Assessing the Synergistic Activity of Clarithromycin and Therapeutic Oils Encapsulated in Sodium Alginate Based Floating Microbeads
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Microbeads
2.3. Characterization
2.3.1. Particle Size Analysis
2.3.2. Swelling Study
2.3.3. In Vitro Buoyancy and Lag Time
2.3.4. Density Measurements
2.3.5. Drug Loading and Drug Entrapment Efficiency
2.3.6. Microscopic Studies
2.3.7. Fourier Transform Infra-Red Spectroscopy (FTIR)
2.3.8. Thermal Analysis
2.3.9. Powder X-ray Diffraction (XRD)
2.3.10. Antibacterial Assay
2.3.11. In Vitro Drug Release and Release Kinetics
3. Results and Discussion
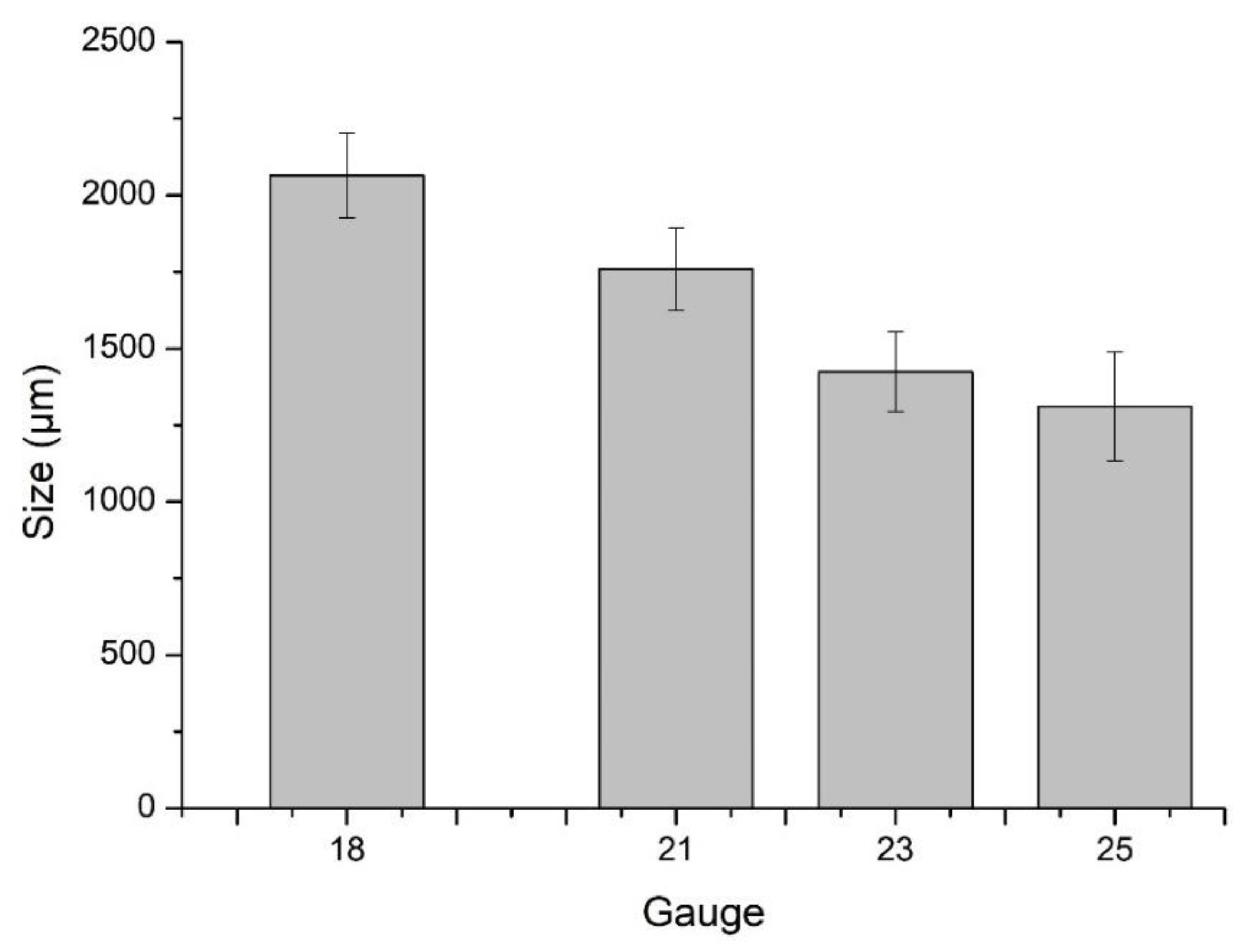
3.1. Particle Size Analysis
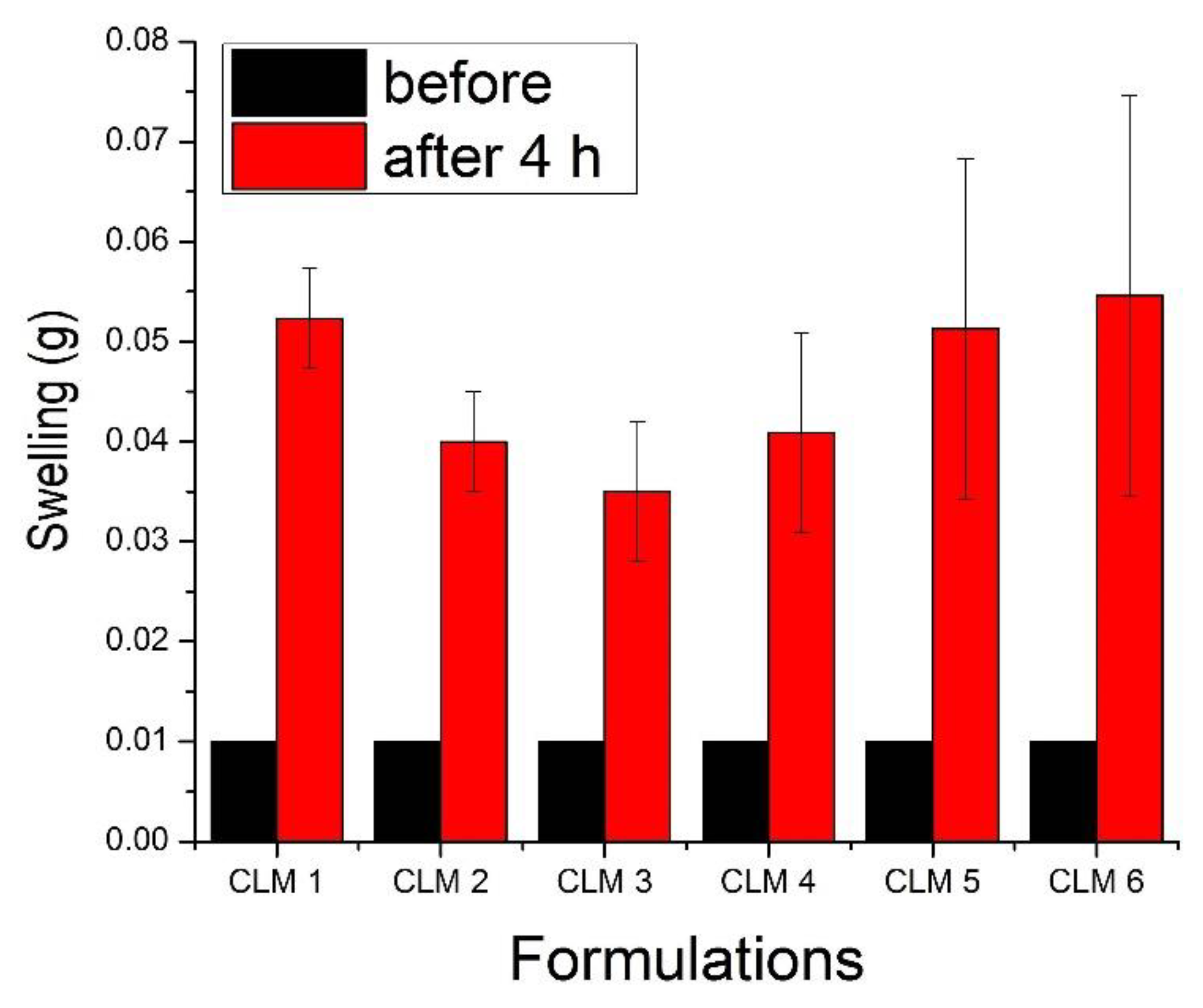
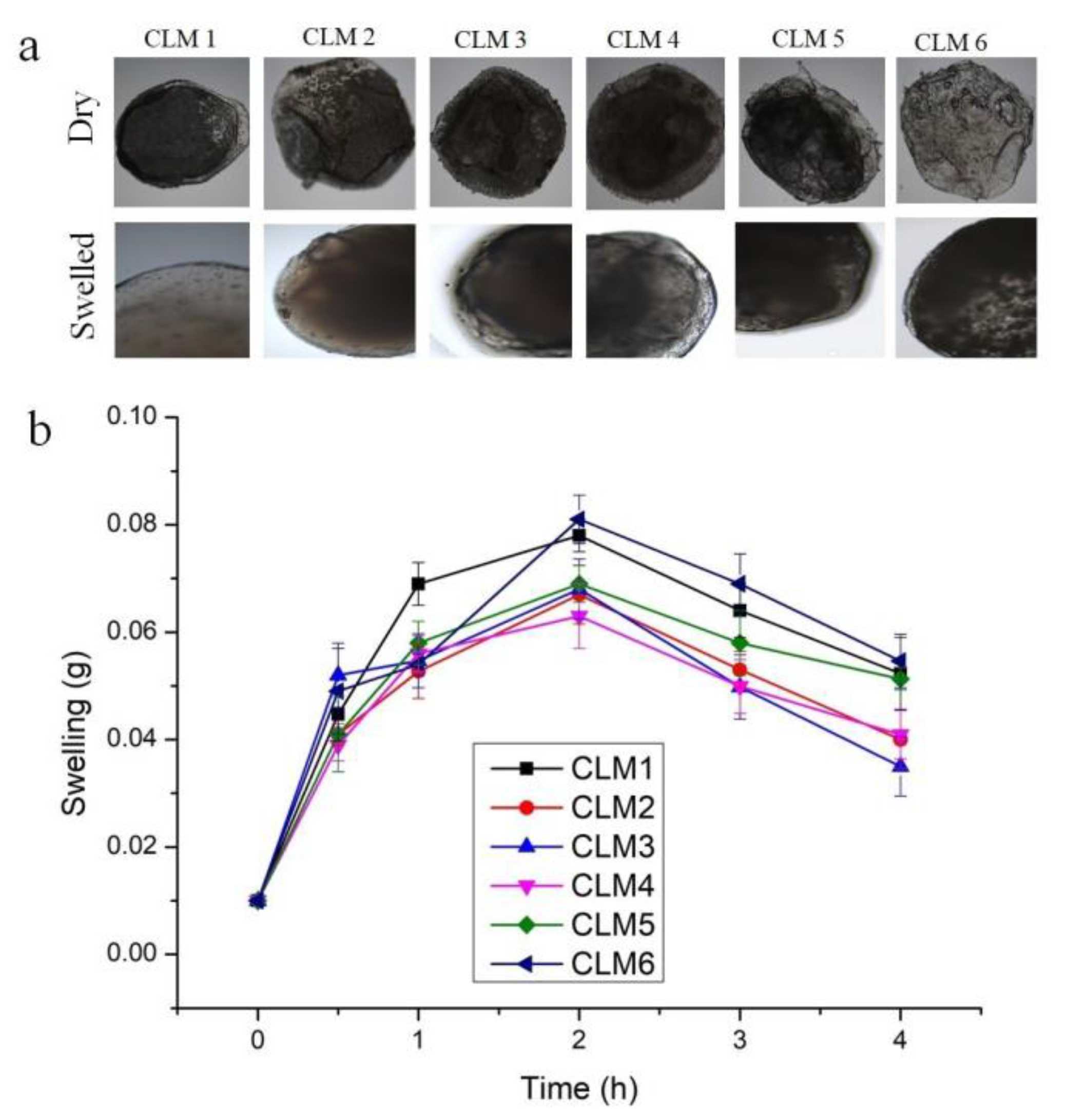
3.2. Swelling Studies
3.3. Buoyancy Studies
3.4. Density Measurement
3.5. Drug Loading and Entrapment Efficiency
3.6. Antibacterial Assay
3.7. Scanning Electron Microscopy (SEM)
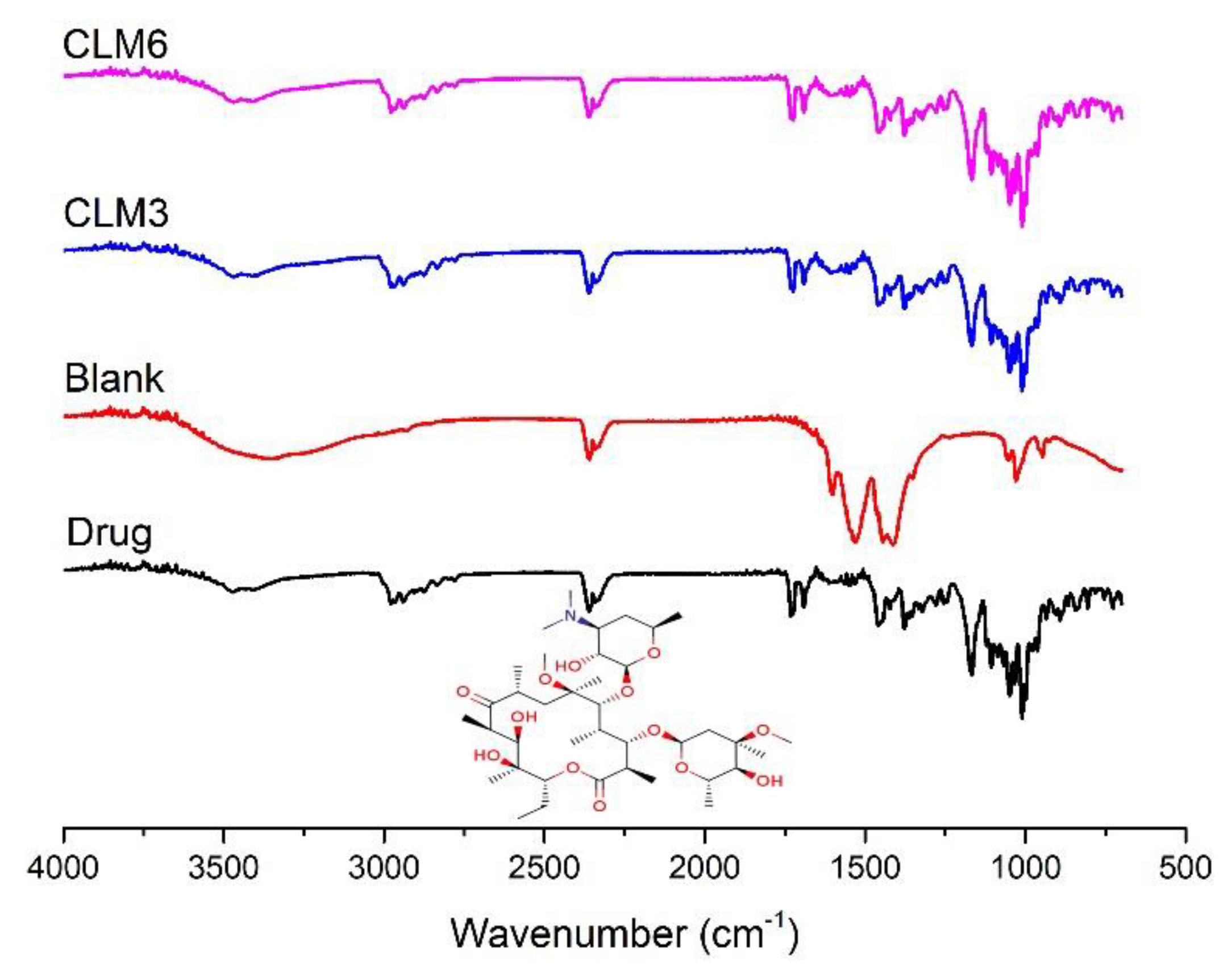
3.8. Fourier Transform Infrared Spectroscopy
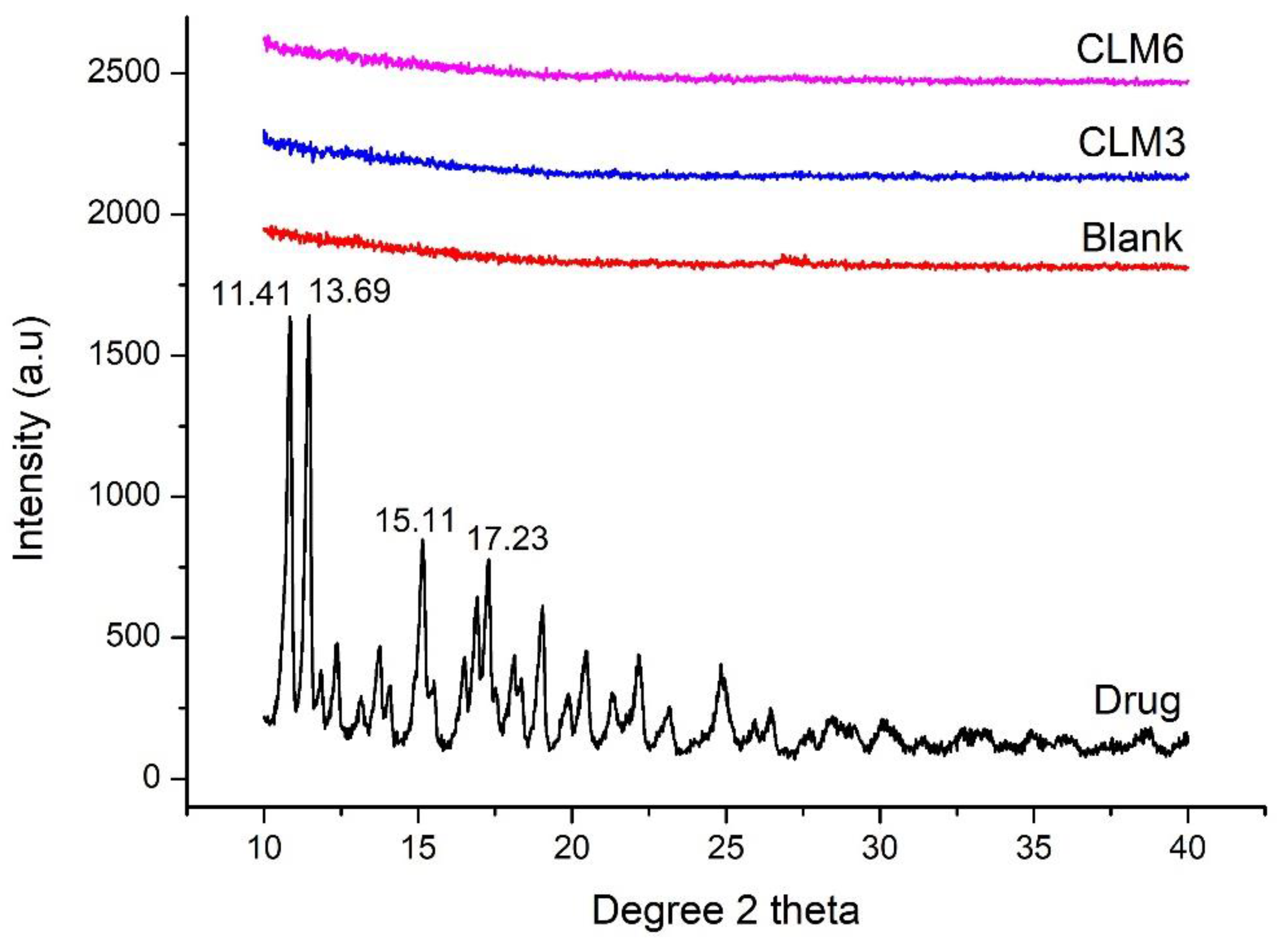
3.9. X-ray Diffraction (XRD)
3.10. Differential Scanning Calorimetry (DSC)
3.11. Thermogravimetric Analysis (TGA)
3.12. In Vitro Drug Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naseem, F.; Shah, S.U.; Rashid, S.A.; Farid, A.; Almehmadi, M.; Alghamdi, S. Metronidazole Based Floating Bioadhesive Drug Delivery System for Potential Eradication of H. pylori: Preparation and In Vitro Characterization. Polymers 2022, 14, 519. [Google Scholar] [CrossRef]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef]
- Kumar, M.; Kaushik, D. An Overview on Various Approaches and Recent Patents on Gastroretentive Drug Delivery Systems. Recent Pat. Drug Deliv. Formul. 2018, 12, 84–92. [Google Scholar] [CrossRef]
- Vrettos, N.N.; Roberts, C.J. Gastroretentive Technologies in Tandem with Controlled-Release Strategies: A Potent Answer to Oral Drug Bioavailability and Patient Compliance Implications. Pharmaceutics 2021, 13, 1591. [Google Scholar] [CrossRef] [PubMed]
