Mycobacterium chimaera Identification Using MALDI-TOF MS Technology: A Practical Approach for the Clinical Microbiology Laboratories
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mycobacterial Strains
2.2. MALDI-TOF: Equipment and Libraries
2.3. MALDI-TOF: Extraction Procedures
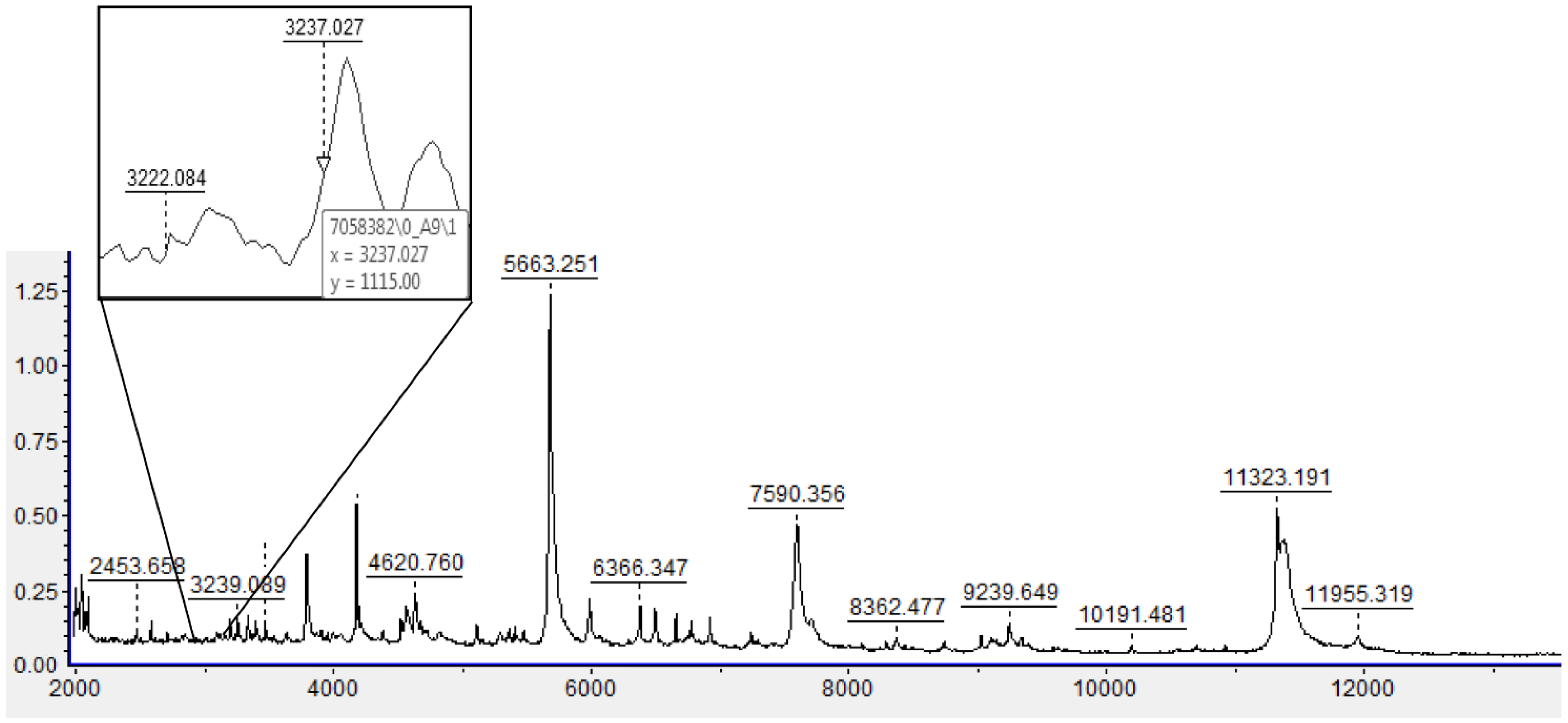
2.4. MALDI-TOF: Analysis
2.5. MALDI-TOF: Reproducibility
2.6. Implementation of the Protocol in the Daily Practice
3. Results
3.1. Mycobacterial Isolates
3.2. MALDI-TOF: Extraction Procedures
3.3. MALDI-TOF: Analysis
3.4. Implementation of the Protocol in the Daily Practice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tortoli, E.; Rindi, L.; Garcia, M.J.; Chiaradonna, P.; Dei, R.; Garzelli, C.; Kroppenstedt, R.M.; Lari, N.; Mattei, R.; Mariottini, A.; et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Siddam, A.D.; Zaslow, S.J.; Wang, Y.; Phillips, K.S.; Silverman, M.D.; Regan, P.M.; Amarasinghe, J.J. Characterization of Biofilm Formation by Mycobacterium chimaera on Medical Device Materials. Front. Microbiol. 2020, 11, 586657. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.P.; Zembower, T.R.; Reddy, S.; Qi, C. Comparison of Clinical Features, Virulence, and Relapse among Mycobacterium avium Complex Species. Am. J. Respir. Crit. Care Med. 2015, 191, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Bacrie, S.; David, M.; Stremler, N.; Dubus, J.C.; Rolain, J.M.; Drancourt, M. Mycobacterium chimaera pulmonary infection complicating cystic fibrosis: A case report. J. Med. Case Rep. 2011, 5, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bills, N.D.; Hinrichs, S.H.; Aden, T.A.; Wickert, R.S.; Iwen, P.C. Molecular identification of Mycobacterium chimaera as a cause of infection in a patient with chronic obstructive pulmonary disease. Diagn. Microbiol. Infect. Dis. 2009, 63, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.; Kuster, S.P.; Bloemberg, G.; Schulthess, B.; Frank, M.; Tanner, F.C.; Rossle, M.; Boni, C.; Falk, V.; Wilhelm, M.J.; et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur. Heart J. 2015, 36, 2745–2753. [Google Scholar] [CrossRef] [Green Version]
- Achermann, Y.; Rossle, M.; Hoffmann, M.; Deggim, V.; Kuster, S.; Zimmermann, D.R.; Bloemberg, G.; Hombach, M.; Hasse, B. Prosthetic valve endocarditis and bloodstream infection due to Mycobacterium chimaera. J. Clin. Microbiol. 2013, 51, 1769–1773. [Google Scholar] [CrossRef] [Green Version]
- Dalvi, S.; Das, P. Prosthetic heart valve surgery and potential risk of -developing Mycobacterium chimaera endocarditis. Clin. Med. 2018, 18, 301–303. [Google Scholar] [CrossRef] [Green Version]
- Ninh, A.; Weiner, M.; Goldberg, A. Healthcare-Associated Mycobacterium chimaera Infection Subsequent to Heater-Cooler Device Exposure During Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1831–1835. [Google Scholar] [CrossRef]
