Natural Protein Kinase Inhibitors, Staurosporine, and Chelerythrine Suppress Wheat Blast Disease Caused by Magnaporthe oryzae Triticum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain, Growth Medium, and Plant Materials
2.2. Chemicals
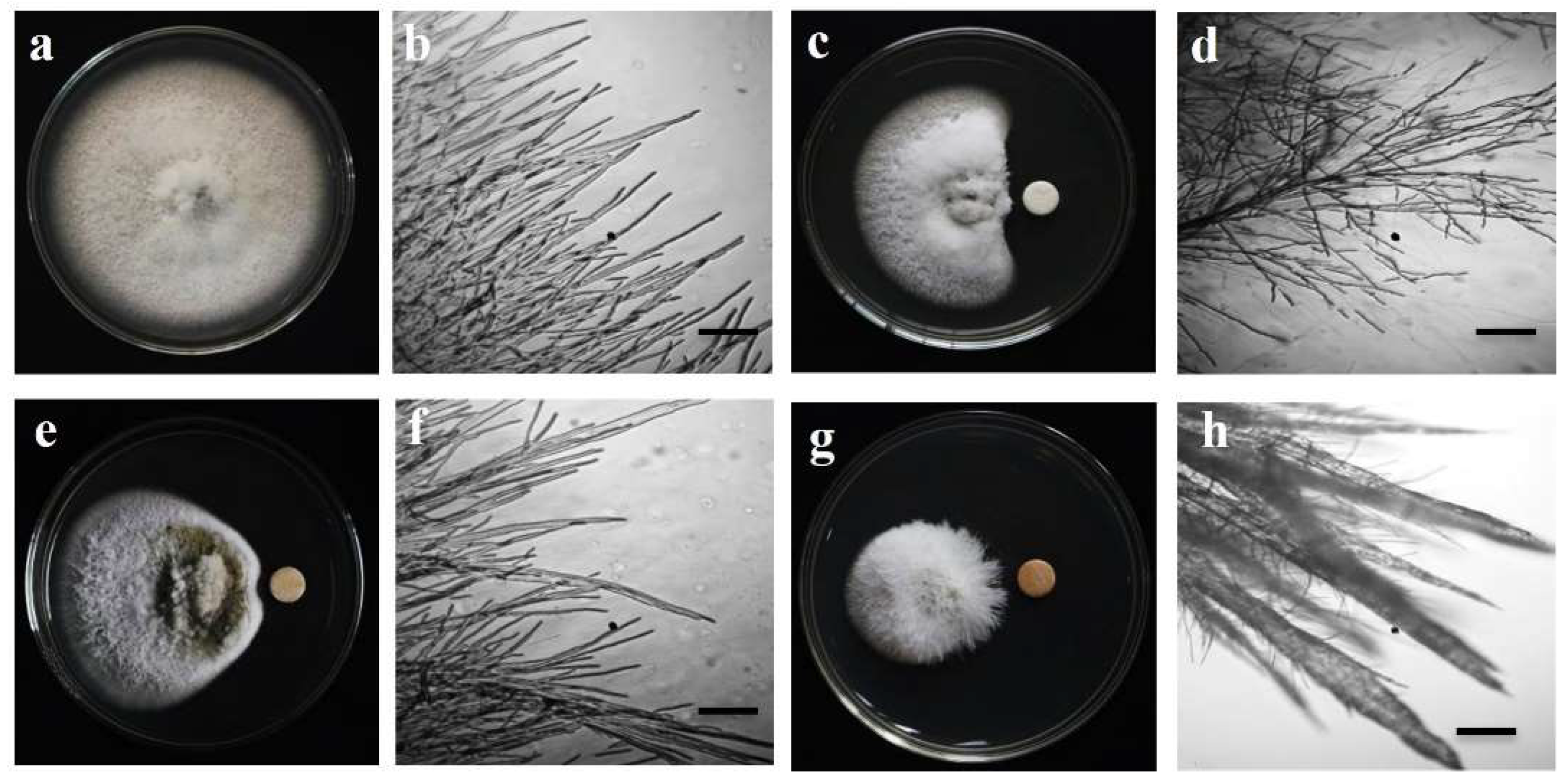
2.3. Mycelial Growth Suppression and Morphological Impacts on Mycelium
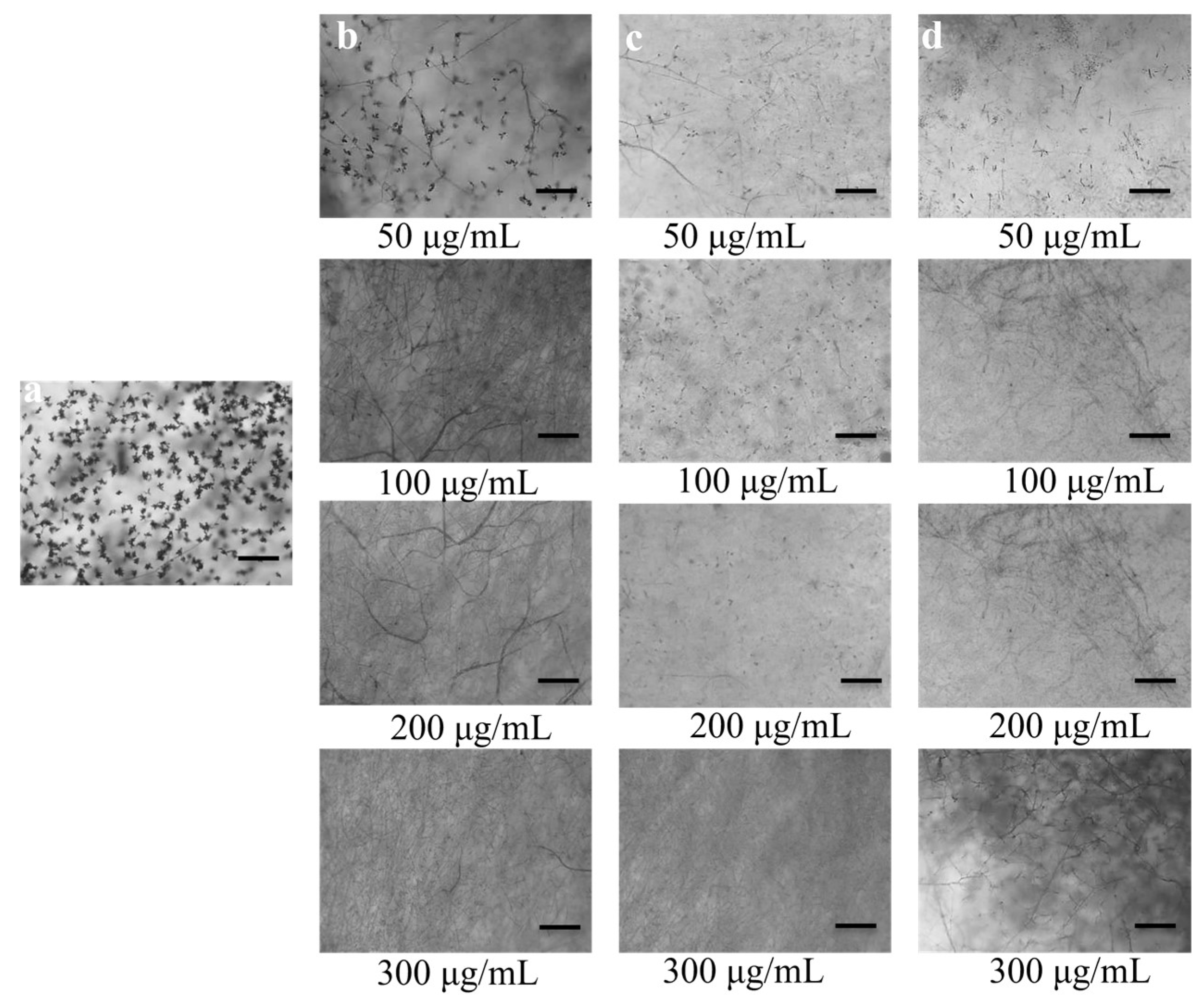
2.4. Conidia Formation (Conidiogenesis) Suppression
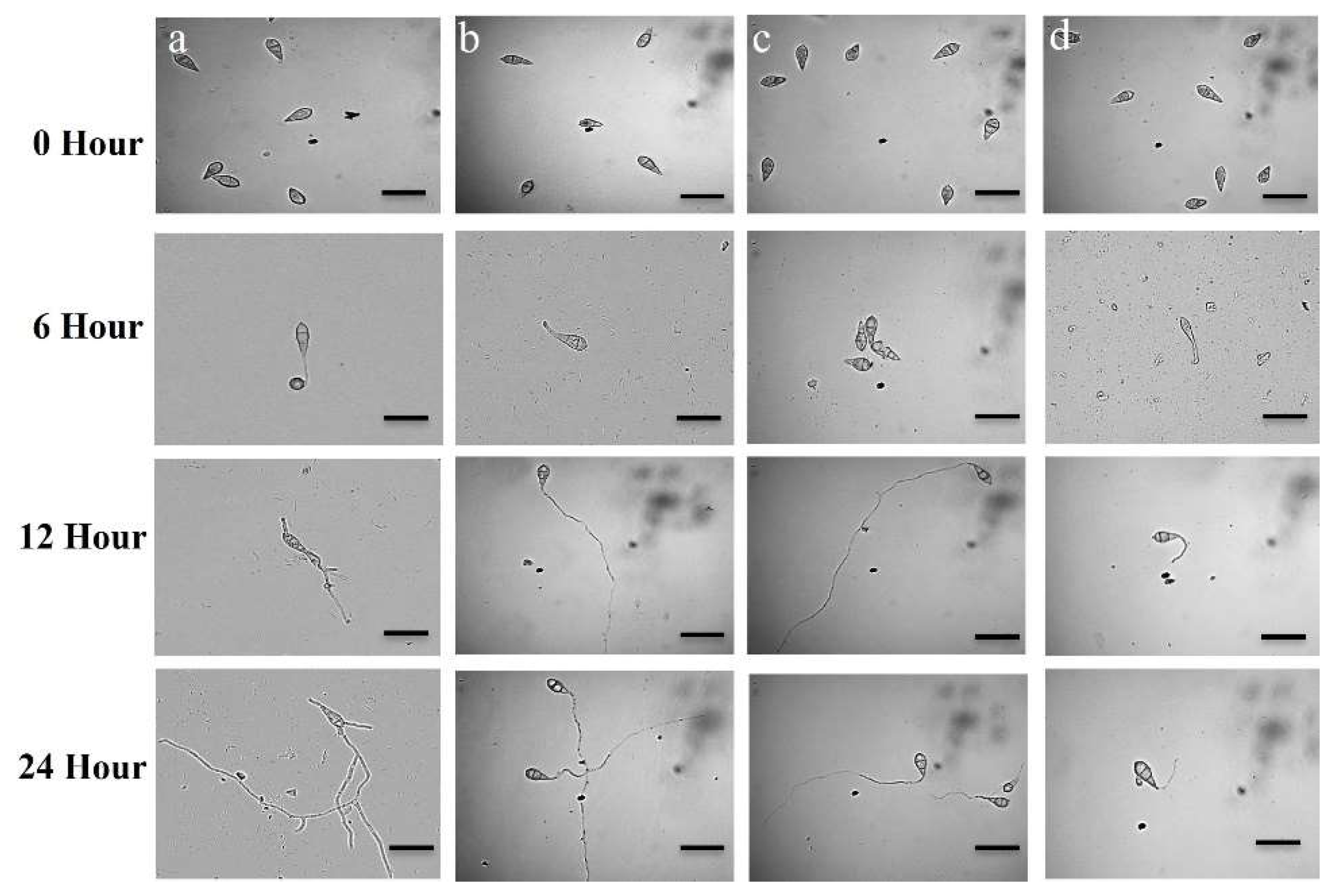
2.5. Conidia Germination Inhibition and Morphological Abnormalities in Germinated Conidia
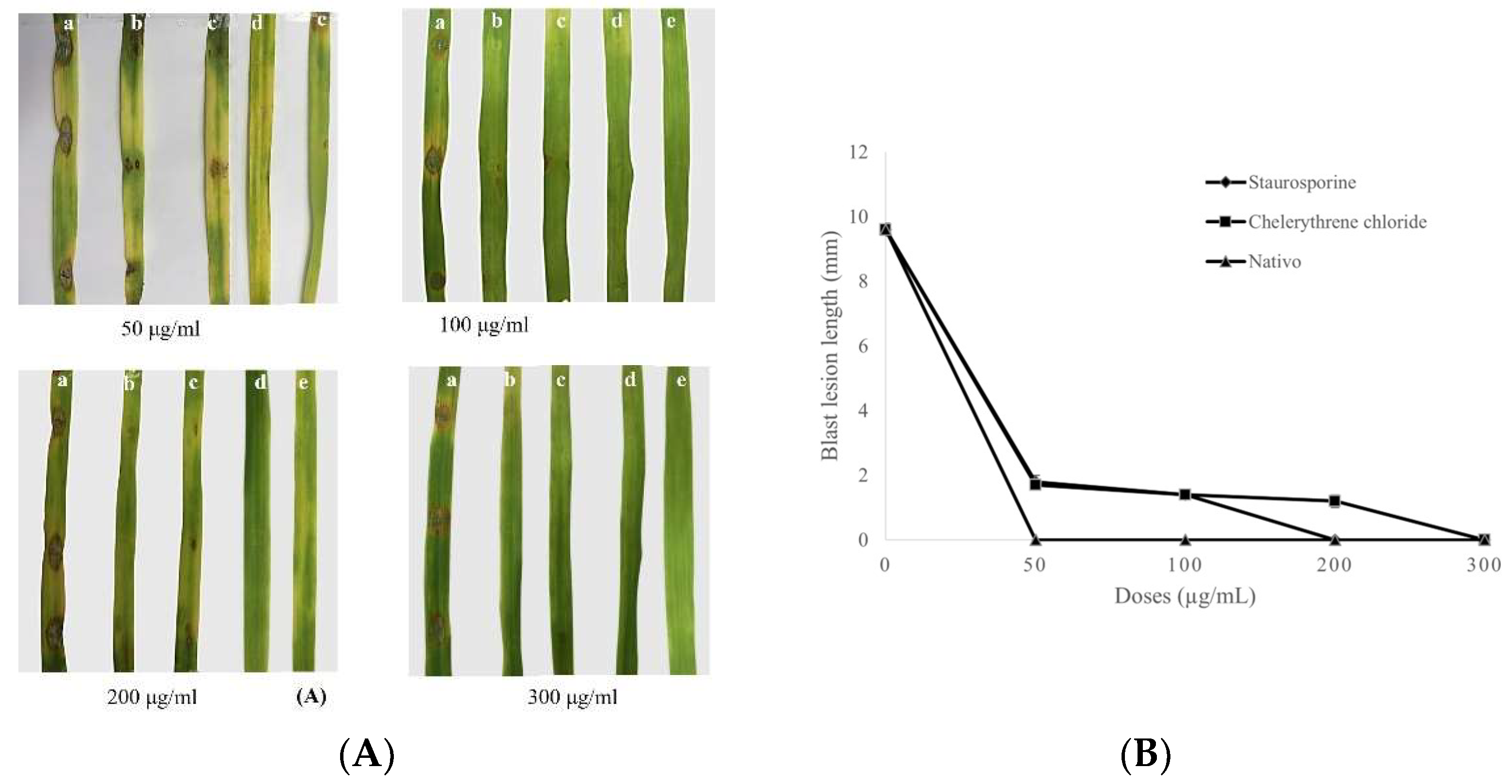
2.6. Progression of Wheat Blast on Separated Wheat Leaves
2.7. Field Evaluation of Staurosporine and Chelerythrine Chloride for Wheat Blast Control
2.7.1. Land Preparation, Seed Sowing, and Plot Maintenance
2.7.2. Infection Assay in the Reproductive Phase of Wheat
2.7.3. Data Collection, Disease Intensity, and Severity Evaluation
- n = number of leaves infected by blast
- v = value score of each category attack
- N = number of leaves observed
- V = value of highest score
2.8. Experimental Design and Statistical Analysis
3. Results
3.1. Hyphal Growth Suppression and Morphological Impacts on Mycelium
3.2. Conidia Formation (Conidiogenesis) Suppression
3.3. Conidia Germination Inhibition and Morphological Abnormalities in Germinated Conidia
3.4. Progression of Wheat Blast on Separated Wheat Leaves
3.5. Wheat Blast Disease Suppression by PKC Inhibitors at the Heading Stage of Wheat in the Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, S.; Nishizuka, Y. Protein kinase C isotypes and their specific functions: Prologue. J. Biochem. 2002, 132, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted terapy. Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P. The development and therapeutic potential of protein kinase inhibitors. Curr. Opin. Chem. Biol. 1999, 3, 459–465. [Google Scholar] [CrossRef]
