Hyphal Fusions Enable Efficient Nutrient Distribution in Colletotrichum graminicola Conidiation and Symptom Development on Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Media and Growth Conditions
2.2. Generation of Plasmids
2.3. Transformation of C. graminicola Strains
2.4. Growth and Conidiation Analyses
2.5. Quantification of Conidial Anastomosis Tube (CAT) Formation
2.6. Acervuli Development
2.7. Microscopy
2.8. Plant Infection-Related Assays
2.9. Statistics
3. Results
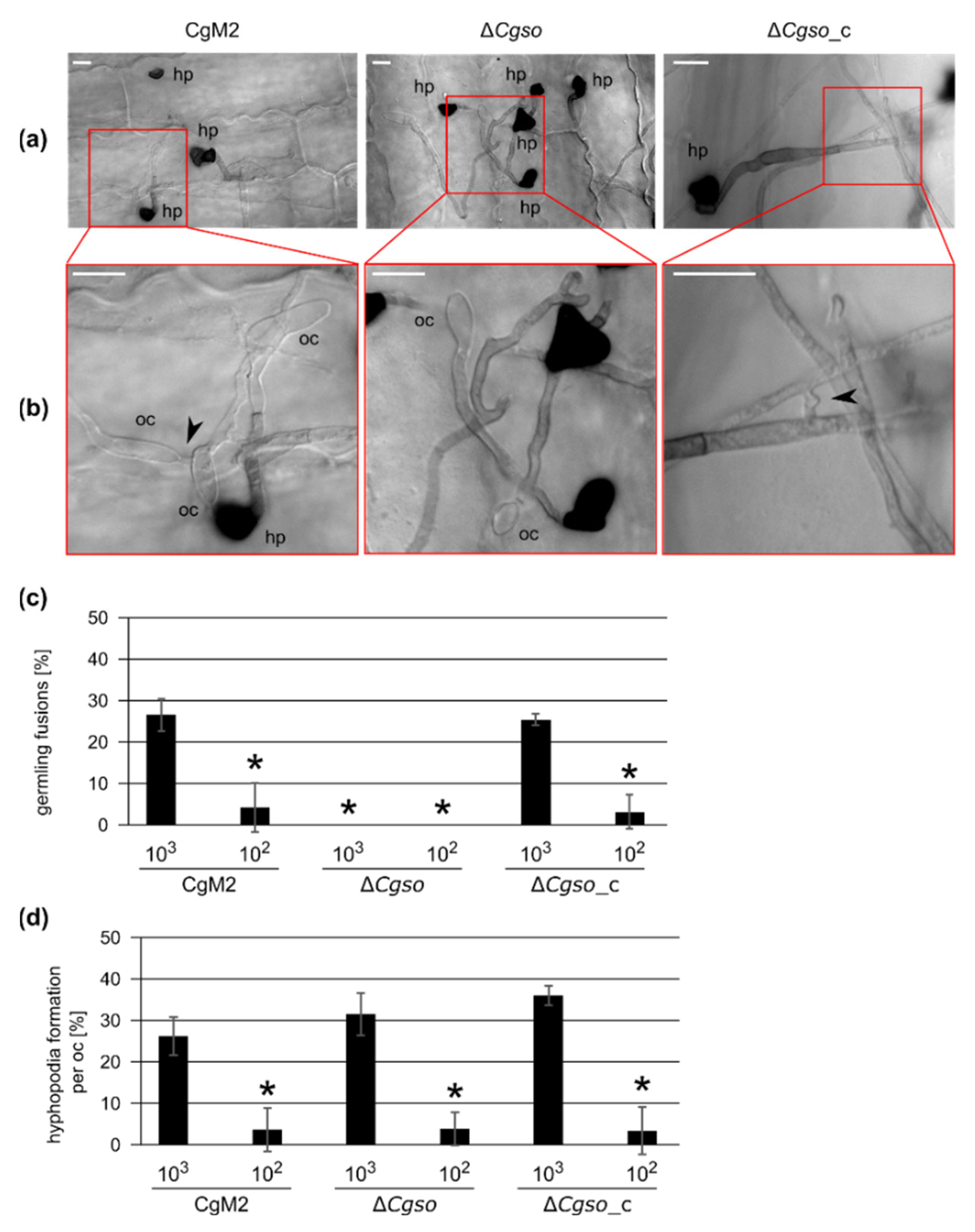
3.1. A Defective Germling Fusion Process Does Not Affect Hyphopodia Formation by Oval Conidia
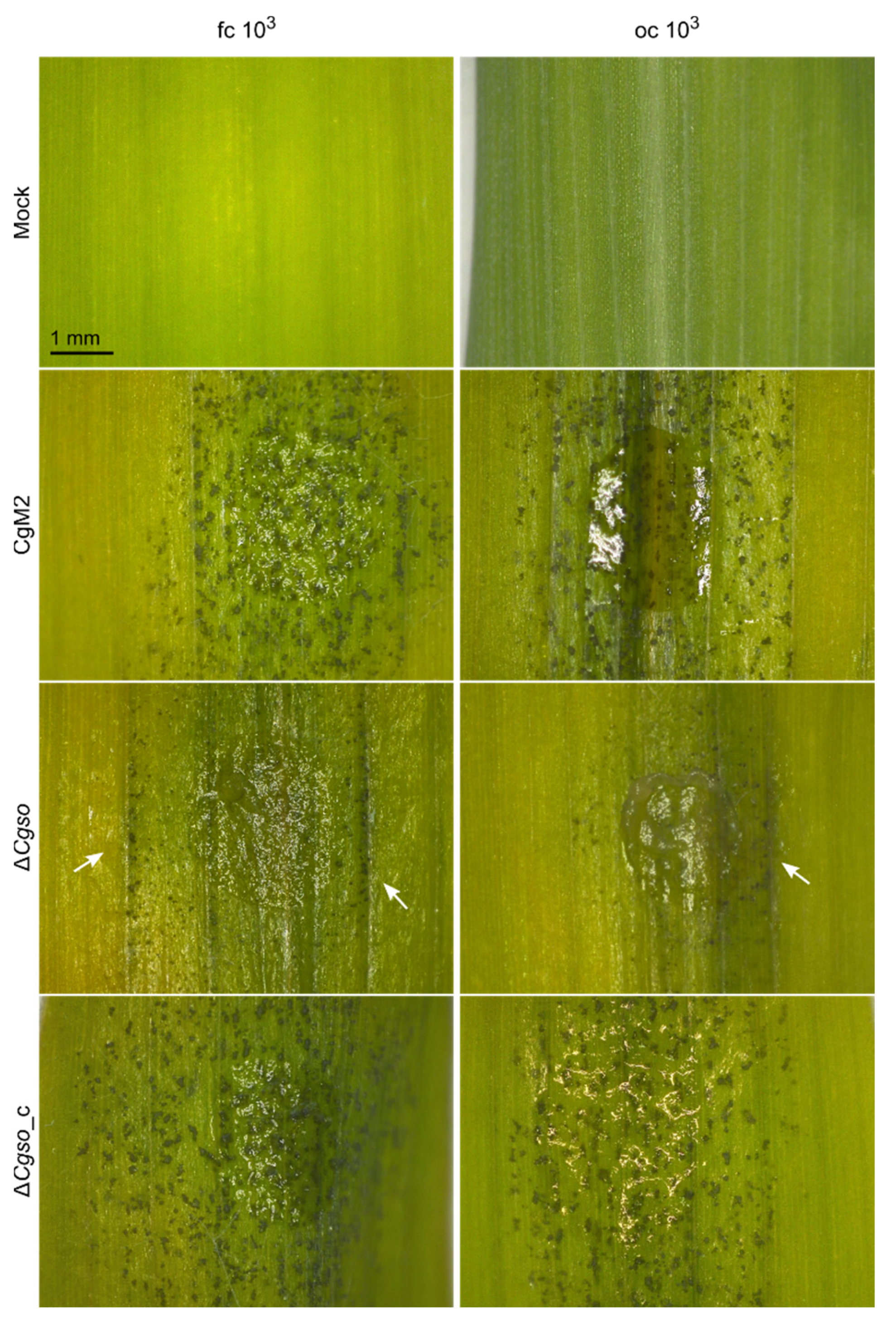
3.2. ∆Cgso Shows Reduced Symptom Development on Z. mays Leaves
3.3. A Cgso Deletion Mutant Is Drastically Reduced in the Conidiation of Falcate Spores
3.4. Autophagy-Degraded Cellular Material Might Serve for the Nutrition of Acervuli
4. Discussion
4.1. An Unknown Quorum-Sensing Mechanism Regulates Hyphopodia Formation from Oval Conidia
4.2. Coordinated Nutrient Recycling and Distribution Might Be the Basis for Acervulus Maturation
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhunjun, C.S.; Phukhamsakda, C.; Jayawardena, R.S.; Jeewon, R.; Promputtha, I.; Hyde, K.D. Investigating species boundaries in Colletotrichum. Fungal Divers. 2021, 107, 107–127. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Crouch, J.A.; Clarke, B.B.; White, J.F., Jr.; Hillman, B.I. Systematic analysis of the falcate-spored graminicolous Colletotrichum and a description of six new species from warm-season grasses. Mycologia 2009, 101, 717–732. [Google Scholar] [CrossRef]
- Karunarathna, A.; Tibpromma, S.; Jayawardena, R.S.; Nanayakkara, C.; Asad, S.; Xu, J.; Hyde, K.D.; Karunarathna, S.C.; Stephenson, S.L.; Lumyong, S. Fungal pathogens in grasslands. Front. Cell. Infect. Microbiol. 2021, 11, 695087. [Google Scholar] [CrossRef]
- Bergstrom, G.C.; Nicholson, R.L. The biology of corn anthracnose: Knowledge to exploit for improved management. Plant Dis. 1999, 83, 596–608. [Google Scholar] [CrossRef] [Green Version]
- Sukno, S.A.; García, V.M.; Shaw, B.D.; Thon, M.R. Root infection and systemic colonization of maize by Colletotrichum graminicola. Appl. Environ. Microbiol. 2008, 74, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Perfect, S.E.; Hughes, H.B.; O’Connell, R.J.; Green, J.R. Colletotrichum: A model genus for studies on pathology and fungal–plant interactions. Fungal Genet. Biol. 1999, 27, 186–198. [Google Scholar] [CrossRef]
- Frey, T.; Weldekidan, T.; Colbert, T.; Wolters, P.; Hawk, J. Fitness evaluation of Rcg1, a locus that confers resistance to Colletotrichum graminicola (Ces.) GW Wils. using near-isogenic maize hybrids. Crop Sci. 2011, 51, 1551–1563. [Google Scholar] [CrossRef]
