Abstract
Annually, approximately 23,000 cases of food poisoning by Staphylococcus aureus enterotoxins are reported worldwide. The aim of this study was to determine the occurrence and characterize S. aureus on beef and beef products in South Africa. Organ meats (n = 169), raw processed meat (n = 110), raw intact (n = 53), and ready-to-eat meats (n = 68) were obtained from 25 retail outlets. S. aureus was isolated and enumerated according to the ISO 6888-1 method. Identification of the strains was performed by MALDI-TOF MS. The antimicrobial resistance was determined using the disc diffusion test. The presence of methicillin-resistance genes and the staphylococcal enterotoxin genes was determined by PCR. Prevalence was low (13/400; CI 1.7–5) and all but one positive sample were from organ meats. Eight isolates were resistant to at least one antibiotic. Two isolates carried the mecC gene. All the isolates tested positive for seg, seh, sei, and sep, whilst 53.8% were positive for sea. None of the isolates was positive for ser, sej, seb, sec, or sed. The prevalence of S. aureus was low, with organ meats being the most contaminated. The presence of mecC-positive MRSA and of enterotoxins warrants further investigation and risk assessment.
1. Introduction
Beef is known for its role in supplying protein, minerals, and vitamins in human nutrition []. Due to its high nutritional content, beef is an excellent substrate for the growth of microorganisms, of which some are leading causes of meat spoilage []. Spoilage of meat is enhanced by inadequately stored or packed meat []. Different storage conditions, such as cold and gaseous composition, on packed meat are most likely to suppress the microflora, among them S. aureus [].
S. aureus causes staphylococcal food poisoning (SFP) through the ingestion of food contaminated with staphylococcal enterotoxins []. This enterotoxaemia is characterized by diarrhea, nausea, abdominal cramping, and vomiting within 24 h of eating []. Contamination of food by S. aureus may originate from the animal, the food handlers, and the environment. It may be a consequence of poor hygiene during processing from slaughter to final product or inappropriate storage and household manipulations; however, contamination of meat is a complicated process which may occur well before the meat reaches retail outlets []. In addition to toxins encoded by the seb, sec, sed, and see genes, in particular, strains that produce the Staphylococcal Enterotoxin A (SEA), encoded by the sea gene, have caused a large number of outbreaks [,].
Antimicrobial resistance (AMR) is an increasing global challenge mainly driven by the overall use of antimicrobials []. In certain S. aureus clones, AMR is a major problem, especially in methicillin-resistant S. aureus (MRSA), of which the prevalence increases globally []. In the last 15 years, MRSA clonal complex 398 was discovered in food-producing animals, while other sequence types (ST), such as ST1, ST5, ST9, ST97, ST130, and ST433, have been reported to a lesser extent []. These strains were subsequently named Livestock Associated (LA)-MRSA. LA-MRSA Clonal complex 398 (CC 398) is mainly prevalent in Europe and North America; however, it has been reported in Asia as well as in Africa []. To a lesser extent, LA-MRSA CC398 has been associated with different infections in humans, including skin and soft tissue illnesses, ventilator-associated pneumonia, and septicemia [].
There are few studies that have been conducted on meat and meat products in Africa so far [,]; most studies did not type the isolates, and only few of the studies have identified AMR genes [,,,]. Therefore, this study aimed to determine the occurrence, AMR, and virulence genes of S. aureus isolated from beef and beef products in retail outlets of the KwaZulu-Natal province, South Africa.
2. Materials and Methods
2.1. Ethical Approval
Ethical approval for this study was obtained from the University of Zululand with certificate number UZREC 171110-030 PMG 2019/112.
2.2. Study Design
The cross-sectional study involved the collection and microbiological analysis of meat and meat products from retail outlets and butcheries from King Cetshwayo and iLembe districts in the KwaZulu-Natal province of South Africa. King Cetshwayo district covers an area of 8213 km2 from the north coast region, whereas iLembe covers an area of 3269 km2 from the south coast region with a population of approximately 885,944 and 606,809, respectively []. The two districts contribute to about a quarter of the total population in KwaZulu-Natal KZN (Figure 1 and Figure 2).
Figure 1.
Geographical map representing King Cetshwayo district, KwaZulu-Natal. Source: https://municipalities.co.za/map/124/king-cetshwayo-district-municipality (accessed on 1 November 2020) [].
Figure 2.
Geographical map representing iLembe district, KwaZulu-Natal. Source: https://municipalities.co.za/map/117/ilembe-district-municipality (accessed on 1 November 2020) [].