- Canduri, F.; Uchoa, H.B.; de Azevedo, W.F., Jr. Molecular models of cyclin-dependent kinase 1 complexed with inhibitors. Biochem. Biophys. Res. Commun. 2004, 324, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Krystof, V.; Cankar, P.; Frysová, I.; Slouka, J.; Kontopidis, G.; Dzubák, P.; Hajdúch, M.; Srovnal, J.; de Azevedo, W.F., Jr.; Orság, M.; et al. 4-Arylazo-3,5-diamino-1H-pyrazole CDK inhibitors: SAR study, crystal structure in complex with CDK2, selectivity, and cellular effects. J. Med. Chem. 2006, 49, 6500–6509. [Google Scholar] [CrossRef]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchiya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 of Streptomyces origin taxomony, fermentation, isolation and preliminary characterization. J. Antibiot. 1977, 30, 275–282. [Google Scholar] [CrossRef]
- Ruegg, U.T.; Burgess, G.M. Staurosporine, K-252 and UCN-01: Potent but nonspecific inhibitors of protein kinases. Trends Pharmacol. Sci. 1989, 10, 218–220. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, J.Y.; Hwang, I.S.; Yun, B.S.; Kim, B.S.; Hwang, B.K. Isolation and antifungal and antioomycete activities of staurosporine from Streptomyces roseoflavus strain LS-A24. J. Agric. Food. Chem. 2006, 54, 3041–3046. [Google Scholar] [CrossRef]
- Chae, H.J.; Kang, J.S.; Byun, J.O.; Han, K.S.; Kim, D.U.; Oh, S.M.; Kim, H.M.; Chae, S.W.; Kim, H.R. Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol. Res. 2000, 42, 373–381. [Google Scholar] [CrossRef]
- Stepczynska, A.; Lauber, K.; Engels, I.H.; Janssen, O.; Kabelitz, D.; Wesselborg, S.; Schulze-Ostho, K. Staurosporine and conventional anticancer drugs induce overlapping, yet distinct pathways of apoptosis and caspase activation. Oncogene 2001, 20, 1193–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yang, Z.; Liu, Z.; Liu, H.; Yin, J. Research progress on natural benzophenanthridine alkaloids and their pharmacological functions: A review. Nat. Prod. Commun. 