- Belisario, R.; Robertson, A.E.; Vaillancourt, L. Maize anthracnose stalk rot in the genomic era. Plant Dis. 2022. [Google Scholar] [CrossRef]
- Nishihara, N. Two types of conidia of Colletotrichum graminicola (Ces.) GW Wils. formed on artificial media, and their pathogenicity. Jpn. J. Phytopathol. 1975, 41, 171–175. [Google Scholar] [CrossRef]
- Panaccione, D.G.; Vaillancourt, L.J.; Hanau, R.M. Conidial dimorphism in Colletotrichum graminicola. Mycologia 1989, 81, 876–883. [Google Scholar] [CrossRef]
- Frost, R. Seta formation in Colletotrichum spp. Nature 1964, 201, 730–731. [Google Scholar] [CrossRef]
- Leite, B.; Nicholson, R.L. Mycosporine-alanine: A self-inhibitor of germination from the conidial mucilage of Colletotrichum graminicola. Exp. Mycol. 1992, 16, 76–86. [Google Scholar] [CrossRef]
- Nordzieke, D.E.; Sanken, A.; Antelo, L.; Raschke, A.; Deising, H.B.; Poggeler, S. Specialized infection strategies of falcate and oval conidia of Colletotrichum graminicola. Fungal Genet. Biol. 2019, 133, 103276. [Google Scholar] [CrossRef]
- Venard, C.; Vaillancourt, L. Colonization of fiber cells by Colletotrichum graminicola in wounded maize stalks. Phytopathology 2007, 97, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Schunke, C.; Pöggeler, S.; Nordzieke, D.E. A 3D printed device for easy and reliable quantification of fungal chemotropic growth. Front Microbiol. 2020, 11, 584525. [Google Scholar] [CrossRef]
- Fleißner, A.; Herzog, S. Signal exchange and integration during self-fusion in filamentous fungi. Semin. Cell Dev. Biol. 2016, 57, 76–83. [Google Scholar] [CrossRef]
- Read, N.D.; Goryachev, A.B.; Lichius, A. The mechanistic basis of self-fusion between conidial anastomosis tubes during fungal colony initiation. Fungal Biol. Rev. 2012, 26, 1–11. [Google Scholar] [CrossRef]
- Fischer, M.S.; Glass, N.L. Communicate and fuse: How filamentous fungi establish and maintain an interconnected mycelial network. Front. Microbiol. 2019, 10, 619. [Google Scholar] [CrossRef]
- Fleißner, A.; Sarkar, S.; Jacobson, D.J.; Roca, M.G.; Read, N.D.; Glass, N.L. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot. Cell 2005, 4, 920–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleißner, A.; Leeder, A.C.; Roca, M.G.; Read, N.D.; Glass, N.L. Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behavior during cell fusion. Proc. Natl. Acad. Sci. USA 2009, 106, 19387–19392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goryachev, A.B.; Lichius, A.; Wright, G.D.; Read, N.D. Excitable behavior can explain the “ping-pong” mode of communication between cells using the same chemoattractant. Bioessays 2012, 34, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Teichert, I.; Steffens, E.K.; Schnaß, N.; Fränzel, B.; Krisp, C.; Wolters, D.A.; Kück, U. PRO40 is a scaffold protein of the cell wall integrity pathway, linking the MAP kinase module to the upstream activator protein kinase C. PLoS Genet. 