- Schreiber, P.W.; Sax, H. Mycobacterium chimaera infections associated with heater-cooler units in cardiac surgery. Curr. Opin. Infect. Dis. 2017, 30, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Garvey, M.I.; Phillips, N.; Bradley, C.W.; Holden, E. Decontamination of an Extracorporeal Membrane Oxygenator Contaminated With Mycobacterium chimaera. Infect. Control Hosp. Epidemiol. 2017, 38, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Gotting, T.; Klassen, S.; Jonas, D.; Benk, C.; Serr, A.; Wagner, D.; Ebner, W. Heater-cooler units: Contamination of crucial devices in cardiothoracic surgery. J. Hosp. Infect. 2016, 93, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Moore, G.; Collins, S.; Parks, S.; Garvey, M.I.; Lamagni, T.; Smith, G.; Dawkin, L.; Goldenberg, S.; Chand, M. Microbiological problems and biofilms associated with Mycobacterium chimaera in heater-cooler units used for cardiopulmonary bypass. J. Hosp. Infect. 2017, 96, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, K.B.; Yuh, D.D.; Schwartz, S.B.; Lange, R.A.; Hopkins, R.; Bauer, K.; Marders, J.A.; Delgado Donayre, J.; Milligan, N.; Wentz, C. Nontuberculous Mycobacterium Infections Associated With Heater-Cooler Devices. Ann. Thorac. Surg. 2017, 104, 1237–1242. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.I.; Shin, S.J.; Shin, M.K. Differential Genotyping of Mycobacterium avium Complex and Its Implications in Clinical and Environmental Epidemiology. Microorganisms 2020, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Kostrzewa, M.; Nagy, E.; Schrottner, P.; Pranada, A.B. How MALDI-TOF mass spectrometry can aid the diagnosis of hard-to-identify pathogenic bacteria—The rare and the unknown. Expert. Rev. Mol. Diagn. 2019, 19, 667–682. [Google Scholar] [CrossRef]
- Pan, S.W.; Shu, C.C.; Feng, J.Y.; Chien, J.Y.; Wang, J.Y.; Chan, Y.J.; Yu, C.J.; Su, W.J. Impact of different subspecies on disease progression in initially untreated patients with Mycobacterium avium complex lung disease. Clin. Microbiol. Infect. 2021, 27, 467.e9–467.e14. [Google Scholar] [CrossRef]
- Schweickert, B.; Goldenberg, O.; Richter, E.; Gobel, U.B.; Petrich, A.; Buchholz, P.; Moter, A. Occurrence and clinical relevance of Mycobacterium chimaera sp. nov., Germany. Emerg. Infect. Dis. 2008, 14, 1443–1446. [Google Scholar] [CrossRef]
- Lecorche, E.; Haenn, S.; Mougari, F.; Kumanski, S.; Veziris, N.; Benmansour, H.; Raskine, L.; Moulin, L.; Cambau, E.; CNR-MyRMA. Comparison of methods available for identification of Mycobacterium chimaera. Clin. Microbiol. Infect. 2018, 24, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Russo, C.; Tortoli, E.; Menichella, D. Evaluation of the new GenoType Mycobacterium assay for identification of mycobacterial species. J. Clin. Microbiol. 2006, 44, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Seng, P.; Rolain, J.M.; Fournier, P.E.; La Scola, B.; Drancourt, M.; Raoult, D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010, 5, 1733–1754. [Google Scholar] [CrossRef] [PubMed]