2016, 11, 1934578X1601100838. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Martínez, Y.; Guan, G.; Rodríguez, R.; Más, D.; Peng, H.; Valdivié Navarro, M.; Liu, G. Analysis of the impact of isoquinoline alkaloids, derived from Macleaya cordata extract, on the development and innate immune response in swine and poultry. BioMed Res. Int. 2016, 2016, 1352146. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Zhao, M.; Li, X. Extraction of chelerythrine and its effects on pathogenic fungus spore germination. Pharm. Mag. 2017, 13, 600–606. [Google Scholar]
- Kobayashi, E.; Ando, K.; Nakano, H.; Iida, T.; Ohno, H.; Morimoto, M.; Tamaoki, T. Calphostins (UCN-1028), novel and specific inhibitors of protein kinase C. I. Fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. 1989, 42, 1470–1474. [Google Scholar] [CrossRef] [Green Version]
- Herbert, J.M.; Augereau, J.M.; Gleye, J.; Maffrand, J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990, 172, 993–999. [Google Scholar] [CrossRef]
- Rotenberg, S.A.; Huang, M.H.; Zhu, J.; Su, L.; Riedel, H. Deletion analysis of protein kinase C inactivation by calphostin C. Mol. Carcinog. 1995, 12, 42–49. [Google Scholar] [CrossRef]
- Hu, B.; Xu, G.; Zheng, Y.; Tong, F.; Shen, R. Chelerythrine attenuates renal ischemia/reperfusion-induced myocardial injury by activating CSE/H2S via PKC/NF-κB pathway in diabetic rats. J. Kidney Blood Press. Res. 2017, 42, 379–388. [Google Scholar] [CrossRef]
- Saavedra, A.; Fernández-García, S.; Cases, S.; Puigdellívol, M.; Alcalá-Vida, R.; Martín-Flores, N.; Alberch, J.; Ginés, S.; Malagelada, C.; Pérez-Navarro, E. Chelerythrine promotes Ca2+-dependent calpain activation in neuronal cells in a PKC-independent manner. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 922–935. [Google Scholar] [CrossRef]