2.3. Sample Size Determination
There are important statistical variables to consider when determining the sample size for a surveillance study []. These include z, α, p, and d, where z (1.96) is the normal deviate for two-tailed alternative hypotheses []. Alpha (α) is the level of significance and it is usually 5%, which implies that having a 5% probability of incorrectly rejecting a null hypothesis is acceptable []. The p-value is the expected prevalence proportion, and a prevalence of 50% (0.50) was assumed in this study based on a national surveillance of foodborne pathogens in South Africa by [], which detected about 56% S. aureus in diverse meat and meat products from various establishments. The value of d is precision, and at the confidence interval of 95%, d is 0.05. In this study, the following formula was used to calculate the sample size of the surveillance study:
However, 400 samples were collected in this study for robust results.
2.4. Sample Collection
A total of 400 samples were collected during the cross-sectional study. Twenty-five retail outlets and butcheries from King Cetshwayo and iLembe districts were included in this study. The beef samples were ready-to-eat beef products (n = 68), raw processed beef (n = 110), raw intact beef (n = 53), and organ meats (n = 169). Samples were packed into sampling bags using strict aseptic techniques, and placed in cooler bags containing ice packs to maintain a temperature of approximately 4 °C. The packaged samples were transported immediately to the Microbiology laboratory at the University of Zululand, Department of Biochemistry and Microbiology, for further bacteriological examination. Samples were analyzed immediately after arrival.
2.4.1. Microbiological Analysis
Control Strains for Quality Control
The S. aureus ATCC 25,923 (Microbiologics, MN, USA) and field strains (positive for tested virulence factors) were included in all laboratory experiments as positive control strains. ATCC 25,922 was used as negative control.
Detection, Enumeration, and Isolation and Identification of S. aureus
The detection, enumeration, and isolation of S. aureus was performed according to the ISO 6888-1:1999 AMD 2018 standard method []. Briefly, each sample was analyzed for the presence of S. aureus by weighing 25 g, followed by addition of 225 mL of buffered peptone water. The samples and buffered peptone water were thoroughly mixed in a homogenizer (Bagmixer 400 cc, Interscience, France) for 2 min at 10 stroke/s. Subsequently, ten-fold serial dilutions were made using sterile pipettes []. From these dilutions, 0.1 mL was inoculated in duplicate onto Baird Parker agar plates (Oxoid, UK) using the spread plate technique, as described by Goja et al. []. Plates were incubated at 37 °C for 24 to 48 h. After incubation, the typical colonies were counted. Typical Staphylococcus spp. appeared as shiny black colonies []. To calculate the number of colony-forming units per gram (CFU/g), the colonies on the countable plate were multiplied by final dilution factor. The presumptive colonies were purified three times through sub-culturing on nutrient agar (Oxoid, UK) and incubation at 37 °C for 24 h. The presumptive S. aureus colonies were subjected to Gram staining, catalase test, mannitol salt, and free and bound coagulase tests (Oxoid, UK) []. Gram-positive cocci that appeared purple with grape-like shape were catalase-positive, appearing yellow on mannitol salt agar due to mannitol fermentation, and where coagulase-positive, were considered to be presumptive S. aureus and the colonies were subjected to further tests. Identification of S. aureus was confirmed using MALDI-TOF MS, according to the manufactures instructions for the MALDI Biotyper® (Bruker Daltonics, Germany) []. All confirmed S. aureus isolates were streaked on 5% sheep blood agar plates to identify the type of hemolysin they produce []. The plates were incubated at 37 °C for 24 h ± 2 [].
2.5. Antimicrobial Susceptibility Testing
For the antimicrobial susceptibility test, the Kirby Bauer disk diffusion method according to Clinical Laboratory Standard Institute guidelines was applied [,]. Briefly, from a pure bacterial culture, 2–5 colonies were suspended in 5 mL sterile saline solution. The bacterial concentration was adjusted to an optical density of 0.5 on the McFarland scale [,]. A sterile cotton swab was dipped into the suspension and excess fluid was removed by squeezing the swab at the top of the bijou bottles. The bacteria were inoculated onto Mueller Hinton agar (Thermofisher, Waltham, MA, USA) by streaking in three different directions to obtain confluent bacterial growth. The medium surface was allowed to dry, followed by placing the following antimicrobial disks: ciprofloxacin (5 µg), cefoxitin (30 µg), clindamycin (2 µg), erythromycin (15 µg), rifampicin (5 µg), oxacillin (1 µg), kanamycin (30 µg), penicillin G (10 units), chloramphenicol (30 µg), gentamicin (10 µg), and trimethoprim (25 µg) (Davies Diagnostics, Randburg, South Africa) [].
