot fungi. Indian J. Exp. Biol. 2003, 41, 1076–1087. [Google Scholar] [PubMed]
- Merouani, D.R. Traitement de Colorants Azoïque et Anthraquinonique par Procédés D’oxydation Avancée (POA). Ph.D. Thesis, Lille 1 à l’Université des Sciences et Technologies de Lille et L’université Abdelhamid Ibn Badis de Mostaganem, Mostaganem, Algeria, 2011. [Google Scholar]
- Hachem, C.; Bocquillon, F.; Zahraa, O.; Bouchy, M. Decolorization of textile industry wastewater by the photocatalytic degradation process. Dyes Pigments 2001, 49, 117–125. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Murugesan, K.; Dhamija, A.; Nam, I.H.; Kim, Y.M.; Chang, Y.S. Decolorization of reactive black 5 by laccase: Optimization by response surface methodology. Dyes Pigments 2007, 75, 176–184. [Google Scholar] [CrossRef]
- Khlifi, R.; Belbahri, L.; Woodward, S.; Ellouz, M.; Dhouib, A.; Sayadi, S.; Mechichi, T. Decolorization and detoxification of textile industry wastewater by the laccase-mediator system. J. Hazard. Mater. 2010, 175, 802–808. [Google Scholar] [CrossRef]
- Warren, C.E. Biology and Water Pollution Control; W.B. Saunders: Philadelphia, PA, USA, 1971; p. 434. [Google Scholar]
- Madhu, A.; Chakraborty, J.N. Developments in application of enzymes for textile processing. J. Clean. Prod. 2017, 145, 114–133. [Google Scholar] [CrossRef]
- Abadulla, E.; Tzanov, T.; Costa, S.; Robra, K.H.; Cavaco-Paulo, A.; Gübitz, G.M. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 2000, 66, 3357–3362. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Viraraghavan, T. Fungal decolorization of dye wastewaters: A review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef]
- Uzan, E.; Nousiainen, P.; Balland, V.; Sipila, J.; Piumi, F.; Navarro, D.; Asther, M.; Record, E.; Lomascolo, A. High redox potential laccases from the lignolytic fungi Pycnoporus coccineus and P. sanguineus suitable for white biotechnology: From gene cloning to enzyme characterization and applications. J. Appl. Microbiol. 2010, 108, 2199–2213. [Google Scholar] [CrossRef]
- Xu, L.; Sun, K.; Wang, F.; Zhao, L.; Hu, J.; Ma, H.; Ding, Z. Laccase production by Trametes versicolor in solid-state fermentation using tea residues as substrate and its application in dye decolorization. J. Environ. Manag. 2020, 270, 110904. [Google Scholar] [CrossRef]
- Kiran, S.; Huma, T.; Jalal, F.; Farooq, T.; Hameed, A.; Gulzar, T.; Bashir, A.; Rahmat, M.; Rahmat, R.; Rafique, M.A. Lignin degrading system of Phanerochaete chrysosporium and its exploitation for degradation of synthetic dyes wastewater. Pol. J. Environ. Stud. 2019, 28, 1749–1757. [Google Scholar] [CrossRef]
- Carbajo, J.M.; Junca, H.; Terrón, M.C.; González, T.; Yagüe, S.; Zapico, E.; González, A.E. Tannic acid induces transcription of laccase gene cglcc1 in the white-rot fungus Coriolopsis gallica. Can. J. Microbiol. 2002, 48, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Daâssi, D.; Rodríguez-Couto, S.; Nasri, M.; Mechichi, T. Biodegradation of textile dyes by immobilized laccase from Coriolopsis gallica into Ca-alginate beads. Int. Biodeterior. Biodegrad. 2014, 90, 71–78. [Google Scholar] [CrossRef]