- Klausner, E.A.; Lavy, E.; Friedman, M.; Hoffman, A. Expandable gastroretentive dosage forms. J. Control. Release 2003, 90, 143–162. [Google Scholar] [CrossRef]
- Talukder, R.; Fassihi, R. Gastroretentive delivery systems: A mini review. Drug Dev. Ind. Pharm. 2004, 30, 1019–1028. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, G. Progress in controlled gastroretentive delivery systems. Trop. J. Pharm. Res. 2008, 7, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Namdev, A.; Jain, D. Floating Drug Delivery Systems: An Emerging Trend for the Treatment of Peptic Ulcer. Curr. Drug Deliv. 2019, 16, 874–886. [Google Scholar] [CrossRef]
- Choudhary, S.; Jain, A.; Amin, M.C.I.M.; Mishra, V.; Agrawal, G.P.; Kesharwani, P. Stomach specific polymeric low density microballoons as a vector for extended delivery of rabeprazole and amoxicillin for treatment of peptic ulcer. Colloids Surf. B Biointerfaces 2016, 141, 268–277. [Google Scholar] [CrossRef]
- Israr, M.; Pugliese, N.; Farid, A.; Ghazanfar, S.; Di Cerbo, A.; Muzammal, M.; Alamri, A.S.; Basheeruddin Asdaq, S.M.; Ahmad, A.; Khan, K.A. Preparation and Characterization of Controlled-Release Floating Bilayer Tablets of Esomeprazole and Clarithromycin. Molecules 2022, 27, 3242. [Google Scholar] [CrossRef]
- Adebisi, A.O.; Laity, P.R.; Conway, B.R. Formulation and evaluation of floating mucoadhesive alginate beads for targeting Helicobacter pylori. J. Pharm. Pharmacol. 2015, 67, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organ. Regions. Gastroenterol. 2018, 155, 1372–1382.e1317. [Google Scholar] [CrossRef] [Green Version]
- Fagoonee, S.; Pellicano, R. Helicobacter pylori: Molecular basis for colonization and survival in gastric environment and resistance to antibiotics. A short review. Infect Dis. 2019, 51, 399–408. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E. In-Depth Study into Polymeric Materials in Low-Density Gastroretentive Formulations. Pharmaceutics 2020, 12, 636. [Google Scholar] [CrossRef]