- Wang, H.; Li, K.; Ma, L.; Wu, S.; Hu, J.; Yan, H.; Jiang, J.; Li, Y. Berberine inhibits enterovirus 71 replication by downregulating the MEK/ERK signaling pathway and autophagy. J. Virol. 2017, 14, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Pan, Y.; Zhang, G.; Wu, Y.; Zhong, W.; Chu, C. Chelerythrine inhibits human hepatocellular carcinoma metastasis in vitro. J. Biol. Pharm. Bull. 2018, 41, b17-00451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Yano, A.; Shinshi, H. Slow and prolonged activation of the p47 protein kinase during hypersensitive cell death in a culture of tobacco cells. Plant Physiol. 1999, 119, 1465–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.Y.; Xue, G.H.; Liu, J.Y.; Li, N. Isolation and purification of Chelerythrine and its anti-fungal activity. J. Hubei Agric. Sci. 2010, 49, 679–682. [Google Scholar]
- Igarashi, S.; Utiamada, C.M.; Igarashi, L.C.; Kazuma, A.H.; Lopes, R.S. Occurrence of Pyrcularia sp. in wheat (Triticum aestivum L.) in the state of Paraná, Brazil. Fitopatol Bras. 1986, 11, 351–352. [Google Scholar]
- Islam, M.T.; Croll, D.; Gladieux, P.; Soanes, D.M.; Persoons, A.; Bhattacharjee, P.; Hossain, M.; Gupta, D.R.; Rahman, M.; Mahboob, M.G.; et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 2016, 14, 84. [Google Scholar] [CrossRef] [Green Version]
- Barea, G.; Toledo, J. Identificación y Zonificación de Pyricularia o Brusone (Pyricularia oryzae) en el Cutivo de Trigo en el Departamento de Santa Cruz; Informe Tecnico. Proyecto de Investigacion Trigo; Centro de Investigación Agrícola Tropical: Santa Cruz de la Sierra, Bolivia, 1996; Volume 347, pp. 76–86. [Google Scholar]
- Viedma, L. Wheat blast occurrence in Paraguay. Phytopathology 2005, 95, 152. [Google Scholar]
- Cabrera, M.; Gutierrez, S. Primer Registro de Pyricularia grisea en Cultivos de Trigo del NE de Argentina; Depto. Proteccio’n Vegetal, Facultad de Ciencias Agrarias, UNNE: Corrientes, Argentina; Available online: www.agr.unne.edu.ar/Extension/Res2007/SanVegetal/SanVegetal_06.pdf.2007 (accessed on 27 December 2021).