2.6. Detection of Selected Resistance and Virulence Genes
2.6.1. DNA Extraction and PCR for Staphylococcal Enterotoxins and mec Genes
The Zymo DNA extraction kit (California, CA, USA) was used for the extraction of the DNA according to the manufacturer’s instructions. The quality and quantity of the DNA were measured using a Nanodrop 2000 spectrophotometer (Thermofisher Scientific, Waltham, MA, USA).
The enterotoxin genes (sea, seb, sed, sec, she, seg, ser, sei, sep, sej) and mec genes (mecA and mecC) were assessed by PCR using the primers listed in Table 1. The 20 µL PCR reaction mixtures contained 10–30 ng of template DNA (in 1 µL), NEB one Taq 2× master mix with standard buffer (10 µL), forward primer (1 µL), reverse primer (1 µL), and nuclease free water (7 µL). PCR amplifications for sea, seb, sec, sed, and ser were carried out in a thermal cycler with the following thermal conditions: initial denaturation for 5 min at 95 °C; 35 cycles of 30 s at 94 °C, 40 s at 56 °C, and 1 min at 68 °C; final extension for 5 min at 68 °C. The PCR conditions for seg, sei, sep, sej and she were similar to the above, except that the annealing stage was performed at 53 °C for 40 s.

Table 1.
Oligonucleotide sequence primers used to target genes for species confirmation in S. aureus.
2.6.2. Agarose Gel Electrophoresis
PCR products were subjected to electrophoresis in 1.5% agarose gels stained with ethidium bromide at 3 volts/cm for approximately 30 min. The 50 bp and 100 bp DNA ladders were used to estimate the size of PCR amplicons. The PCR amplicons were visualized under ultraviolet light and the gel images were documented using a gel documentation system (E-Box).
3. Results
3.1. Prevalence of S. Aureus in Meat
Out of the 400 beef and beef products that were analyzed, 3.25% (n = 13; CI 1.7–5) tested positive for S. aureus (Table 2). From each of the 13 positive samples, one isolate was retained for further investigation. The S. aureus-positive samples were predominantly organ meats (n = 10/13; CI 46.2–95), followed by raw intact beef (n = 2/13; 1.9–45). Only one of the 13 S. aureus-positive samples was from ready-to-eat beef. No S. aureus was detected in raw processed beef. The 13 S. aureus from 13 positive samples showed alpha, beta, and gamma hemolysis reactions on 5% sheep blood agar.

Table 2.
Prevalence of S. aureus from beef-based products in selected districts from KZN.
3.2. Enumeration of Staphylococcus aureus
Table 3 shows the results of Staphylococcus aureus enumeration of the 13 positive samples. The S. aureus counts from beef-based products ranged from 2.65 log10 CFU/g to 4.1 log10 CFU/g (Table 4). S. aureus from 1 of the 13 positive samples (ox kidneys) were too numerous to count.

Table 3.
S. aureus counts from beef and beef products.

Table 4.
Antimicrobial resistance among 13 S. aureus (including MRSA) from meat and meat products in selected KZN province municipalities.
3.3. Antimicrobial Susceptibility Testing
AMR of the S. aureus isolates are shown in Table 4. Eight out of 13 (61.54%) isolates were resistant to at least one antibiotic. Less than 50% of the isolates exhibited resistance to penicillin G (38.46%; n = 5/13; CI 13.9–68), cefoxitin (7.69%; n = 1/13; CI 0.2–36), tetracycline (7.69%; n = 1/13; 0.2–36), oxacillin (7.69%; n = 1/13; CI 0.2–36), clindamycin (30.76%; n = 4/13; CI 9.1–61), erythromycin (23.07%; n = 3/13; CI 5–54), ciprofloxacin (15.38%; n = 2/13; CI 1.9–45), and rifampicin with a resistance percentage of 7.6%. (n = 1/13; CI 0.2–36). Multi-drug resistance (MDR), which is the lack of susceptibility to at least three antimicrobial classes [], was observed in two S. aureus isolates. Two MDR profiles were observed, namely, PG-FOX-OX-RP-CD-E-TET (n = 1) and PG-E-CD (n = 1).