- Mandal, U.K.; Chatterjee, B.; Senjoti, F.G. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J. Pharm. Sci. 2016, 11, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Cornaglia, A.I.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Rajinikanth, P.S.; Mishra, B. Stomach-site specific drug delivery system of clarithromycin for eradication of Helicobacter pylori. Chem. Pharm. Bull. 2009, 57, 1068–1075. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.-X.; Li, Y.F. Preparation and characterization of crosslinked porous cellulose beads. Carbohydr. Polym. 2006, 64, 402–407. [Google Scholar] [CrossRef]
- El Nashar, N.F.; Donia, A.A.; Mady, O.Y.; El Maghraby, G.M. Formulation of clarithromycin floating microspheres for eradication of Helicobacter pylori. J. Drug Deliv. Sci. Technol. 2017, 41, 213–221. [Google Scholar] [CrossRef]
- Rajbhar, P.; Sahu, A.K.; Gautam, S.S.; Prasad, R.K.; Singh, V.; Nair, S.K. Formulation and Evaluation of Clarithromycin Co-Crystals Tablets Dosage Forms to Enhance the Bioavailability. Pharma Innov. 2016, 5, 5. [Google Scholar]
- Morakul, B.; Suksiriworapong, J.; Chomnawang, M.T.; Langguth, P.; Junyaprasert, V.B. Dissolution enhancement and in vitro performance of clarithromycin nanocrystals produced by precipitation–lyophilization–homogenization method. Eur. J. Pharm. Biopharm. 2014, 88, 886–896. [Google Scholar] [CrossRef]
- Peppas, N. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Amsden, B.; Goosen, M. An examination of factors affecting the size, distribution and release characteristics of polymer microbeads made using electrostatics. J. Control. Release 1997, 43, 183–196. [Google Scholar] [CrossRef]
- Hadley, D.J.; Campbell, K.T.; Gabriel, M.H.; Silva, E.A. Open-source 3D printed air-jet for generating monodispersed alginate microhydrogels. bioRxiv 2019, 804849. [Google Scholar] [CrossRef]