- Reyes, P.; Pickard, M.A.; Vazquez-Duhalt, R. Hydroxybenzotriazole increases the range of textile dyes decolorized by immobilized laccase. Biotechnol. Lett. 1999, 21, 875–880. [Google Scholar] [CrossRef]
- Daâssi, D.; Mechichi, T.; Nasri, M.; Rodriguez-Couto, S. Decolorization of the metal textile dye Lanaset Grey G by immobilized white-rot fungi. J. Environ. Manag. 2013, 129, 324–332. [Google Scholar] [CrossRef]
- Daâssi, D.; Lozano-Sánchez, J.; Borrás-Linares, I.; Belbahri, L.; Woodward, S.; Zouari-Mechichi, H.; Nasri, M.; Segura-Carretero, A. Olive oil mill wastewaters: Phenolic content characterization during degradation by Coriolopsis gallica. Chemosphere 2014, 113, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Bressler, D.C.; Fedorak, P.M.; Pickard, M.A. Oxidation of carbazole, N-ethylcarbazole, fluorene, and dibenzothiophene by the laccase of Coriolopsis gallica. Biotechnol. Lett. 2000, 22, 1119–1125. [Google Scholar] [CrossRef]
- Zhuo, R.; Zhang, J.; Yu, H.; Ma, F.; Zhang, X. The roles of Pleurotus ostreatus HAUCC 162 laccase isoenzymes in decolorization of synthetic dyes and the transformation pathways. Chemosphere 2019, 234, 733–745. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [Green Version]
- Berthet, S.; Thevenin, J.; Baratiny, D.; Demont-Caulet, N.; Debeaujon, I.; Bidzinski, P.; Leple, J.C.; Huis, R.; Hawkins, S.; Gomez, L.; et al. Role of plant laccases in lignin polymerization. Adv. Bot. Res. 2012, 61, 145–172. [Google Scholar] [CrossRef]
- Claus, H. Laccases and their occurrence in prokaryotes. Arch. Microbiol. 2003, 179, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, N.T.; Kanost, M.R. Insect multicopper oxidases: Diversity, properties, and physiological roles. Insect Biochem. Mol. Biol. 2010, 40, 179–188. [Google Scholar] [CrossRef]
- Gochev, V.K.; Krastanov, A.I. Fungal laccases. Bulg. J. Agric. Sci. 2007, 13, 75–83. [Google Scholar]
- Mikolasch, A.; Timo, H.J.N.; Michael, L.; Sabine, W.; Simone, S.; Elke, H.; Frieder, S.; Manuela, G.; Susanne, H.; Wolf-Dieter, J.; et al. Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem. Pharm. Bull. 2006, 54, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Pezzella, C.; Lucia, G.; Alessandra, P. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Aracri, E.; Josep, F.C.; Teresa, V. Application of laccase-natural mediator systems to sisal pulp: An effective approach to biobleaching or functionalizing pulp fibres? Bioresour. Technol. 2009, 100, 5911–5916. [Google Scholar] [CrossRef] [PubMed]
- Minussi, R.C.; Gláucia, M.P.; Nelson, D. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Hammami, A.; Bayoudh, A.; Hadrich, B.; Abdelhedi, O.; Jridi, M.; Nasri, M. Response-surface methodology for the production and the purification of a new H2O2-tolerant alkaline protease from Bacillus invictae AH1 strain. Biotechnol. Prog. 2020, 36, e2965. [Google Scholar] [CrossRef]
- Rmili, F.; Achouri, N.; Smichi, N.; Krayem, N.; Bayoudh, A.; Gargouri, Y.; Chamkha, M.; Fendri, A. Purification and biochemical characterization of an organic solvent-tolerant and detergent-stable Lipase from Staphylococcus capitis. Biotechnol. Prog. 2019, 35, e2833. [Google Scholar] [CrossRef]