3.4. Detection of Selected Resistance and Virulence Genes
3.4.1. Methicillin-Resistant Determinants
All isolates were tested for the presence of mecA and mecC genes. None of the isolates were positive for mecA genes. Two isolates (15.4%; CI 1.9–45) tested positive for mecC gene (Figure 3).
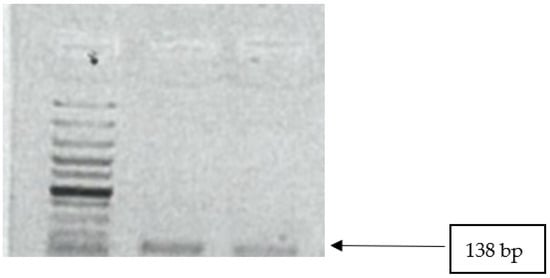
Figure 3.
Image showing mecC gene amplicons observed on agarose gel. Lane 1: 100 bp DNA ladder; lanes 2–3 show positive band for mecC genes (138 bp).
3.4.2. S. aureus Enterotoxin Genes
In Table 5, Figure 4 and Figure 5, the results of the virulence genes are shown. Out of eleven enterotoxin genes that were tested, five (sep, seh, sei, sej, sea) were detected. All S. aureus strains were positive for seg, seh, and sei, sep. The sea gene was detected in 7 of the 13 S. aureus (53.84%).

Table 5.
PCR amplification results for S. aureus methicillin-resistance and enterotoxin genes.
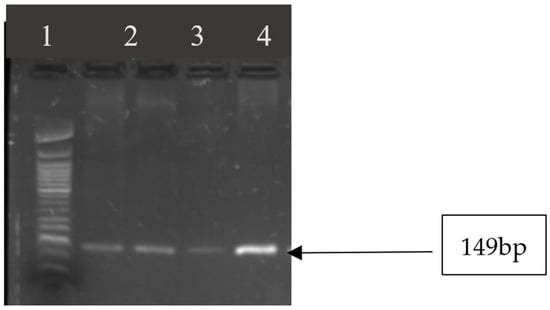
Figure 4.
Image showing seg gene amplicons observed on agarose gel. Lane 1: 50 bp DNA ladder; lanes 2–5 show amplicon sizes for samples that were positive for seg gene (149 bp).
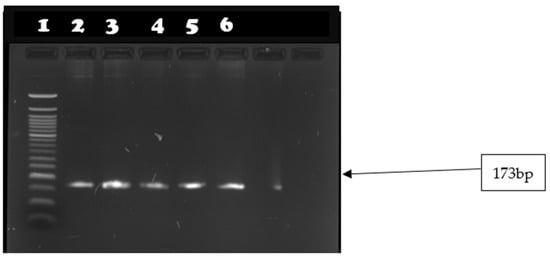
Figure 5.
Image showing seh gene amplicons observed on agarose gel. Lane 1: 50 bp DNA ladder; lanes 2–6 show amplicon sizes for samples that were positive for seh gene (165 bp).
4. Discussion
The aim of this study was to determine the occurrence, AMR, and virulence characteristics of S. aureus from products of bovine origin in retail outlets of selected municipalities. Though most studies in Africa found prevalences of between 15 and 40%, with the exception of 55% S. aureus detection in Algeria, the overall occurrence of 3.25% from this study is lower compared to most of the previous African studies [,,,,]. Higher prevalences of up to 65% have also been found in non-African countries such as Turkey, Jordan, United States of America (USA), and several countries in Europe [,,,,,,]. It is important to note that differences in methodology and sample size should be taken into account and may explain, in part, the differences seen [,]. It is important to apply a system such as the ISO standard, used in this study, to allow direct comparison of the prevalence between different studies.
The relatively high S. aureus prevalence in organ meats (kidneys, livers, lungs) compared to raw intact beef meat and ready to-eat meat was conspicuous in this study (though not to a significant extent). Probably the organ meat may be more prone to cross contamination compared to other meat types, and S. aureus can be found in the intestines []. S. aureus occurrence was also observed in ready to-eat meats and this may be attributed to cross contamination and growth due to further preparation [,]. The differences in preparation and the type of preparation of the ready-to-eat (RTE) beef, as well as conservation of the product, may play a large influence.