- Nama, M.; Gonugunta, C.S.R.; Veerareddy, P.R. Formulation and evaluation of gastroretentive dosage forms of clarithromycin. AAPS PharmSciTech 2008, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of black seed oil in alginate beads as a ph-sensitive carrier for intestine-targeted drug delivery: In vitro, in vivo and ex vivo study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef] [Green Version]
- Marzoug, H.N.B.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Khouja, M.L.; Bouajila, J. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Khan, I.N.; Khan, I.U.; Yousaf, A.M.; Shahzad, Y. Gellan Gum-Based Bilayer Mucoadhesive Films Loaded with Moxifloxacin Hydrochloride and Clove Oil for Possible Treatment of Periodontitis. Drug Des. Dev. Ther. 2021, 15, 3937. [Google Scholar] [CrossRef]
- Mahmood, H.; Khan, I.U.; Asif, M.; Khan, R.U.; Asghar, S.; Khalid, I.; Khalid, S.H.; Irfan, M.; Rehman, F. Shahzad, Y.; et al. In vitro and in vivo evaluation of gellan gum hydrogel films: Assessing the co impact of therapeutic oils and ofloxacin on wound healing. Int. J. Biol. Macromol. 2021, 166, 483–495. [Google Scholar] [CrossRef]
- Wang, Q.; Geil, P.; Padua, G. Role of hydrophilic and hydrophobic interactions in structure development of zein films. J. Polym. Environ. 2004, 12, 197–202. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Rao, K.C. Fabrication and characterization of smart karaya gum/sodium alginate semi-IPN microbeads for controlled release of D-penicillamine drug. Polym. Polym. Compos. 2020, 29, 163–175. [Google Scholar] [CrossRef]
- Patel, H.; Srinatha, A.; Sridhar, B.K. External Cross-linked Mucoadhesive Microbeads for Prolonged Drug Release: Development and In vitro Characterization. Indian J. Pharm. Sci. 2014, 76, 437–444. [Google Scholar] [PubMed]