- Louati, I.; Elloumi-Mseddi, J.; Cheikhrouhou, W.; Hadrich, B.; Nasri, M.; Aifa, S.; Woodward, S.; Mechichi, T. Simultaneous clean-up of Reactive Black 5 and cadmium by a desert soil bacterium. Ecotoxicol. Environ. Saf. 2020, 190, 110103. [Google Scholar] [CrossRef]
- Ghariani, B.; Hadrich, B.; Louati, I.; Mtibaà, R.; Daâssi, D.; Rodriguez-Couto, S.; Nasri, M.; Mechichi, T. Porous heat-treated fungal biomass: Preparation, characterization and application for removal of textile dyes from aqueous solutions. J. Porous Mater. 2019, 26, 1475–1488. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Laccases for denim bleaching: An eco-friendly alternative. Sigma 2012, 1, 10–12. [Google Scholar] [CrossRef]
- Hu, M.R.; Chao, Y.P.; Zhang, G.Q.; Xue, Z.Q.; Qian, S. Laccase-mediator system in the decolorization of different types of recalcitrant dyes. J. Ind. Microbiol. Biotechnol. 2009, 36, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, H.; Huang, L.; Pan, Y.; Huang, J.; Liu, Y. Electrochemical characteristics of the decolorization of three dyes by laccase mediator system (LMS) with synthetic and natural mediators. Chemosphere 2020, 239, 124779. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Gentili, P. Chemical messengers: Mediated oxidations with the enzyme laccase. J. Phys. Org. Chem. 2004, 17, 973–977. [Google Scholar] [CrossRef]
- Li, X.; La, G.; Cheng, Q.; Wang, F.; Feng, F.; Zhang, B.; Zhang, Z. Profile of natural redox mediators’ production of laccase-producing fungus Pleurotus ostreatus. Bull. Environ. Contam. Toxicol. 2014, 93, 478–482. [Google Scholar] [CrossRef]
- Moldes, D.; Díaz, M.; Tzanov, T.; Vidal, T. Comparative study of the efficiency of synthetic and natural mediators in laccase-assisted bleaching of eucalyptus kraft pulp. Bioresour. Technol. 2008, 99, 7959–7965. [Google Scholar] [CrossRef]
- Mechichi, T.; Mhiri, N.; Sayadi, S. Remazol Brilliant Blue R decolorization by the laccase from Trametes trogii. Chemosphere 2006, 64, 998–1005. [Google Scholar] [CrossRef]
- Vanaja, K.; Shobha Rani, R.H. Design of experiments: Concept and applications of Plackett Burman design. Clin. Res. Regul. Aff. 2007, 24, 1–23. [Google Scholar] [CrossRef]
- Goupy, J.; Creighton, L. Introduction aux Plans D’expériences: Avec Applications; Dunod Technique Et Ingenierie; Dunod, L’Usine Nouvelle: Paris, France, 2013. [Google Scholar]
- Hasanbeigi, A.; Price, L. A technical review of emerging technologies for energy and water efficiency and pollution reduction in the textile industry. J. Clean. Prod. 2015, 95, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, M.A. Water conservation in textile industry. Pak. Text. J. 2009, 58, 48–51. [Google Scholar]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lim, J.S.; Lee, Y.; Lee, B.; Kim, S.W.; Lee, J.; Kim, S. Optimization and morphology for decolorization of reactive black 5 by Funalia trogii. Enzyme Microbiol. Technol. 2007, 40, 1758–1764. [Google Scholar] [CrossRef]