- Saleeb, P.G.; Drake, S.K.; Murray, P.R.; Zelazny, A.M. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2011, 49, 1790–1794. [Google Scholar] [CrossRef] [Green Version]
- Alcaide, F.; Amlerova, J.; Bou, G.; Ceyssens, P.J.; Coll, P.; Corcoran, D.; Fangous, M.S.; Gonzalez-Alvarez, I.; Gorton, R.; Greub, G.; et al. How to: Identify non-tuberculous Mycobacterium species using MALDI-TOF mass spectrometry. Clin. Microbiol. Infect. 2018, 24, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Temporal, D.; Rodriguez-Sanchez, B.; Alcaide, F. Evaluation of MALDI Biotyper Interpretation Criteria for Accurate Identification of Nontuberculous Mycobacteria. J. Clin. Microbiol. 2020, 58, e01103-20. [Google Scholar] [CrossRef] [PubMed]
- Pranada, A.B.; Witt, E.; Bienia, M.; Kostrzewa, M.; Timke, M. Accurate differentiation of Mycobacterium chimaera from Mycobacterium intracellulare by MALDI-TOF MS analysis. J. Med. Microbiol. 2017, 66, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Epperson, L.E.; Timke, M.; Hasan, N.A.; Godo, P.; Durbin, D.; Helstrom, N.K.; Shi, G.; Kostrzewa, M.; Strong, M.; Salfinger, M. Evaluation of a Novel MALDI Biotyper Algorithm to Distinguish Mycobacterium intracellulare from Mycobacterium chimaera. Front. Microbiol. 2018, 9, 3140. [Google Scholar] [CrossRef] [PubMed]
- Ghodousi, A.; Borroni, E.; Peracchi, M.; Palu, G.; Fallico, L.; Rassu, M.; Manfrin, V.; Mantegani, P.; Monzillo, V.; Manganelli, R.; et al. Genomic analysis of cardiac surgery-associated Mycobacterium chimaera infections in Italy. PLoS ONE 2020, 15, e0239273. [Google Scholar] [CrossRef]
- Russello, G.; Visiello, R.; Barbarini, D.; Bardaro, M.; Del Bianco, F.; De Vitis, D.; Matteucci, M.; Sambri, V.; Carretto, E. Application of MALDI-TOF for identification of nontuberculous mycobacteria in a clinical setting. In Proceedings of the 27th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Mather, C.A.; Rivera, S.F.; Butler-Wu, S.M. Comparison of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry systems for identification of mycobacteria using simplified protein extraction protocols. J. Clin. Microbiol. 2014, 52, 130–138. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.A.; Lynch-Healy, M.; Corcoran, D.; O’Reilly, B.; O’Mahony, J.; Lucey, B. Improved Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS)-Based Identification of Mycobacterium spp. by Use of a Novel Two-Step Cell Disruption Preparatory Technique. J. Clin. Microbiol. 2016, 54, 495–496. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Sanchez, B.; Ruiz-Serrano, M.J.; Ruiz, A.; Timke, M.; Kostrzewa, M.; Bouza, E. Evaluation of MALDI Biotyper Mycobacteria Library v3.0 for Identification of Nontuberculous Mycobacteria. J. Clin. Microbiol. 2016, 54, 1144–1147. [Google Scholar] [CrossRef] [Green Version]