The average counts of the S. aureus-positive samples in this study ranged from 2.65 log10 and 4.07 log10 per gram in organ meat. These S. aureus counts are lower than those that were previously observed for organ beef meat in South Africa (5.1 log–log 5.6) []. When considering the S. aureus limit of 100 CFU/g in RTE, proposed by the guidelines for environmental health officers on the interpretation of microbiological analysis data of food [], the 13 positive samples were not within the compliance limits, though they were sold at retail level. The situation is concerning for RTE biltong, which is not processed further prior to consumption. The contaminated samples were mainly plucked meats that might not be subjected to similar strict hygiene scrutiny as beef cuts. It is possible that the S. aureus counts may have increased in the pluck meats during transportation, probably due to an inadequate cold chain, or the level of preservation at the retail level may have contributed to an increase in bacterial numbers.
As we found only few strains, comparing with other studies is difficult. The isolates from this study were, in general, more susceptible to antimicrobials than those from other studies on beef in South Africa [] and other African countries [,], but are similar to studies in Europe [], and higher than what has been detected in the United States of America [].
Interestingly, two isolates were MRSA, with only one detected phenotypically. Phenotypic methicillin resistance should always be confirmed by PCR, as false positive and false negative results may be obtained by the phenotypic tests. While this may not seem a lot, it may have a significant public health impact. The MRSA isolates from this study were mecC-positive. While this resistance gene has not been associated extensively with MRSA either in humans or animals, it has, however, been isolated mainly from animals, including wildlife []. In most countries, the mecA gene is mostly found in MRSA [,,,,,]. However, in South Africa, the mecC gene has also been shown as the sole methicillin-resistance gene in strains from different food-producing animal species as well as wild birds []. This might indicate a very specific and unique situation in South Africa and urges for a more large-scale study of MRSA on food-producing animals, wild animals, and foods derived from animals in South Africa so as to determine the human health hazard. These studies should include whole-genome sequencing to determine their true epidemiology.
Staphylococcal enterotoxins (SEs) types SEA to SEE have been reported to account for approximately 95% of food poisoning outbreaks caused by staphylococci [], whilst the remainder may be due to the other SE types, including SEG, SEH, SEI, SEJ, SEK, SEL, SEM, SEN, and SEO []. Based on the positive enterotoxin genes, it is clear that many S. aureus isolates from this study are enterotoxigenic. Some of the genes found in this study, such as sea, seh, seg, and sei, have been associated with outbreaks of food poisoning in different parts of the world [,,,,]. However, seg and sei have not frequently been isolated from food isolates and are, rather, associated with staphylococcal scarlet fever and toxic shock syndrome []. The seg, sei, and seh have also been identified in patients with other S. aureus-associated infections [].
In the current study, all the 13 S. aureus isolates tested positive for seg, sei, and seh genes. The seg and sei genes are components of the egc operon, together with sem, sen, and seo enterotoxin genes []. The egc operon is located on a mobile genetic element (MGE) [] and can thus be transferred to non-pathogenic S. aureus []. This combination is, however, rarely found in strains involved in toxi-infections [,,,].
5. Conclusions
In conclusion, the current study contributes to the knowledge about S. aureus on beef in South African markets. While the overall prevalence was relatively low, care should, however, be taken when handling pluck meats to avoid cross contamination with utensils, working surfaces, and RTE. Few S. aureus isolates exhibited antimicrobial resistance; however, the presence of mecC-positive S. aureus strains is worrisome. Five classical staphylococcal enterotoxin genes were identified from these isolates, which indicate a health risk to the consumers. The observation of mecC-positive MRSA that are present on food and have been reported also in food-producing animals warrants a One Health study on MRSA in food-producing animals, pet animals, wildlife, and foods in South Africa. These studies should include whole-genome sequencing so as to determine the epidemiology and origins of mecC-positive MRSA.