2014, 10, e1004582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammadeh, H.H.; Serrano, A.; Wernet, V.; Stomberg, N.; Hellmeier, D.; Weichert, M.; Brandt, U.; Sieg, B.; Kanofsky, K.; Hehl, R.; et al. A dialogue-like cell communication mechanism is conserved in filamentous ascomycete fungi and mediates interspecies interactions. Proc. Natl. Acad. Sci. USA 2022, 119, e2112518119. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Baghela, A. Quorum sensing-mediated inter-specific conidial anastomosis tube fusion between Colletotrichum gloeosporioides and C. siamense. IMA Fungus 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Roca, M.G.; Davide, L.C.; Davide, L.M.C.; Mendes-Costa, M.C.; Schwan, R.F.; Wheals, A.E. Conidial anastomosis fusion between Colletotrichum species. Mycol. Res. 2004, 108, 1320–1326. [Google Scholar] [CrossRef]
- Forgey, W.M.; Blanco, M.H.; Loegering, W.Q. Differences in pathological capabilities and host specificity of Colletotrichum graminicola on Zea mays. Plant Dis. Report. 1979, 62, 573–576. [Google Scholar]
- Sambrook, J.; Fritsch, E.; Maniatis, T. (Eds.) Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Bloemendal, S.; Bernhards, Y.; Bartho, K.; Dettmann, A.; Voigt, O.; Teichert, I.; Seiler, S.; Wolters, D.A.; Pöggeler, S.; Kück, U. A homologue of the human STRIPAK complex controls sexual development in fungi. Mol. Microbiol. 2012, 84, 310–323. [Google Scholar] [CrossRef]
- Klix, V.; Nowrousian, M.; Ringelberg, C.; Loros, J.; Dunlap, J.; Pöggeler, S. Functional characterization of MAT1-1-specific mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and nonessential sexual regulators. Eukaryot. Cell 2010, 9, 894–905. [Google Scholar] [CrossRef] [Green Version]
- Pöggeler, S.; Masloff, S.; Hoff, B.; Mayrhofer, S.; Kück, U. Versatile EGFP reporter plasmids for cellular localization of recombinant gene products in filamentous fungi. Curr. Genet. 2003, 43, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Groth, A.; Schunke, C.; Reschka, E.J.; Pöggeler, S.; Nordzieke, D.E. Tracking fungal growth: Establishment of Arp1 as a marker for polarity establishment and active hyphal growth in filamentous ascomycetes. J. Fungi 2021, 7, 580. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2019, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruxton, G.D. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behav. Ecol. 2006, 17, 688–690. [Google Scholar] [CrossRef]
- Behr, M.; Humbeck, K.; Hause, G.; Deising, H.B.; Wirsel, S.G. The hemibiotroph Colletotrichum graminicola locally induces photosynthetically active green islands but globally accelerates senescence on aging maize leaves. Mol. Plant Microbe Interact. 2010, 23, 879–892. [Google Scholar] [CrossRef]