- Torres-Sangiao, E.; Leal Rodriguez, C.; Garcia-Riestra, C. Application and Perspectives of MALDI-TOF Mass Spectrometry in Clinical Microbiology Laboratories. Microorganisms 2021, 9, 1539. [Google Scholar] [CrossRef] [PubMed]
- Ceyssens, P.J.; Soetaert, K.; Timke, M.; Van den Bossche, A.; Sparbier, K.; De Cremer, K.; Kostrzewa, M.; Hendrickx, M.; Mathys, V. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Combined Species Identifi-cation and Drug Sensitivity Testing in Mycobacteria. J. Clin. Microbiol. 2017, 55, 624–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Temporal, D.; Perez-Risco, D.; Struzka, E.A.; Mas, M.; Alcaide, F. Evaluation of Two Protein Extraction Protocols Based on Freezing and Mechanical Disruption for Identifying Nontuberculous Mycobacteria by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry from Liquid and Solid Cultures. J. Clin. Microbiol. 2018, 56, e01548-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Temporal, D.; Alcaide, F.; Marekovic, I.; O’Connor, J.A.; Gorton, R.; van Ingen, J.; Van den Bossche, A.; Hery-Arnaud, G.; Beauruelle, C.; Orth-Holler, D.; et al. Multicentre study on the reproducibility of MALDI-TOF MS for nontuberculous mycobacteria identification. Sci. Rep. 2022, 12, 1237. [Google Scholar] [CrossRef]
- Alcolea-Medina, A.; Fernandez, M.T.C.; Montiel, N.; Garcia, M.P.L.; Sevilla, C.D.; North, N.; Lirola, M.J.M.; Wilks, M. An improved simple method for the identification of Mycobacteria by MALDI-TOF MS (Matrix-Assisted Laser Desorption-Ionization mass spectrometry). Sci. Rep. 2019, 9, 20216. [Google Scholar] [CrossRef]
- López Medrano, R.; Burgos Asurmendi, I.; Rivero Lezcano, O.M. A rapid proteomic system (MALDI-TOF) for nontuberculous-mycobacteria identification. Enferm. Infecc. Microbiol. Clin. 2022. [Google Scholar] [CrossRef]
| TEMPLATE SPREADSHEET FOR THE CALCULATION OF THE SCORE FOR THE ISOLATE IDENTIFICATION | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCORE VALUES for different replicates of the same strain | Relative intensities (a.u.) of specific peaks (m/z) | Sum intensities of M. chimaera (SIC, ki) | Sum intensities of M. intracellulare (SS, ji) | RATIO SIC/SII (ki/ji) | IQ (LOG) | Mean | |||||
| 3222 (m/z) | 3237 (m/z) | 6448 (m/z) | 6476 (m/z) | 6904 (m/z) | 7358 (m/z) | ||||||
| Higher score (A) | k1A | j1A | k2A | j2A | j3A | k3A | kiA = k1A + k2A + k3A | jiA = j1A + j2A + j3A | m1 = kiA/jiA | log m1 | |