Author Contributions
Conceptualization, E.M. and P.B.; methodology, T.T., E.M., P.B. and T.S.M.; validation, E.M. and P.B.; formal analysis, T.T., E.M. and P.B.; investigation, T.T.; resources, E.M.; data curation, E.M.; writing—original draft preparation, T.T.; writing—review and editing, E.M., P.B., A.K.B. and K.M.; supervision, E.M., A.K.B. and P.B.; project administration, E.M.; funding acquisition, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Red Meat Research and Development South Africa (RMRD SA), and the Department of Trade Industry and Competition grant number THRIP/22/30/11/2017.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the relevant municipalities that were involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biesalski, H.K. Meat as a component of a healthy diet-are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005, 70, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.A.F.; Nero, L.A.; Monteiro, A.A.; Beloti, V. Technology, Identification of main contamination points by hygiene indicator microorganisms in beef processing plants. J. Food Sci. Technol. 2007, 27, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Koutsoumanis, K.; Sofos, J.N. Microbial Contamination of carcasses and cuts. In Encyclopedia of Meat Sciences; Jensen, W.K., Devine, C., Dikeman, M., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 727–737. [Google Scholar]
- Moutiq, R.; Misra, N.; Mendonca, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef]
- Omoe, K.; Ishikawa, M.; Shimoda, Y.; Hu, D.L.; Ueda, S.; Shinagawa, K. Detection of seg, seh, and sei genes in Staphylococcus aureus isolates and determination of the enterotoxin productivities of S. aureus isolates harboring seg, seh, or sei genes. J. Clin. Microbiol. 2002, 40, 857–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalrahman, L.S.; Fakhr, M.K. Incidence, antimicrobial susceptibility, and toxin genes possession screening of Staphylococcus aureus in retail chicken livers and gizzards. Foods 2015, 4, 115–129. [Google Scholar] [CrossRef] [Green Version]
- Madoroba, E.; Gelaw, A.K.; Kapeta, D. Salmonella contamination, serovars and antimicrobial resistance profiles of cattle slaughtered in South Africa. J. Vet. Res. 2016, 83, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [Green Version]
- Omwenga, I.; Aboge, G.O.; Mitema, E.S.; Obiero, G.; Ngaywa, C.; Ngwili, N.; Wamwere, G.; Wainaina, M.; Bett, B. Staphylococcus aureus enterotoxin genes detected in milk from various livestock species in northern pastoral region of Kenya. Food Control 2019, 103, 126–132. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heuer, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Stefani, S.; Goglio, A. Methicillin-resistant Staphylococcus aureus: Related infections and antibiotic resistance. Int. J. Infect. Dis. 2010, 14 (Suppl. S4), S19–S22. [Google Scholar] [CrossRef] [Green Version]
- Butaye, P.; Argudín, M.A.; Smith, T.C. Livestock-Associated MRSA and its current evolution. Curr. Clin. Microbiol. Rep. 2016, 3, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Cuny, C.; Abdelbary, M.; Layer, F.; Werner, G.; Witte, W. Prevalence of the immune evasion gene cluster in Staphylococcus aureus CC398. Vet. Microbiol. 2015, 177, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Jaja, I.F.; Green, E.; Muchenje, V. Aerobic mesophilic, coliform, Escherichia coli, and Staphylococcus aureus counts of raw meat from the formal and informal meat sectors in South Africa. Int. J. Environ. Res. Public Health 2018, 15, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekana, A.; Green, E. Antimicrobial resistance profiles of Staphylococcus aureus isolated from meat carcasses and bovine milk in abattoirs and dairy farms of the Eastern Cape, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 2223. [Google Scholar] [CrossRef] [Green Version]
- Tanih, N.F.; Sekwadi, E.; Ndip, R.N.; Bessong, P.O. Detection of pathogenic Escherichia coli and Staphylococcus aureus from cattle and pigs slaughtered in abattoirs in Vhembe District, South Africa. J. World Sci. 2015, 2015, 195972. [Google Scholar] [CrossRef] [Green Version]
- Dweba, C.C.; Zishiri, O.T.; El Zowalaty, M.E. Isolation and molecular identification of virulence, antimicrobial and heavy metal resistance genes in livestock-associated methicillin-resistant Staphylococcus aureus. Pathogens 2019, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Statistics South Africa. Formal Census 2011. Available online: https://www.statssa.gov.za/?page_id=3839 (accessed on 15 July 2020).
- King Cetshwayo District Municipality (DC28). In The Local Government Handbook: South Africa 2022, 20th ed.; Main, O. (Ed.) Yes! Media: Mowbray, South Africa, 2022; p. 109. Available online: https://municipalities.co.za/map/124/king-cetshwayo-district-municipality (accessed on 1 November 2020).
- Suresh, K.; Chandrashekara, S. Sample size estimation and power analysis for clinical research studies. J. Human Rep. Sci. 2012, 5, 7. [Google Scholar] [CrossRef]
- Naing, L.; Winn, T.; Rusli, B.N. Practical issues in calculating the sample size for prevalence studies. J. Arch. Sci. 2006, 1, 9–14. [Google Scholar]
- Lenth, R. Some practical guidelines for effective sample size determination. J. Am. Stats. 2001, 55, 187–193. [Google Scholar] [CrossRef]
- Madoroba, E.; Magwedere, K.; Chaora, N.S.; Matle, I.; Muchadeyi, F.; Mathole, M.A.; Pierneef, R. Microbial communities of meat and meat roducts: An exploratory analysis of the product quality and safety at selected enterprises in South Africa. Microorganisms 2021, 9, 507. [Google Scholar] [CrossRef]
- ISO 6888-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Technique Using Baird-Parker Agar. ISO: Geneva, Switzerland, 1999; pp. 1–11.