- Wen, X.; Klionsky, D.J. An overview of macroautophagy in yeast. J. Mol. Biol. 2016, 428, 1681–1699. [Google Scholar] [CrossRef] [Green Version]
- Voigt, O.; Pöggeler, S. Autophagy genes Smatg8 and Smatg4 are required for fruiting-body development, vegetative growth and ascospore germination in the filamentous ascomycete Sordaria macrospora. Autophagy 2013, 9, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Felkner, I.C.; Wyss, O. A substance produced by competent Bacillus cereus 569 cells that affects transformability. Biochem. Biophys. Res. Commun. 1964, 16, 94–99. [Google Scholar] [CrossRef]
- Tomasz, A.; Hotchkiss, R.D. Regulation of the transformability of pneumococcal cultures by macromolecular cell products. Proc. Natl. Acad. Sci. USA 1964, 51, 480. [Google Scholar] [CrossRef] [Green Version]
- Hogan, D.A. Talking to themselves: Autoregulation and quorum sensing in fungi. Eukaryot. Cell 2006, 5, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Barriuso, J.; Hogan, D.A.; Keshavarz, T.; Martínez, M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbiol. Rev. 2018, 42, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Padder, S.A.; Prasad, R.; Shah, A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018, 210, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Roca, G.M.; Read, N.D.; Wheals, A.E. Conidial anastomosis tubes in filamentous fungi. FEMS Microbiol. Lett. 2005, 249, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, N.; Patil, R.; Baghela, A. Differential physiological prerequisites and gene expression profiles of conidial anastomosis tube and germ tube formation in Colletotrichum gloeosporioides. J. Fungi 2021, 7, 509. [Google Scholar] [CrossRef]
- Kurian, S.M.; Di Pietro, A.; Read, N.D. Live-cell imaging of conidial anastomosis tube fusion during colony initiation in Fusarium oxysporum. PLoS ONE 2018, 13, e0195634. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Sanogo, S.; Stevenson, R.; Pennypacker, S. Appressorium formation and tomato fruit infection by Colletotrichum coccodes. Plant Dis. 2003, 87, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Vitale, S.; Di Pietro, A.; Turrà, D. Autocrine pheromone signalling regulates community behaviour in the fungal pathogen Fusarium oxysporum. Nat. Microbiol. 2019, 4, 1443–1449. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Li, L.; Wu, M.; Liang, S.; Shi, H.-B.; Liu, X.-H.; Lin, F.-C. Current opinions on autophagy in pathogenicity of fungi. Virulence 2019, 10, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Huang, W.