| Intermediate score (B) | k1B | j1B | k2B | j2B | j3B | k3B | kiB = k1B + k2B + k3B | jiB = j1B + j2B + J3B | m2 = kiB/jiB | log m2 | |
| Lowest score (C) | k1C | j1C | k2C | j2C | j3C | k3C | kiC = k1C + k2C + k3C | jiC = j1C + j2C + j3C | m3 = kiC/jiC | log m3 | |
| EXAMPLE OF APPLICATION | |||||||||||
| SCORE VALUES for different replicates of the same strain | Relative intensities (a.u.) of specific peaks (m/z) | Sum intensities of M. chimaera (SIC, ki) | Sum intensities of M. intracellulare (SS, ji) | RATIO SIC/SII (ki/ji) | IQ (LOG) | Mean | |||||
| 3222 (m/z) | 3237 (m/z) | 6448 (m/z) | 6476 (m/z) | 6904 (m/z) | 7358 (m/z) | −0.177 | |||||
| Higher score (A) | 922 | 1.424 | 751 | 1.592 | 988 | 552 | 2.225 | 4.004 | 0.5557 | −0.255 | |
| Intermediate score (B) | 636 | 953 | 633 | 959 | 747 | 440 | 1.709 | 2.659 | 0.6427 | −0.192 | |
| Lowest score (C) | 1.016 | 1.089 | 1.017 | 1.367 | 1.232 | 1.017 | 3050 | 3.688 | 0.8270 | −0.082 | |
| Samples | ID (Gold Standard) | SCORES AND LOGs OBTAINED AT | D (IQ) | |||
|---|---|---|---|---|---|---|
| SITE A | SITE B | |||||
| Score Average | log(IQ) | Score Average | log(IQ) | |||
| A1 | M. chimaera | 2.38 | 0.32 | 2.43 | 0.38 | 0.06 |
| A2 | M. chimaera | 2.55 | 0.28 | 2.56 | 0.41 | 0.13 |
| A3 | M. chimaera | 2.02 | 0.14 | 2.51 | 0.42 | 0.28 |
| A4 | M. chimaera | 2.29 | 0.24 | 2.63 | 0.15 | 0.09 |
| A5 | M. chimaera | 2.36 | 0.16 | 2.63 | 0.12 | 0.04 |
| A6 | M. chimaera | 2.58 | 0.35 | 2.56 | 0.44 | 0.09 |
| A7 | M. chimaera | 2.58 | 0.4 | 2.55 | 0.43 | 0.03 |
| A8 | M. chimaera | 2.59 | 0.36 | 2.55 | 0.33 | 0.03 |
| A9 | M. chimaera | 2.22 | 0.12 | 2.55 | 0.41 | 0.29 |
| A10 | M. chimaera | 2.46 | 0.46 | 2.58 | 0.47 | 0.01 |
| A11 | M. chimaera | 2.53 | 0.34 | 2.61 | 0.07 | 0.27 |
| A12 | M. chimaera | 2.20 | 0.16 | 2.60 | 0.14 | 0.02 |
| A13 | M. chimaera | 2.52 | 0.36 | 2.65 | 0.12 | 0.24 |
| A14 | M. chimaera | 2.00 | 0.12 | 2.68 | 0.07 | 0.05 |
| A15 | M. chimaera | 2.33 | 0.43 | 2.45 | 0.53 | 0.10 |
| A16 | M. intracellulare | 2.52 | −0.32 | 2.49 | −0.45 | 0.13 |
| A17 | M. intracellulare | 2.51 | −0.14 | 2.50 | −0.32 | 0.18 |
| A18 | M. intracellulare | 2.3 | −0.11 | 2.50 | −0.02 | 0.09 |
| A19 | M. intracellulare | 1.85 | −0.27 | 2.47 | −0.48 | 0.21 |
| A20 | M. intracellulare | 2.39 | −0.26 | 2.52 | −0.43 | 0.17 |
| A21 | M. intracellulare | 2.49 | −0.24 | 2.51 | −0.15 | 0.09 |
| A22 | M. chimaera | 2.55 | 0.36 | 2.48 | 0.05 | 0.31 |
| B1 | M. chimaera | 2.47 | 0.36 | 2.45 | 0.11 | 0.25 |
| B2 | M. intracellulare | 2.37 | −0.38 | 2.48 | −0.35 | 0.03 |
| B3 | M. intracellulare | 2.27 | −0.29 | 2.42 | −0.23 | 0.06 |
| B4 | M. chimaera | 2.39 | 0.4 | 2.63 | 0.47 | 0.07 |
| B5 | M. chimaera | 2.32 | 0.39 | 2.33 | 0.24 | 0.15 |
| B6 | M. chimaera | 2.39 | 0.38 | 2.5 | 0.21 | 0.17 |