- Goja, A.; Ahmed, T.; Saeed, S.; Dirar, H. Isolation and identification of Staphylococcus spp. in fresh beef. Pak. J. Nutr. 2013, 12, 114. [Google Scholar] [CrossRef] [Green Version]
- Govender, V.; Madoroba, E.; Magwedere, K.; Fosgate, G.; Kuonza, L. Prevalence and risk factors contributing to antibiotic-resistant Staphylococcus aureus isolates from poultry meat products in South Africa, 2015–2016. J. S. Afr. Vet. Assoc. 2019, 90, e1–e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholomew, J.W.; Mittwer, T. The gram stain. J. Bacteriol. 1952, 16, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, M.; Perry, J.; Middleton, J.R.; Chaffer, M.; Lewis, J.; Keefe, G.P. Evaluation of MALDI-TOF mass spectrometry and a custom reference spectra expanded database for the identification of bovine-associated coagulase-negative staphylococci. J. Dairy Sci. 2018, 101, 590–595. [Google Scholar] [CrossRef]
- Savini, V.; Paparella, A.; Serio, A.; Marrollo, R.; Carretto, E.; Fazii, P. Staphylococcus pseudintermedius for CAMP-test. Int. J. Clin. Exp. Pathol. 2014, 7, 1733. [Google Scholar]
- Hanson, A. CAMP Test Protocols. Microbe Library Curriculum; Website of The American Society for Microbiology: Washington, DC, USA, 2006; Available online: http://www.microbelibrary.org (accessed on 7 January 2022).
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2016, 16, 1–23. [Google Scholar]
- Bauer, A.W. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Spolaczyk, R.; Harnack, K. Measuring System for Optically Determining Concentration of Turbid Liquid Samples. U.S. Patents US6803594B2, 12 October 2004. [Google Scholar]
- Clinical and Laboratory Standard Institute. Performance standard for antimicrobial susceptibility testing. In Nineteenth Informational Supplement (M100-S19), 27th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Arfatahery, N.; Davoodabadi, A.; Abedimohtasab, T. Characterization of toxin genes and antimicrobial susceptibility of Staphylococcus aureus isolates in fishery products in Iran. J. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, M.; Wang, G.; Johnson, W.M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol. 2000, 38, 1032–1035. [Google Scholar] [CrossRef] [Green Version]
- Petróczki, F.M.; Pásztor, Á.; Szűcs, K.D.; Pál, K.; Kardos, G.; Albert, E.; Horváth, B.; Ungvári, E.; Béri, B.; Peles, F. Occurrence and characteristics of Staphylococcus aureus in a Hungarian dairy Farm during a control program. Pathogens 2021, 10, 104. [Google Scholar] [CrossRef]
- Lima, M.C.; de Barros, M.; Scatamburlo, T.M.; Polveiro, R.C.; de Castro, L.K.; Guimarães, S.H.S.; da Costa, S.L.; da Costa, M.M.; Moreira, M.A.S. Profiles of Staphyloccocus aureus isolated from goat persistent mastitis before and after treatment with enrofloxacin. BMC Microbiol. 2020, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Tema, A.T. Microbiological Characterization of Unpasteurized Sheep Milk. Doctoral Dissertation, University Of Debrecen, Debrecen, Hungary, 2021. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. J. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bounar-Kechih, S.; Taha Hamdi, M.; Aggad, H.; Meguenni, N.; Cantekin, Z. Carriage Methicillin-Resistant Staphylococcus aureus in poultry and cattle in Northern Algeria. Vet. Med. Int. 2018, 2018, 4636121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attien, P.; Sina, H.; Moussaoui, W.; Dadieacute, T.; Chabi, S.K.; Djeacute ni, T.; Bankole, H.S.; Kotchoni, S.O.; Edoh, V.; Preacute vost, G.; et al. Prevalence and antibiotic resistance of Staphylococcus strains isolated from meat products sold in Abidjan streets (Ivory Coast). Afr. J. Microbiol. Res. 2013, 7, 3285–3293. [Google Scholar] [CrossRef] [Green Version]