-P.; Klionsky, D.J. Membrane recruitment of Aut7p in the autophagy and cytoplasm to vacuole targeting pathways requires Aut1p, Aut2p, and the autophagy conjugation complex. J. Cell Biol. 2001, 152, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Kirisako, T.; Kamada, Y.; Mizushima, N.; Noda, T.; Ohsumi, Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001, 20, 5971–5981. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ohsumi, Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007, 581, 2156–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, B.; Yuan, J. Autophagy in cell death: An innocent convict? J. Clin. Investig. 2005, 115, 2679–2688. [Google Scholar] [CrossRef] [PubMed]
- Teter, S.A.; Eggerton, K.P.; Scott, S.V.; Kim, J.; Fischer, A.M.; Klionsky, D.J. Degradation of lipid vesicles in the yeast vacuole requires function of Cvt17, a putative lipase. J. Biol. Chem. 2001, 276, 2083–2087. [Google Scholar] [CrossRef] [Green Version]
- Epple, U.D.; Suriapranata, I.; Eskelinen, E.-L.; Thumm, M. Aut5/Cvt17p, a putative lipase essential for disintegration of autophagic bodies inside the vacuole. J. Bacteriol. 2001, 183, 5942–5955. [Google Scholar] [CrossRef] [Green Version]
- Voigt, O.; Pöggeler, S. Self-eating to grow and kill: Autophagy in filamentous ascomycetes. Appl. Microbiol. Biotechnol. 2013, 97, 9277–9290. [Google Scholar] [CrossRef]
- Inoue, Y.; Klionsky, D.J. Regulation of macroautophagy in Saccharomyces cerevisiae. Semin. Cell Dev. Biol. 2010, 27, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.; Klionsky, D.J. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 2008, 9, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Kirisako, T.; Ichimura, Y.; Okada, H.; Kabeya, Y.; Mizushima, N.; Yoshimori, T.; Ohsumi, M.; Takao, T.; Noda, T.; Ohsumi, Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 2000, 151, 263–276. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Kolattukudy, P.E. Early expression of the calmodulin gene, which precedes appressorium formation in Magnaporthe grisea, is inhibited by self-inhibitors and requires surface attachment. J. Bacteriol. 1999, 181, 3571–3577. [Google Scholar] [CrossRef] [Green Version]
- Teichert, I.; Pöggeler, S.; Nowrousian, M. Sordaria macrospora: 25 years as a model organism for studying the molecular mechanisms of fruiting body development. Appl. Microbiol. Biotechnol. 2020, 104, 3691–3704. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.Z.; Ramos-Pamplona, M.; Naqvi, N.I. Autophagy-assisted glycogen catabolism regulates asexual differentiation in Magnaporthe oryzae. Autophagy 2009, 5, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veneault-Fourrey, C.; Barooah, M.; Egan, M.; Wakley, G.; Talbot, N.J. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 2006, 312, 580–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, M.J.; Talbot, N.J. Genome-wide functional analysis reveals that infection-associated fungal autophagy is necessary for rice blast disease. Proc. Natl. Acad. Sci. USA 2009, 106, 15967–15972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuma, T.; Arioka, M.; Kitamoto, K. Autophagy during conidiation and conidial germination in filamentous fungi. Autophagy 2007, 3, 128–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitsche, B.M.; Burggraaf-van Welzen, A.-M.; Lamers, G.; Meyer, V.; Ram, A.F. Autophagy promotes survival in aging submerged cultures of the filamentous fungus Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 8205–8218. [Google Scholar] [CrossRef]
- Kikuma, T.; Ohneda, M.; Arioka, M.; Kitamoto, K. Functional analysis of the ATG8 homologue Aoatg8 and role of autophagy in differentiation and germination in Aspergillus oryzae. Eukaryot. Cell 2006, 5, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Asakura, M.; Ninomiya, S.; Sugimoto, M.; Oku, M.; Yamashita, S.; Okuno, T.; Sakai, Y.; Takano, Y. Atg26-mediated pexophagy is required for host invasion by the plant pathogenic fungus Colletotrichum orbiculare. Plant Cell 2009, 21, 1291–1304. [Google Scholar] [CrossRef] [Green Version]
- Josefsen, L.; Droce, A.; Sondergaard, T.E.; Sørensen, J.L.; Bormann, J.; Schäfer, W.; Giese, H.; Olsson, S. Autophagy provides nutrients for nonassimilating fungal structures and is necessary for plant colonization but not for infection in the necrotrophic plant pathogen Fusarium graminearum. Autophagy 2012, 8, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Xiong, M.; Jagernath, J.S.; Wang, C.; Qiu, J.; Shi, H.; Kou, Y. UvAtg8-mediated autophagy regulates fungal growth, stress responses, conidiation, and pathogenesis in Ustilaginoidea virens. Rice 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Roca, M.G.; Davide, L.C.; Mendes-Costa, M.C.; Wheals, A. Conidial anastomosis tubes in Colletotrichum. Fungal Genet. Biol. 2003, 40, 138–145. [Google Scholar] [CrossRef]
- Bloemendal, S.; Lord, K.M.; Rech, C.; Hoff, B.; Engh, I.; Read, N.D.; Kück, U. A mutant defective in sexual development produces aseptate ascogonia. Eukaryot. Cell 2010, 9, 1856–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirschnabel, D.E.; Nowrousian, M.; Cano-Domínguez, N.; Aguirre, J.; Teichert, I.; Kück, U. New insights into the roles of NADPH oxidases in sexual development and ascospore germination in Sordaria macrospora. Genetics 2014, 196, 729–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kück, U. A Sordaria macrospora mutant lacking the leu1 gene shows a developmental arrest during fruiting body formation. Mol. Genet. Genom. 2005, 274, 307–315. [Google Scholar] [CrossRef]