- Azad, A.K.; Al-Mahmood, S.M.A.; Kennedy, J.F.; Chatterjee, B.; Bera, H. Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int. J. Biol. Macromol. 2021, 185, 861–875. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Thirawong, N.; Puttipipatkhachorn, S. Morphology and buoyancy of oil-entrapped calcium pectinate gel beads. AAPS J. 2004, 6, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Hardikar, S.; Bhosale, A. Formulation and evaluation of gastro retentive tablets of clarithromycin prepared by using novel polymer blend. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 147–157. [Google Scholar] [CrossRef]
- Stops, F.; Fell, J.T.; Collett, J.H.; Martini, L.G. Floating dosage forms to prolong gastro-retention—The characterisation of calcium alginate beads. Int. J. Pharm. 2008, 350, 301–311. [Google Scholar] [CrossRef]
- Elhesaisy, N. l Swidan, S. Trazodone loaded lipid core poly (ε-caprolactone) nanocapsules: Development, characterization and in vivo antidepressant effect evaluation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Basso, J.; Mendes, M.; Cova, T.; Sousa, J.; Pais, A.; Fortuna, A.; Vitorino, R.; Vitorino, C. A Stepwise Framework for the Systematic Development of Lipid Nanoparticles. Biomolecules 2022, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Vitali, C.; De Laurentis, N.; Armenise, D.; Milillo, M.A. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine 2007, 14, 727–732. [Google Scholar] [CrossRef]
- Malik, T.; Singh, P.; Pant, S.; Chauhan, N.; Lohani, H. Potentiation of antimicrobial activity of ciprofloxacin by Pelargonium graveolens essential oil against selected uropathogens. Phytother. Res. 2011, 25, 1225–1228. [Google Scholar] [CrossRef]
- Duarte, A.; Ferreira, S.; Silva, F.; Domingues, F.C. Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomedicine 2012, 19, 236–238. [Google Scholar] [CrossRef]
- Lahmar, A.; Bedoui, A.; Mokdad-Bzeouich, I.; Dhaouifi, Z.; Kalboussi, Z.; Cheraif, I.; Ghedira, K.; Chekir-Ghedira, L. Reversal of resistance in bacteria underlies synergistic effect of essential oils with conventional antibiotics. Microb. Pathog. 2017, 106, 50–59. [Google Scholar] [CrossRef]