- Sayahi, E.; Ladhari, N.; Mechichi, T.; Sakli, F. Azo dyes decolorization by the laccase from Trametes trogii. J. Text. Inst. 2016, 107, 1478–1482. [Google Scholar] [CrossRef]
- Daâssi, D.; Frikha, F.; Zouari-Mechichi, H.; Belbahri, L.; Woodward, S.; Mechichi, T. Application of response surface methodology to optimize decolorization of dyes by the laccase-mediator system. J. Environ. Manag. 2012, 108, 84–91. [Google Scholar] [CrossRef]
- Tavares, A.P.; Cristóvão, R.O.; Loureiro, J.M.; Boaventura, R.A.; Macedo, E.A. Application of statistical experimental methodology to optimize reactive dye decolorization by commercial laccase. J. Hazard. Mater. 2009, 162, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.P.A.; Agapito, M.S.M.; Coelho, M.A.M.; Da Silva, J.L.; Barros-Timmons, A.; Coutinho, J.A.J.; Xavier, A.M.R.B. Selection and optimization of culture medium for exopolysaccharide production by Coriolus (Trametes) versicolor. World J. Microbiol. Biotechnol. 2005, 21, 1499–1507. [Google Scholar] [CrossRef]
- Fernandes, C.D.; Nascimento, V.R.S.; Meneses, D.B.; Vilar, D.S.; Torres, N.H.; Leite, M.S.; Baudrit, J.R.V.; Bilal, M.; Iqbal, H.M.N.; Bharagava, R.N.; et al. Fungal biosynthesis of lignin-modifying enzymes from pulp wash and Luffa cylindrica for azo dye RB5 biodecolorization using modelling by response surface methodology and artificial neural network. J. Hazard. Mater. 2020, 399, 123094. [Google Scholar] [CrossRef]
- Songulashvili, G.; Flahaut, S.; Demarez, M.; Tricot, C.; Bauvois, C.; Debaste, F.; Penninckx, M.J. High yield production in seven days of Coriolopsis gallica 1184 laccase at 50 L scale; enzyme purification and molecular characterization. Fungal Biol. 2016, 120, 481–488. [Google Scholar] [CrossRef]
- Hadibarata, T.; Adnan, L.A.; Yusoff, A.R.M.; Yuniarto, A.; Zubir, M.M.F.A.; Khudhair, A.B.; Teh, Z.C.; Naser, M.A. Microbial decolorization of an azo dye reactive black 5 using white-rot fungus Pleurotus eryngii F032. Water Air Soil Pollut. 2013, 224, 1595. [Google Scholar] [CrossRef]
- Martínez-Sánchez, J.; Membrillo-Venegas, I.; Martínez-Trujillo, A.; García-Rivero, A.M. Decolorization of reactive black 5 by immobilized Trametes versicolor. Rev. Mex. Ing. Quím. 2018, 17, 107–121. [Google Scholar] [CrossRef] [Green Version]
- Aracagök, Y.D.; Cihangir, N. Decolorization of reactive black 5 by Yarrowia lipolytica NBRC 1658. Am. J. Microbiol. Res. 2013, 1, 16–20. [Google Scholar] [CrossRef] [Green Version]
- El Bouraie, M.; El Din, W.S. Biodegradation of Reactive Black 5 by Aeromonas hydrophila strain isolated from dye-contaminated textile wastewater. Sustain. Environ. Res. 2016, 26, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Shah, J.A.; Riaz, N.; Butt, T.A.; Khan, A.J.; Khalifa, W.; Gasmi, H.H.; Enamur, R.L.; Arshad, M.; Al Naghi, A.A.A.; et al. Synthesis and Characterization of Fe-TiO2 Nanomaterial: Performance Evaluation for RB5 Decolorization and In Vitro Antibacterial Studies. Nanomaterials 2021, 11, 436. [Google Scholar] [CrossRef]
- Bonugli-Santos, R.C.; Vieira, G.A.; Collins, C.; Fernandes, T.C.C.; Marin-Morales, M.A.; Murray, P.; Sette, L.D. Enhanced textile dye decolorization by marine-derived basidiomycete Peniophora sp. CBMAI 1063 using integrated statistical design. Environ. Sci. Pollut. Res. 2016, 23, 8659–8668. [Google Scholar] [CrossRef]