- Perello, A.; Martinez, L.; Molina, M. First report of virulence and effects of Magnaporthe oryzae isolates causing wheat blast in Argentina. Plant Dis. 2015, 99, 1177–1178. [Google Scholar] [CrossRef]
- Malaker, P.K.; Barma, N.C.D.; Tiwari, T.P.; Collis, W.J.; Duveiller, E.; Singh, P.K.; Braun, H.J.; Peterson, G.L.; Pedley, K.F. First report of wheat blast caused by Magnaporthe oryzae pathotype Triticum in Bangladesh. Plant Dis. 2016, 100, 2330. [Google Scholar] [CrossRef]
- Islam, M.T.; Gupta, D.R.; Hossain, A.; Roy, K.K.; He, X.; Kabir, M.R.; Singh, P.K.; Khan, M.A.R.; Rahman, M.; Wang, G.L. Wheat blast: A new threat to food security. Phytopathol. Res. 2020, 2, 28. [Google Scholar] [CrossRef]
- Tembo, B.; Mulenga, R.M.; Sichilima, S.; M’siska, K.K.; Mwale, M.; Chikoti, P.C.; Singh, P.K.; He, X.; Pedley, K.F.; Peterson, G.L.; et al. Detection, and characterization of fungus (Magnaporthe oryzae pathotype Triticum) causing wheat blast disease on rain-fed grown wheat (Triticum aestivum L.) in Zambia. PLoS ONE 2020, 15, e0238724. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Tufan, H.A.; McGrann, G.R.D.; Magusin, A.; Morel, J.B.; Miche, L.; Boyd, L.A. Wheat blast: Histopathology and transcriptome reprogramming in response to adapted and non-adapted Magnaporthe isolates. New Phytol. 2009, 184, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Mahmud, N.U.; Ullah, C.; Rahman, M.; Islam, T. Biological and biorational management of blast diseases in cereals caused by Magnaporthe oryzae. Crit. Rev. Biotechnol. 2021, 41, 994–1022. [Google Scholar] [CrossRef]
- Kato, H. Rice blast disease. Pestic. Outlook 2001, 12, 23–25. [Google Scholar] [CrossRef]
- Inoue, K.; Suzuki, T.; Ikeda, K.; Jiang, S.; Hosogi, N.; Hyon, G.S.; Hida, S.; Yamada, T.; Park, P. Extracellular matrix of Magnaporthe oryzae may have a role in host adhesion during fungal penetration and is digested by matrix metalloproteinases. J. Gen. Plant Pathol. 2007, 73, 388–398. [Google Scholar] [CrossRef]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Rice blast: A disease with implications for global food security. Agronomy 2019, 9, 451. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, S. Update on Wheat Blast (Pyricularia oryzae) in Brazil. In A Proceeding of the International Conference-Wheat for the Nontraditional Warm Areas; Saunders, D.A., Ed.; CIMMYT: México-Veracruz, Mexico, 1990; pp. 480–483. [Google Scholar]
- Islam, M.T.; Kim, K.H.; Choi, J. Wheat blast in Bangladesh: The current situation and future impacts. Plant Pathol. J. 2019, 35, 1–10. [Google Scholar] [CrossRef]
- Urashima, A.S.; Leite, S.F.; Galbieri, R. Efficiency of aerial dissemination of Pyricularia grisea. Summa Phytopathol. 2007, 33, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Goulart, A.C.P.; Paiva, F.A. Associated fungi in wheat (Triticum aestivum L.) seeds produced in Mato Grosso do Sul, in 1990 and 1991. Rev. Bras. Sementes 1992, 14, 221–225. [Google Scholar] [CrossRef]
- Goulart, A.C.P.; Paiva, F.A.; Andrade, P.J.M. Relationship between incidence of blast in wheat seeds and the presence of Pyricularia grisea in the harvested seeds. Fitopatol. Bras. 1995, 20, 184–189. [Google Scholar]
- Gomes, D.P.; Rocha, V.S.; Pereira, O.L.; de Souza, M.A. Damage of wheat blast on the productivity and quality of seeds as a function of the initial inoculum in the field. J. Seed Sci. 2017, 39, 66–74. [Google Scholar] [CrossRef]
- Urashima, A.S.; Hashimoto, Y.; Le Don, D.; Kusaba, M.; Tosa, Y.; Nakayashiki, H.; Mayama, S. Molecular analysis of the wheat blast population in Brazil with a homolog of retrotransposon MGR583. Jpn. J. Phytopathol. 1999, 65, 429–436. [Google Scholar] [CrossRef] [Green Version]
- De Waard, M.A.; Georgopoulos, S.G.; Hollomon, D.W.; Ishii, H.; Leroux, P.; Ragsdale, N.N.; Schwinn, F.J. Chemical control of plant disease: Problems and prospects. Annu. Rev. Phytopathol. 1993, 31, 403–421. [Google Scholar] [CrossRef]
- Zhang, X.; Ryu, K.; Kim, J.; Cheon, J.; Kim, B. Changes in the sensitivity to metalaxyl, dimethomorph and ethaboxam of Phytophthora infestans in Korea. Plant Pathol. J. 2005, 21, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Dorigan, A.F.; Carvalho, G.D.; Poloni, N.M.; Negrisoli, M.M.; Maciel, J.L.N.; Ceresini, P.C. Resistance to triazole fungicides in Pyricularia species associated with invasive plants from wheat fields in Brazil. Acta Sci. Agron. 2019, 41, 39332. [Google Scholar] [CrossRef] [Green Version]
- Costa, T.R.; Fernandes, F.L.F.; Santos, S.C.; Oliveria, C.M.A.; Liao, L.M.; Ferri, P.H.; Paulo, J.R.; Ferreira, H.D.; Sales, B.H.N.; Silva, M.R.R. Antifungal activity of volatile constituents of Eugenia dysenterica leaf oil. J. Ethnopharmcol. 2000, 72, 111–117. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef]