- Öztürk, A.A.; Yenilmez, E.; Özarda, M.G. Clarithromycin-Loaded Poly (Lactic-co-glycolic Acid) (PLGA) Nanoparticles for Oral Administration: Effect of Polymer Molecular Weight and Surface Modification with Chitosan on Formulation, Nanoparticle Characterization and Antibacterial Effects. Polymers 2019, 11, 1632. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.K.; Alhamdany, A.T. Floating Microspheres of Enalapril Maleate as a Developed Controlled Release Dosage Form: Investigation of the Effect of an Ionotropic Gelation Technique. Turk. J. Pharm. Sci. 2020, 17, 159–171. [Google Scholar] [CrossRef]
- Bani-Jaber, A.; Aideh, K.; Hamdan, I.; Maraqa, R. Drug-loaded casein beads: Influence of different metal-types as cross-linkers and oleic acid as a plasticizer on some properties of the beads. J. Drug Deliv. Sci. Technol. 2009, 19, 125–131. [Google Scholar] [CrossRef]
- Gattani, S.G.; Savaliya, P.J.; Belgamwar, V.S. Floating-mucoadhesive beads of clarithromycin for the treatment of Helicobacter pylori infection. Chem. Pharm. Bull. 2010, 58, 782–787. [Google Scholar] [CrossRef] [Green Version]
- Rasel, M.A.T.; Hasan, M. Formulation and evaluation of floating alginate beads of diclofenac sodium. Dhaka Univ. J. Pharm. Sci. 2012, 11, 29–35. [Google Scholar] [CrossRef]
- Van der Weerd, J.; Kazarian, S.G. Release of poorly soluble drugs from HPMC tablets studied by FTIR imaging and flow-through dissolution tests. J. Pharm. Sci. 2005, 94, 2096–2109. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Rao, K.C. Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. 2021, 11, 191–199. [Google Scholar] [CrossRef]
- Chung, D.; Song, Y.G.; Kang, I.M.; Choi, W.; Park, C.; Song, Y. Development and characterization of clarithromycin-smectite hybrid for burst release at high pH condition. Mater. Res. Express 2018, 5, 115408. [Google Scholar] [CrossRef]