| B7 | M. chimaera | 2.48 | 0.38 | 2.56 | 0.35 | 0.03 |
| B8 | M. chimaera | 2.25 | 0.25 | 2.65 | 0.42 | 0.17 |
| B9 | M. chimaera | 2.39 | 0.31 | 2.61 | 0.19 | 0.12 |
| B10 | M. chimaera | 2.22 | 0.4 | 2.53 | 0.29 | 0.11 |
| B11 | M. chimaera | 2.33 | 0.31 | 2.41 | 0.2 | 0.11 |
| B12 | M. intracellulare | 2.37 | −0.17 | 2.25 | −0.12 | 0.05 |
| B13 | M. intracellulare | 2.09 | −0.17 | 2.28 | −0.2 | 0.03 |
| B14 | M. intracellulare | 1.96 | −0.03 | 2.24 | −0.01 | 0.02 |
| B15 | M. chimaera | 2.26 | 0.28 | 2.63 | 0.42 | 0.14 |
| B16 | M. chimaera | 2.16 | 0.26 | 2.41 | 0.34 | 0.08 |
| B17 | M. chimaera | 2.43 | 0.37 | 2.59 | 0.39 | 0.02 |
| B18 | M. intracellulare | 2.13 | −0.18 | 2.56 | −0.26 | 0.08 |
| B19 | M. intracellulare | 2.26 | −0.13 | 2.51 | −0.30 | 0.17 |
| B20 | M. intracellulare | 2.21 | −0.15 | 2.55 | −0.25 | 0.10 |
| B21 | M. intracellulare | 2.37 | −0.33 | 2.55 | −0.22 | 0.11 |
| B22 | M. chimaera | 2.47 | 0.38 | 2.33 | 0.13 | 0.25 |
| B23 | M. intracellulare | 2.29 | −0.28 | 2.56 | −0.22 | 0.06 |
| B24 | M. intracellulare | 1.86 | −0.42 | 1.79 | −0.11 | 0.31 |
| B25 | M. intracellulare | 2.27 | −0.19 | 2.51 | −0.31 | 0.12 |
| B26 | M. chimaera | 2.34 | 0.33 | 2.39 | 0.22 | 0.11 |
| B27 | M. chimaera | 2.57 | 0.21 | 2.08 | 0.12 | 0.09 |
| B28 | M. intracellulare | 1.86 | −0.27 | 1.77 | −0.23 | 0.04 |
| B29 | M. intracellulare | 2.45 | −0.19 | 2.14 | −0.14 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagnarino, J.; Barbarini, D.; Russello, G.; Siciliano, M.; Monzillo, V.; Baldanti, F.; Carretto, E. Mycobacterium chimaera Identification Using MALDI-TOF MS Technology: A Practical Approach for the Clinical Microbiology Laboratories. Microorganisms 2022, 10, 1184. https://doi.org/10.3390/microorganisms10061184
Bagnarino J, Barbarini D, Russello G, Siciliano M, Monzillo V, Baldanti F, Carretto E. Mycobacterium chimaera Identification Using MALDI-TOF MS Technology: A Practical Approach for the Clinical Microbiology Laboratories. Microorganisms. 2022; 10(6):1184. https://doi.org/10.3390/microorganisms10061184
Chicago/Turabian StyleBagnarino, Jessica, Daniela Barbarini, Giuseppe Russello, Mariangela Siciliano, Vincenzina Monzillo, Fausto Baldanti, and Edoardo Carretto. 2022. "Mycobacterium chimaera Identification Using MALDI-TOF MS Technology: A Practical Approach for the Clinical Microbiology Laboratories" Microorganisms 10, no. 6: 1184. https://doi.org/10.3390/microorganisms10061184
APA StyleBagnarino, J., Barbarini, D., Russello, G., Siciliano, M., Monzillo, V., Baldanti, F., & Carretto, E. (2022). Mycobacterium chimaera Identification Using MALDI-TOF MS Technology: A Practical Approach for the Clinical Microbiology Laboratories. Microorganisms, 10(6), 1184. https://doi.org/10.3390/microorganisms10061184