- Subbanna, A.R.N.S.; Stanley, J.; Rajasekhara, H.; Mishra, K.K.; Pattanayak, A.; Bhowmick, R. Perspectives of Microbial Metabolites as Pesticides in Agricultural Pest Management. In Co-Evolution of Secondary Metabolites; Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 925–952. [Google Scholar]
- Urashima, A.S.; Igarashi, S.; Kato, H. Host range, mating type, and fertility of Pyricularia grisea from wheat in Brazil. Plant Dis. 1993, 77, 1211–1216. [Google Scholar] [CrossRef]
- He, Y.; Zhu, M.; Huang, J.; Hsiang, T.; Zheng, L. Biocontrol potential of a Bacillus subtilis strain BJ-1 against the rice blast fungus Magnaporthe oryzae. Can. J. Plant Pathol. 2019, 41, 47–59. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mahmud, N.U.; Gupta, D.R.; Tareq, F.S.; Shin, H.J.; Islam, T. Inhibitory effects of linear lipopeptides from a marine Bacillus subtilis on the wheat blast fungus Magnaporthe oryzae Triticum. Front. Microbiol. 2020, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Mahmud, N.; Muzahid, A.N.M.; Rabby, S.M.F.; Islam, T. Oligomycins inhibit Magnaporthe oryzae Triticum and suppress wheat blast disease. PLoS ONE 2020, 15, e0233665. [Google Scholar] [CrossRef] [PubMed]
- Riungu, G.M.; Muthorni, J.W.; Narla, R.D.; Wagacha, J.M.; Gathumbi, J.K. Management of Fusarium head blight of wheat and deoxynivalenol accumulation using antagonistic microorganisms. Plant Pathol. J. 2008, 7, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Laatsch, H.; von Tiedemann, A. Inhibitory effects of macrotetrolides from Streptomyces spp. on zoosporogenesis and motility of Peronosporomycete zoospores are likely linked with enhanced ATPase activity in mitochondria. Front. Microbiol. 2016, 7, 1824. [Google Scholar] [CrossRef]
- FRG. Fertilizer Recommendation Guide; Bangladesh Agricultural Research Council (BARC): Farmgate, Dhaka, Bangladesh, 2012; p. 274. [Google Scholar]
- Schumacher, R.W.; Talmage, S.C.; Miller, S.A.; Sarris, K.E.; Davidson, B.S.; Goldberg, A. Isolation and structure determination of an antimicrobial ester from a marine sediment-derived bacterium. J. Nat. Prod. 2003, 66, 1291–1293. [Google Scholar] [CrossRef]
- Romero, D.; de Vicente, A.; Olmos, J.L.; Davila, J.C.; Perez-Garcia, A. Effect of lipopeptides of antagonistic strains of Bacillus subtilis on the morphology and ultrastructure of the cucurbit fungal pathogen Podosphaera fusca. J. Appl. Microbiol. 2007, 103, 969–976. [Google Scholar] [CrossRef]
- Kopecká, M.; Ilkovics, L.; Ramíková, V.; Yamaguchi, M. Effect of cytoskeleton inhibitors on conidiogenesis and capsule in the long neck yeast Fellomyces examined by scanning electron microscopy. Chemotherapy 2010, 56, 197–202. [Google Scholar] [CrossRef]
- Tebbets, B.; Yu, Z.; Stewart, D.; Zhao, L.-X.; Jiang, Y.; Xu, L.-H.; Andes, D.; Shen, B.; Klein, B. Identification of antifungal natural products via Saccharomyces cerevisiae bioassay: Insights into macrotetrolide drug spectrum, potency, and mode of action. Sabouraudia 2013, 51, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sun, C. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018, 84, e00445-18. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.Y.; Zuo, G.Y.; Hao, X.Y.; Wang, G.C.; Xiao, H.T.; Zhang, J.Q.; Xu, G. Antifungal activity of the benzo [c] phenanthridine alkaloids from Chelidonium majus Linn against resistant clinical yeast isolates. J. Ethnopharmacol. 2009, 125, 494–496. [Google Scholar] [CrossRef]
- Miao, F.; Yang, X.J.; Zhou, L.; Hu, H.J.; Zheng, F.; Ding, X.D.; Sun, D.M.; Zhou, C.D.; Sun, W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011, 25, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Lenfeld, J.; Kroutil, M.; Marsálek, E.; Slavík, J.; Preininger, V.; Simánek, V. Antiinflammatory activity of quaternary benzophenanthridine alkaloids from Chelidonium majus. Planta Med. 1981, 43, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Dolezel, P.; Vicar, J.; Ulrichová, J. Cytotoxic activity of sanguinarine and dihydrosanguinarine in human promyelocytic leukemia HL-60 cells. Toxicol. In Vitro 2009, 23, 580–588. [Google Scholar] [CrossRef]
- Eun, J.P.; Koh, G.Y. Supression of angiogenesis by the plant alkaloid, sanguinarine. Biochem. Biophys. Res. Commun. 2004, 317, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Yoo, I.D.; Kim, W.G. 8-hydroxydihydrochelerythrine and 8-hydroxydihydrosanguinarine with a potent acetylcholinesterase inhibitory activity from Chelidonium majus L. Biol. Pharm. Bull. 2006, 29, 2317–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T.; Hashidoko, Y.; Deora, A.; Ito, T.; Tahara, S. Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne Peronosporomycetes. Appl. Environ. Microbiol. 2005, 71, 3786–3796. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T. Disruption of ultrastructure and cytoskeletal network is involved with biocontrol of damping-off pathogen Aphanomyces cochlioides by Lysobacter sp. strain SB-K88. Biol. Control 2008, 46, 312–321. [Google Scholar] [CrossRef]