- Masloff, S.; Pöggeler, S.; Kück, U. The pro1+ gene from Sordaria macrospora encodes a C6 zinc finger transcription factor required for fruiting body development. Genetics 1999, 152, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Nowrousian, M.; Frank, S.; Koers, S.; Strauch, P.; Weitner, T.; Ringelberg, C.; Dunlap, J.C.; Loros, J.J.; Kück, U. The novel ER membrane protein PRO41 is essential for sexual development in the filamentous fungus Sordaria macrospora. Mol. Microbiol. 2007, 64, 923–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowrousian, M.; Masloff, S.; Pöggeler, S.; Kück, U. Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity. Mol. Cell. Biol. 1999, 19, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Nowrousian, M.; Teichert, I.; Masloff, S.; Kück, U. Whole-genome sequencing of Sordaria macrospora mutants identifies developmental genes. G3 Genes Genomes Genet. 2012, 2, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Pöggeler, S.; Kück, U. A WD40 repeat protein regulates fungal cell differentiation and can be replaced functionally by the mammalian homologue striatin. Eukaryot. Cell 2004, 3, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Bernhards, Y.; Pöggeler, S. The phocein homologue SmMOB3 is essential for vegetative cell fusion and sexual development in the filamentous ascomycete Sordaria macrospora. Curr. Genet. 2011, 57, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Nordzieke, S.; Zobel, T.; Fränzel, B.; Wolters, D.A.; Kück, U.; Teichert, I. A fungal sarcolemmal membrane-associated protein (SLMAP) homolog plays a fundamental role in development and localizes to the nuclear envelope, endoplasmic reticulum, and mitochondria. Eukaryot. Cell 2015, 14, 345–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, A.; Herzog, B.; Frey, S.; Pöggeler, S. Autophagy-associated protein SmATG12 is required for fruiting-body formation in the filamentous ascomycete Sordaria macrospora. PLoS ONE 2016, 11, e0157960. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordzieke, D.E. Hyphal Fusions Enable Efficient Nutrient Distribution in Colletotrichum graminicola Conidiation and Symptom Development on Maize. Microorganisms 2022, 10, 1146. https://doi.org/10.3390/microorganisms10061146
Nordzieke DE. Hyphal Fusions Enable Efficient Nutrient Distribution in Colletotrichum graminicola Conidiation and Symptom Development on Maize. Microorganisms. 2022; 10(6):1146. https://doi.org/10.3390/microorganisms10061146
Chicago/Turabian StyleNordzieke, Daniela Elisabeth. 2022. "Hyphal Fusions Enable Efficient Nutrient Distribution in Colletotrichum graminicola Conidiation and Symptom Development on Maize" Microorganisms 10, no. 6: 1146. https://doi.org/10.3390/microorganisms10061146
APA StyleNordzieke, D. E. (2022). Hyphal Fusions Enable Efficient Nutrient Distribution in Colletotrichum graminicola Conidiation and Symptom Development on Maize. Microorganisms, 10(6), 1146. https://doi.org/10.3390/microorganisms10061146