- Chen, K.C.; Wu, J.Y.; Liou, D.J.; Hwang, S.C.J. Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol. 2003, 101, 57–68. [Google Scholar] [CrossRef]
- Wanyonyi, W.C.; Onyari, J.M.; Shiundu, P.M.; Mulaa, F.J. Effective biotransformation of reactive black 5 dye using crude protease from Bacillus cereus strain KM201428. Energy Procedia 2019, 157, 815–824. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Daâssi, D.; Zouari-Mechichi, H.; Frikha, F.; Martinez, M.J.; Nasri, M.; Mechichi, T. Decolorization of the azo dye Acid Orange 51 by laccase produced in solid culture of a newly isolated Trametes trogii strain. 3 Biotech 2013, 3, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Neifar, M.; Jaouani, A.; Kamoun, A.; Ellouze-Ghorbel, R.; Ellouze-Chaabouni, S. Decolorization of solophenyl red 3BL polyazo dye by laccase-mediator system: Optimization through response surface methodology. Enzym. Res. 2011, 2011, 179050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzina, O.; Daâssi, D.; Zouari-Mechichi, H.; Frikha, F.; Woodward, S.; Belbahri, L.; Rodriguez-Couto, S.; Mechichi, T. Decolorization and detoxification of two textile industry effluents by the laccase/1-hydroxybenzotriazole system. Environ. Sci. Pollut. Res. 2013, 20, 5177–5187. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.N.; Lu, L.; Li, G.F.; Li, J.; Xu, T.F.; Zhao, M. Decolorization of the azo dye reactive black 5 using laccase mediator system. Afr. J. Biotechnol. 2011, 10, 17186–17191. [Google Scholar] [CrossRef]
- Jamal, F.; Qidwai, T.; Pandey, P.K.; Singh, D. Catalytic potential of cauliflower (Brassica oleracea) bud peroxidase in decolorization of synthetic recalcitrant dyes using redox mediator. Catal. Commun. 2011, 15, 93–98. [Google Scholar] [CrossRef]
- Ali, W.B.; Chaduli, D.; Navarro, D.; Lechat, C.; Turbé-Doan, A.; Bertrand, E.; Faulds, B.; Sciara, G.; Lesage-Meessen, L.; Record, E.; et al. Screening of five marine-derived fungal strains for their potential to produce oxidases with laccase activities suitable for biotechnological applications. BMC Biotechnol. 2020, 20, 27. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Kenawy, E.R.; Sun, J.; Ali, S.S. Performance of a newly isolated salt-tolerant yeast strain Sterigmatomyces halophilus SSA-1575 for azo dye decolorization and detoxification. Front. Microbiol. 2020, 11, 1163. [Google Scholar] [CrossRef]
- Pavko, A. Fungal decolorization and degradation of synthetic dyes. Some chemical engineering aspects. Waste Water Treat. Reutil. 2011, 4, 65–89. [Google Scholar]
- Garg, S.K.; Tripathi, M.; Lal, N. Response surface methodology for optimization of process variable for reactive orange 4 dye discoloration by Pseudomonas putida SKG-1 strain and bioreactor trial for its possible use in large-scale bioremediation. Desalin. Water Treat. 2015, 54, 3122–3133. [Google Scholar] [CrossRef]