- Ijaz, Q.A.; Latif, S.; Rashid, M.; Arshad, M.S.; Hussain, A.; Bukhari, N.I.; Riaz, S.; Abbas, N. Preparation and Characterization of pH-Independent Sustained-Release Tablets Containing Hot Melt Extruded Solid Dispersions of Clarithromycin. AAPS PharmSciTech 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Balanč, B.; Kalušević, A.; Drvenica, I.; Coelho, M.T.; Djordjević, V.; Alves, V.D.; Sousa, I.; Moldão-Martins, M.; Rakić, V.; Nedović, V.; et al. Calcium–alginate–inulin microbeads as carriers for aqueous carqueja extract. J. Food Sci. 2016, 81, E65–E75. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Lalwani, D.; Gollmer, S.; Injeti, E.; Sari, Y.; Nesamony, J. Development and evaluation of a calcium alginate based oral ceftriaxone sodium formulation. Prog. Biomater. 2016, 5, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, G.; Singh, S. Formulation and In Vitro Evaluation of pH-sensitive oil-entrapped buoyant beads of Clarithromycin. Trop. J. Pharm. Res. 2010, 9, 533–539. [Google Scholar] [CrossRef] [Green Version]





| Code | Sodium Alginate (% w/v) | HPMC (% w/v) | CaCO3 (% w/v) | Eucalyptus Oil (% w/v) | Oleic Acid (% w/v) | Clarithromycin (mg) |
|---|---|---|---|---|---|---|
| CLM 1 | 0.5 | 0.25 | 0.5 | 0.3 | - | 120 |
| CLM 2 | 0.5 | 0.25 | 0.5 | 0.4 | - | 120 |
| CLM 3 | 0.5 | 0.25 | 0.5 | 0.5 | - | 120 |
| CLM 4 | 0.5 | 0.25 | 0.5 | - | 0.20 | 60 |
| CLM 5 | 0.5 | 0.25 | 0.5 | - | 0.25 | 60 |
| CLM 6 | 0.5 | 0.25 | 0.5 | - | 0.30 | 60 |
| CLM 7 | 0.5 | 0.25 | 0.5 | - | - | - |
| Code | 1 h | 2 h | 3 h | 4 h | 24 h | Floating Lag Time |
|---|---|---|---|---|---|---|
| CLM 1 | 60% | 10% | 6% | 2% | - | <1 min |
| CLM 2 | 50% | 24% | 18% | 8% | - | <1 min |
| CLM 3 | 72% | 64% | 64% | 60% | 6% | <1 min |
| CLM 4 | 52% | 52% | 52% | 48% | - | <1 min |
| CLM 5 | 50% | 48% | 44% | 44% | - | <1 min |
| CLM 6 | 66% | 60% | 40% | 40% | 4% | <1 min |
| Code | Bulk Density (g/cm3) | % Drug Loading | % Entrapment Efficiency |