- Islam, M.T. Mode of antagonism of a biocontrol bacterium Lysobacter sp. SB-K88 toward a damping-off pathogen Aphanomyces cochlioides. World J. Microb. Biotechnol. 2010, 26, 629–637. [Google Scholar] [CrossRef]
- Islam, M.T.; Fukushi, Y. Growth inhibition and excessive branching in Aphanomyces cochlioides induced by 2,4-diacetylphloroglucinol is linked to disruption of filamentous actin cytoskeleton in the hyphae. World J. Microb. Biotechnol. 2010, 26, 1163–1170. [Google Scholar] [CrossRef]
- Magae, Y.; Magae, J. Effect of staurosporine on growth and hyphal morphology of Pleurotus ostreatus. J. Gen. Microbiol. 1992, 139, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.H.; Cui, D.Z.; Liu, X.F.; Chai, Y.Y.; Zhao, N.; Wang, J.Y.; Zhao, M. In vitro antifungal activity and possible mechanisms of action of chelerythrine. Pestic. Biochem. Physiol. 2020, 164, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Miao, F.; Yao, Y.; Cao, F.-J.; Yang, R.; Ma, Y.-N.; Qin, B.-F.; Zhou, L. In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules 2012, 17, 13026–13035. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T. REN1 is required for development of microconidia and macroconidia, but not of Chlamydospores, in the plant pathogenic fungus Fusarium oxysporum. Genetics 2004, 166, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T.; von Tiedemann, A.; Laatsch, H. Protein kinase C is likely to be involved in zoosporogenesis and maintenance of flagellar motility in the Peronosporomycete zoospores. Mol. Plant-Microbe Interact. 2011, 24, 938–947. [Google Scholar] [CrossRef] [Green Version]
- Homma, Y.; Takahashi, H.; Arimoto, Y. Studies on the Mode of Action of Soybean Lecithin Part 3. Effects on the Infection Process of Rice Blast Fungus, Pyricularia oryzae. Jpn J. Phytopathol. 1992, 58, 514–521. [Google Scholar] [CrossRef]
- Ansary, M.W.R.; Hoque, E.; West, H.M.; Rahman, M.M.; Akanda, A.M.; Wang, Y.; Islam, M.T. Medicinal plant extracts and protein kinase C inhibitor suppress zoosporogenesis and impair motility of Phytophthora capsici zoospores. Plant Prot. Sci. 2016, 52, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Park, S.B.; Kim, S.H.; Yang, S.H.; Suh, J.-W.; Lee, C.H.; Kim, J.G. Suppressing activity of staurosporine from Streptomyces sp. MJM4426 against rice bacterial blight disease. J. Appl. Microbiol. 2016, 120, 975–985. [Google Scholar] [CrossRef] [Green Version]
- He, N.; Wang, P.; Wang, P.; Ma, C.; Kang, W. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018, 18, 261. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Bower, M.J.; McDevitt, P.J.; Zhao, H.; Davis, S.T.; Johanson, K.O.; Green, S.M.; Concha, N.O.; Zhou, B.B. Structural basis for Chk1 inhibition by UCN-01. J. Biol. Chem. 2002, 277, 46609–46615. [Google Scholar] [CrossRef] [Green Version]
- Atwell, S.; Adams, J.M.; Badger, J.; Buchanan, M.D.; Feil, I.K.; Froning, K.J.; Gao, X.; Hendle, J.; Keegan, K.; Leon, B.C.; et al. A novel mode of Gleevec binding is revealed by the structure of spleen tyrosine kinase. J. Biol. Chem. 2004, 279, 55827–55832. [Google Scholar] [CrossRef] [Green Version]
- Fernández, A.; Maddipati, S. A priori inference of cross reactivity for drug-targeted kinases. J. Med. Chem. 2006, 49, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, T.; Nomoto, H.; Takahashi, I.K.; Ato, Y.; Morimotm, O.; Tomita, F. Staurosporine, a potent inhibitor of phospholipid/ Ca++-dependent protein kinase. Biochem. Biophys. Res. Commun. 1986, 135, 397402. [Google Scholar] [CrossRef]
- Nakano, H.; Kobayashe, I.; Takahashi, I.; Amaokti, T.; Kuzuu, Y.; Iba, H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p 60. J. Antibiot. 1987, 50, 706708. [Google Scholar]
- Yamaguchi, Y.F.; Kathuria, S. Characterization of receptor tyrosine-specific protein kinases by the use of inhibitors. Staurosporine is a 100-times more potent inhibitor of insulin receptor than IGF-I receptor. Biochem. Biophys. Res. Commun. 1988, 157, 955–962. [Google Scholar] [CrossRef]
- Tamaoki, T. Use and specificity of staurosporine, UCN-01 and calphostin C as protein kinase inhibitors. Methods Enzymol. 1991, 201, 340–347. [Google Scholar] [PubMed]
- Gupta, S.; Gomez-Munoz, A.; Matowe, W.C.; Brindley, D.N.; Ginsberg, J. Thyroid-stimulating hormone activates phospholipase D in FRTL-5 thyroid cells via stimulation of protein kinase C. Endocrinology 1995, 136, 3794–3799. [Google Scholar] [CrossRef]
- Nixon, P.A.; Orenstein, D.M.; Kelsey, S.F.; Doershuk, C.F. The prognostic value of exercise testing in patients with cystic fibrosis. N. Engl. J. Med. 1992, 327, 1785–1788. [Google Scholar] [CrossRef]