- Khalik, W.F.; Ho, L.N.; Ong, S.A.; Voon, C.H.; Wong, Y.S.; Yusoff, N.; Lee, S.L.; Yusuf, S.Y. Optimization of degradation of Reactive Black 5 (RB5) and electricity generation in a solar photocatalytic fuel cell system. Chemosphere 2017, 184, 112–119. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, X.; Sun, D.; Qi, H. Biodecolorization and partial mineralization of Reactive Black 5 by a strain of Rhodopseudomonas palustris. J. Environ. Sci. 2008, 20, 1218–1225. [Google Scholar] [CrossRef]
- Tavares, A.P.; Cristóvão, R.O.; Loureiro, J.M.; Boaventura, R.A.; Macedo, E.A. Optimisation of reactive textile dyes degradation by laccase-mediator system. J. Chem. Technol. Biotechnol. 2008, 83, 1609–1615. [Google Scholar] [CrossRef]
| Properties | Reactive Black 5 |
|---|---|
| CAS number | 17095-24-8 |
| Molecular weight (gmol−1) | 991.82 |
| EC Number | 241-164-5 |
| CI | 20505 |
| Dye content | ≥50% |
| Chemical formula | C26H21N5Na4O19S6 |
| λmax (nm) | 598 |
| Molecular structure | 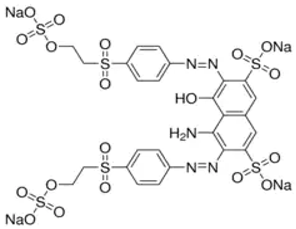 |
| Symbol Code | Factor | Unit | Coded and Uncoded Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| x1 | Laccase-like activity | U mL−1 | 0.02 | 0.26 | 0.5 |
| x2 | Initial dye concentration | mg L−1 | 25 | 75 | 125 |
| x3 | HBT concentration | mM | 0.5 | 2.5 | 4.5 |
| x4 | pH | - | 3.0 | 4.5 | 6.0 |
| x5 | Temperature | °C | 25 | 40 | 55 |
| Run | Symbol Code of Factors | Decolorization (%) | ||||
|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | x5 | ||
| 1 | +1 | −1 | +1 | −1 | −1 | 74.8 ± 0.4 |
| 2 | +1 | +1 | −1 | +1 | −1 | 1.0 ± 0.2 |
| 3 | −1 | +1 | +1 | −1 | +1 | 1.3 ± 0.6 |
| 4 | +1 | −1 | +1 | +1 | −1 | 5.2 ± 0.3 |
| 5 | +1 | +1 | −1 | +1 | +1 | 1.3 ± 0.6 |
| 6 | +1 | +1 | +1 | −1 | +1 | 55.8 ± 1.2 |
| 7 | −1 | +1 | +1 | +1 | −1 | 1.3 ± 0.4 |
| 8 | −1 | −1 | +1 | +1 | +1 | 6.0 ± 0.7 |
| 9 | −1 | −1 | −1 | +1 | +1 | 3.1 ± 0.1 |
| 10 | +1 | −1 | −1 | −1 | +1 | 65.7 ± 3.2 |
| 11 | −1 | +1 | −1 | −1 | −1 | 1.2 ± 0.4 |
| 12 | −1 | −1 | −1 | −1 | −1 | 3.7 ± 1.1 |
| 13 | 0 | 0 | 0 | 0 | 0 | 62.0 ± 0.2 |
| 14 | 0 | 0 | 0 | 0 | 0 | 62.3 ± 0.3 |
| 15 | 0 | 0 | 0 | 0 | 0 | 63.4 ± 0.2 |
| Symbol Code of Factors | Decolorization (%) | |||||
|---|---|---|---|---|---|---|
| Run | x1 | x2 | x3 | x4 | x5 | |
| 1 | −1 | −1 | 0 | 0 | 0 | 77.6 ± 1.3 |
| 2 | +1 | −1 | 0 | 0 | 0 | 80.9 ± 0.7 |
| 3 | −1 | +1 | 0 | 0 | 0 | 1.3 ± 0.3 |
| 4 | +1 | +1 | 0 | 0 | 0 | 43.7 ± 1.8 |
| 5 | 0 | 0 | −1 | −1 | 0 | 0.8 ± 0.3 |
| 6 | 0 | 0 | +1 | −1 | 0 | 60.9 ± 0.3 |
| 7 | 0 | 0 | −1 | +1 | 0 | 1.7 ± 0.2 |
| 8 | 0 | 0 | +1 | +1 | 0 | 2.0 ± 1.0 |
| 9 | 0 | −1 | 0 | 0 | −1 | 75.6 ± 0.1 |
| 10 | 0 | +1 | 0 | 0 | −1 | 29.0 ± 1.9 |
| 11 | 0 | −1 | 0 | 0 | +1 | 85.4 ± 2.1 |
| 12 | 0 | +1 | 0 | 0 | +1 | 56.2 ± 4.1 |
| 13 | −1 | 0 | −1 | 0 | 0 | 0.8 ± 0.3 |
| 14 | +1 | 0 | −1 | 0 | 0 | 53.5 ± 1.0 |
| 15 | −1 | 0 | +1 | 0 | 0 | 0.6 ± 0.1 |
| 16 | +1 | 0 | +1 | 0 | 0 | 69.9 ± 1.8 |
| 17 | 0 | 0 | 0 | −1 | −1 | 52.6 ± 1.3 |
| 18 | 0 | 0 | 0 | +1 | −1 | 1.6 ± 0.3 |
| 19 | 0 | 0 | 0 | −1 | +1 | 63.7 ± 3.7 |
| 20 | 0 | 0 | 0 | +1 | +1 | 1.5 ± 0.2 |
| 21 | 0 | −1 | −1 | 0 | 0 | 74.7 ± 0.4 |
| 22 | 0 | +1 | −1 | 0 | 0 | 23.9 ± 2.8 |
| 23 | 0 | −1 | +1 | 0 | 0 | 82.8±0.8 |
| 24 | 0 | +1 | +1 | 0 | 0 | 37.0 ± 7.7 |