|---|---|---|---|
| CLM 1 | 0.33 ± 0.0006 | 2.51 | 26.10 |
| CLM 2 | 0.32 ± 0.0009 | 2.76 | 28.71 |
| CLM 3 | 0.29 ± 0.001 | 3.09 | 32.24 |
| CLM 4 | 0.28 ± 0.033 | 2.85 | 29.78 |
| CLM 5 | 0.31 ± 0.017 | 2.36 | 24.66 |
| CLM 6 | 0.26 ± 0.03 | 2.04 | 21.26 |
| Code | Korsemeyer–Peppas Model | |
|---|---|---|
| Correlation Coefficients (R2) | Release Exponent (n) | |
| CLM 3 | 0.95 | 0.04 |
| CLM 6 | 0.99 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.U.; Shoukat, M.; Asif, M.; Khalid, S.H.; Asghar, S.; Munir, M.U.; Irfan, M.; Rasul, A.; Qari, S.H.; Qumsani, A.T.; et al. Assessing the Synergistic Activity of Clarithromycin and Therapeutic Oils Encapsulated in Sodium Alginate Based Floating Microbeads. Microorganisms 2022, 10, 1171. https://doi.org/10.3390/microorganisms10061171
Khan IU, Shoukat M, Asif M, Khalid SH, Asghar S, Munir MU, Irfan M, Rasul A, Qari SH, Qumsani AT, et al. Assessing the Synergistic Activity of Clarithromycin and Therapeutic Oils Encapsulated in Sodium Alginate Based Floating Microbeads. Microorganisms. 2022; 10(6):1171. https://doi.org/10.3390/microorganisms10061171
Chicago/Turabian StyleKhan, Ikram Ullah, Mehwish Shoukat, Muhammad Asif, Syed Haroon Khalid, Sajid Asghar, Muhammad Usman Munir, Muhammad Irfan, Akhtar Rasul, Sameer H. Qari, Alaa T. Qumsani, and et al. 2022. "Assessing the Synergistic Activity of Clarithromycin and Therapeutic Oils Encapsulated in Sodium Alginate Based Floating Microbeads" Microorganisms 10, no. 6: 1171. https://doi.org/10.3390/microorganisms10061171
APA StyleKhan, I. U., Shoukat, M., Asif, M., Khalid, S. H., Asghar, S., Munir, M. U., Irfan, M., Rasul, A., Qari, S. H., Qumsani, A. T., Hassan, M. M., Alahdal, M. A., Usman, M., & Khan, Z. (2022). Assessing the Synergistic Activity of Clarithromycin and Therapeutic Oils Encapsulated in Sodium Alginate Based Floating Microbeads. Microorganisms, 10(6), 1171. https://doi.org/10.3390/microorganisms10061171