- Chmura, S.J.; Dolan, M.E.; Cha, A.; Mauceri, H.J.; Kufe, D.W.; Weichselbaum, R.R. In Vitro and in vivo activity of protein kinase C inhibitor chelerythrine chloride induces tumor cell toxicity and growth delay in vivo. Clin. Cancer Res. 2000, 6, 737–742. [Google Scholar]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- McNamara, N.P.; Black, H.I.J.; Beresford, N.A.; Parekh, N.R. Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl. Soil Ecol. 2003, 24, 117–132. [Google Scholar] [CrossRef]
- Chan, S.-L.; Lee, M.C.; Tan, K.O.; Yang, L.-K.; Lee, A.S.Y.; Flotow, H.; Fu, N.Y.; Butler, M.S.; Soejarto, D.D.; Buss, A.D.; et al. Identification of chelerythrine as an inhibitor of BclXL Function. J. Biol. Chem. 2003, 278, 20453–20456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T.; Tahara, S. Chemotaxis of fungal zoospores, with special reference to Aphanomyces cochlioides. Biosci. Biotechnol. Biochem. 2001, 65, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- IRRI. Standard Evaluation System for Rice, 4th ed.; International Rice Research Institute (IRRI): Los Banos, Philippines, 1996; p. 52. [Google Scholar]
- Pring, R.J. Effects of triadimefon on the ultrastructure of rust fungi infecting leaves of wheat and broad bean (Vicia faba). Pestic. Biochem. Phys. 1984, 21, 127–137. [Google Scholar] [CrossRef]
- Sauter, H.; Steglich, W.; Anke, T. Strobilurins: Evolution of a new class of active substances. Angew. Chem. Int. Ed. Engl. 1999, 38, 1328–1349. [Google Scholar] [CrossRef]
- Wedge, D.E.; Camper, N.D. Biologically Active Natural Products. In Agrochemicals and Pharmaceuticals; Cutler, H.G., Cutler, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–15. [Google Scholar]



| Compound | Time (h) | Effects of Natural Compounds on Developmental Transitions of Conidia of Wheat Blast Fungus M. oryzae Triticum (MoT) | |
|---|---|---|---|
| Germinated Conidia (% ± SE a) | Major Morphological Change/Developmental Transitions in the Treated Conidia | ||
| Water | 0 | 0 ± 0 e | No germination |
| 6 | 100 ± 0 a | Germinated with normal germ tube and normal appressoria developed | |
| 12 | 100 ± 0 a | Mycelial growth took place | |
| 24 | 100 ± 0 a | Huge mycelial growth took place | |
| Staurosporine | 0 | 0 ± 0 e | No germination |
| 6 | 27.1 ± 0.8 d | Germinated with a short germ tube | |
| 12 | 22.8 ± 0.6 d | 4.7 ± 0.8% Normal and 18.1 ± 0.6% abnormally long hyphae-like germ tube formed | |
| 24 | 0 ± 0 b | No appressoria formation and mycelial growth took place | |
| Chelerythrine Chloride | 0 | 0 ± 0 e | No germination |
| 6 | 67.5 ± 0.7 b | Germinated with 52.3 ± 0.8% short germ tube and 15.2± 0.7% conidia lysis occurred | |
| 12 | 45.8 ± 0.5 c | 13.3 ± 0.8% Normal and 32.5± 0.6% abnormally long hyphae-like germ tube formed | |
| 24 | 0 ± 0 b | No appressoria formation and mycelial growth took place | |
| Nativo®75WG | 0 | 0 ± 0 e | No germination |
| 6 | 51.6 ± 0.8 c | Germinated with a short germ tube | |
| 12 | 51.6 ± 0.8 b | Normal germ tube formed | |
| 24 | 0 ± 0 b | No appressoria formation or mycelial growth took place | |
| Treatment | Yield/Plot (gm) * | 1000-Grain WEIGHT (gm) * | Disease Incidence (%) * | Disease Severity (%) * |
|---|---|---|---|---|
| Healthy control | 136.60 ± 0.3.98 a | 50.90 ± 0.81 a | 0.00 ± 0.00 d | 0.00 ± 0.00 e |
| Untreated control | 56.40 ± 3.67 c | 34.12 ± 1.74 cd | 91.33 ± 2.03 a | 83.00 ± 3.06 a |
| Staurosporine | 120.60 ± 2.62 b | 42.06 ± 1.71 bc | 36.33 ± 1.76 c | 28.67 ± 1.45 c |
| Chelerythrine chloride | 117.20 ± 3.56 b | 36.10 ± 2.48 dcd | 51.33 ± 1.74 b | 40.33 ± 1.76 b |
| Nativo®75WG | 131.00 ± 6.63 ab | 45.28 ± 2.35 ab | 30.00 ± 2.35 c | 14.33 ± 2.40 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, M.; Rabby, S.M.F.; Gupta, D.R.; Rahman, M.; Paul, S.K.; Mahmud, N.U.; Rahat, A.A.M.; Jankuloski, L.; Islam, T. Natural Protein Kinase Inhibitors, Staurosporine, and Chelerythrine Suppress Wheat Blast Disease Caused by Magnaporthe oryzae Triticum. Microorganisms 2022, 10, 1186. https://doi.org/10.3390/microorganisms10061186
Chakraborty M, Rabby SMF, Gupta DR, Rahman M, Paul SK, Mahmud NU, Rahat AAM, Jankuloski L, Islam T. Natural Protein Kinase Inhibitors, Staurosporine, and Chelerythrine Suppress Wheat Blast Disease Caused by Magnaporthe oryzae Triticum. Microorganisms. 2022; 10(6):1186. https://doi.org/10.3390/microorganisms10061186
Chicago/Turabian StyleChakraborty, Moutoshi, S. M. Fajle Rabby, Dipali Rani Gupta, Mahfuzur Rahman, Sanjoy Kumar Paul, Nur Uddin Mahmud, Abdullah Al Mahbub Rahat, Ljupcho Jankuloski, and Tofazzal Islam. 2022. "Natural Protein Kinase Inhibitors, Staurosporine, and Chelerythrine Suppress Wheat Blast Disease Caused by Magnaporthe oryzae Triticum" Microorganisms 10, no. 6: 1186. https://doi.org/10.3390/microorganisms10061186