| 25 | −1 | 0 | 0 | −1 | 0 | 0.6 ± 0.0 |
| 26 | +1 | 0 | 0 | −1 | 0 | 56.6 ± 0.3 |
| 27 | −1 | 0 | 0 | +1 | 0 | 0.9 ± 0.2 |
| 28 | +1 | 0 | 0 | +1 | 0 | 0.3 ± 0.1 |
| 29 | 0 | 0 | −1 | 0 | −1 | 43.8 ± 1.5 |
| 30 | 0 | 0 | +1 | 0 | −1 | 15.0 ± 1.8 |
| 31 | 0 | 0 | −1 | 0 | +1 | 62.9 ± 5.1 |
| 32 | 0 | 0 | +1 | 0 | +1 | 76.4 ± 4.3 |
| 33 | −1 | 0 | 0 | 0 | −1 | 1.4 ± 0.2 |
| 34 | +1 | 0 | 0 | 0 | −1 | 55.1 ± 2.9 |
| 35 | −1 | 0 | 0 | 0 | +1 | 8.6 ± 5.0 |
| 36 | +1 | 0 | 0 | 0 | +1 | 74.9 ± 4.4 |
| 37 | 0 | −1 | 0 | −1 | 0 | 76.2 ± 0.2 |
| 38 | 0 | +1 | 0 | −1 | 0 | 31.5 ± 1.9 |
| 39 | 0 | −1 | 0 | +1 | 0 | 4.3 ± 0.5 |
| 40 | 0 | +1 | 0 | +1 | 0 | 0.8 ± 0.3 |
| 41 | 0 | 0 | 0 | 0 | 0 | 62.3 ± 0.7 |
| 42 | 0 | 0 | 0 | 0 | 0 | 64.0 ± 1.7 |
| 43 | 0 | 0 | 0 | 0 | 0 | 65.2 ± 0.4 |
| 44 | 0 | 0 | 0 | 0 | 0 | 64.2 ± 1.0 |
| 45 | 0 | 0 | 0 | 0 | 0 | 64.5 ± 1.0 |
| 46 | 0 | 0 | 0 | 0 | 0 | 64.2 ± 1.4 |
| Source | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value Probability > F |
|---|---|---|---|---|---|
| Regression | 20 | 102,350 | 5117.5 | 46.6 | <0.001 |
| x1 | 1 | 17,064 | 17,063.8 | 155.2 | <0.001 |
| x2 | 1 | 19,526 | 19,525.6 | 177.7 | <0.001 |
| x3 | 1 | 931 | 931.0 | 8.5 | 0.004 |
| x4 | 1 | 19,003 | 19,003.2 | 172.9 | <0.001 |
| x5 | 1 | 4585 | 4584.6 | 41.7 | <0.001 |
| x1² | 1 | 8115 | 8115.4 | 73.8 | <0.001 |
| x2² | 1 | 59 | 59.1 | 0.5 | 0.465 |
| x3² | 1 | 3340 | 3339.9 | 30.4 | <0.001 |
| x4² | 1 | 27,057 | 27,056.6 | 246.2 | <0.001 |
| x5² | 1 | 323 | 323.3 | 2.9 | 0.089 |
| x1 × x2 | 1 | 1148 | 1148.1 | 10.5 | 0.002 |
| x1 × x3 | 1 | 162 | 161.6 | 1.5 | 0.228 |
| x1 × x4 | 1 | 1477 | 1477.5 | 13.4 | <0.001 |
| x1 × x5 | 1 | 59 | 59.4 | 0.5 | 0.464 |
| x2 × x3 | 1 | 37 | 37.4 | 0.3 | 0.561 |
| x2 × x4 | 1 | 1300 | 1300.1 | 11.8 | 0.001 |
| x2 × x5 | 1 | 225 | 225.4 | 2.1 | 0.155 |
| x3 × x4 | 1 | 1891 | 1890.7 | 17.2 | <0.001 |
| x3 × x5 | 1 | 1341 | 1340.9 | 12.2 | 0.001 |
| x4 × x5 | 1 | 32 | 31.8 | 0.3 | 0.592 |
| Residual Error | 106 | 11,649 | 109.9 | ||
| Total | 126 | 114,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ayed, A.; Hadrich, B.; Sciara, G.; Lomascolo, A.; Bertrand, E.; Faulds, C.B.; Zouari-Mechichi, H.; Record, E.; Mechichi, T. Optimization of the Decolorization of the Reactive Black 5 by a Laccase-like Active Cell-Free Supernatant from Coriolopsis gallica. Microorganisms 2022, 10, 1137. https://doi.org/10.3390/microorganisms10061137
Ben Ayed A, Hadrich B, Sciara G, Lomascolo A, Bertrand E, Faulds CB, Zouari-Mechichi H, Record E, Mechichi T. Optimization of the Decolorization of the Reactive Black 5 by a Laccase-like Active Cell-Free Supernatant from Coriolopsis gallica. Microorganisms. 2022; 10(6):1137. https://doi.org/10.3390/microorganisms10061137
Chicago/Turabian StyleBen Ayed, Amal, Bilel Hadrich, Giuliano Sciara, Anne Lomascolo, Emmanuel Bertrand, Craig B. Faulds, Héla Zouari-Mechichi, Eric Record, and Tahar Mechichi. 2022. "Optimization of the Decolorization of the Reactive Black 5 by a Laccase-like Active Cell-Free Supernatant from Coriolopsis gallica" Microorganisms 10, no. 6: 1137. https://doi.org/10.3390/microorganisms10061137