- El Tawab, A.A.A.; Maarouf, A.A.; El-Rais, E.M. Bacteriological and molecular studies on antibiotic resistant Staphylococcus aureus isolated from meat and its products in Qaliobaya, Egypt. Vet. Med. J. 2018, 34, 360–373. [Google Scholar]
- Bissong, M.E.A.; Tahnteng, B.F.; Ateba, C.N.; Akoachere, J.T.K. Pathogenic potential and antimicrobial resistance profile of Staphylococcus aureus in milk and beef from the Northwest and Southwest Regions of Cameroon. BioMed Res. Int. 2020, 2020, 6015283. [Google Scholar] [CrossRef]
- Adesiji, Y.O.; Alli, O.T.; Adekanle, M.A.; Jolayemi, J.B. Prevalence of Arcobacter, Escherichia coli, Staphylococcus aureus and Salmonella species in retail raw chicken, pork, beef and goat meat in Osogbo, Nigeria. J. Biomed. Res. 2011, 3, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Aydin, A.; Sudagidan, M.; Muratoglu, K. Prevalence of staphylococcal enterotoxins, toxin genes and genetic-relatedness of foodborne Staphylococcus aureus strains isolated in the Marmara Region of Turkey. Int. J. Food Microbiol. 2011, 148, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Heo, H.J.; Ku, B.K.; Bae, D.H.; Park, C.K.; Lee, Y. Antimicrobial resistance of Staphylococcus aureus isolated from domestic and imported raw meat in Korea. J. Vet. Res. 2008, 48, 75–81. [Google Scholar]
- Quddoumi, S.S.; Bdour, S.M.; Mahasneh, A.M. Isolation and characterization of methicillin-resistant Staphylococcus aureus from livestock and poultry meat. Ann. Microbiol. 2006, 56, 155–161. [Google Scholar] [CrossRef]
- Thapaliya, D.; Forshey, B.M.; Kadariya, J.; Quick, M.K.; Farina, S.; O’Brien, A.; Nair, R.; Nworie, A.; Hanson, B.; Kates, A.; et al. Prevalence and molecular characterization of Staphylococcus aureus in commercially available meat over a one-year period in Iowa, USA. Food Microbiol. 2017, 65, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesavento, G.; Ducci, B.; Comodo, N.; Nostro, A.L. Antimicrobial resistance profile of Staphylococcus aureus isolated from raw meat: A research for methicillin resistant Staphylococcus aureus (MRSA). Food Control 2007, 18, 196–200. [Google Scholar] [CrossRef]
- Abdalrahman, L.S.; Wells, H.; Fakhr, M.K. Staphylococcus aureus is more prevalent in retail beef livers than in pork and other beef cuts. Pathogens 2015, 4, 182–198. [Google Scholar] [CrossRef] [Green Version]
- De Boer, E.; Zwartkruis-Nahuis, J.; Wit, B.; Huijsdens, X.; De Neeling, A.; Bosch, T.; Van Oosterom, R.; Vila, A.; Heuvelink, A.E. Prevalence of methicillin-resistant Staphylococcus aureus in meat. Inter. J. Food Microbiol. 2009, 134, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 1–23. [Google Scholar] [CrossRef]
- Lutz, J.K.; Van Balen, J.; Mac Crawford, J.; Wilkins III, J.R.; Lee, J.; Nava-Hoet, R.C.; Hoet, A.E. Methicillin-resistant Staphylococcus aureus in public transportation vehicles (buses): Another piece to the epidemiologic puzzle. Am. J. Infect. Control 2014, 42, 1285–1290. [Google Scholar] [CrossRef]
- Alvseike, O.; Prieto, M.; Bjørnstad, P.H.; Mason, A. Intact gastro-intestinal tract removal from pig carcasses in a novel Meat Factory Cell approach. J. Vet. Scan. 2020, 62, 1–5. [Google Scholar] [CrossRef]
- Burfoot, D.; Everis, L.; Mulvey, L.; Wood, A.; Campden, R.B. Literature Review on Microbiological Hazards Associated with Biltong and Similar Dried Meat Products; Report to Food Standard Agency: London, UK, 2010; pp. 1–87. [Google Scholar]
- Tshipamba, M.E.; Lubanza, N.; Adetunji, M.C.; Mwanza, M. Molecular characterization and antibiotic resistance of foodborne pathogens in street-vended ready-to-eat meat sold in South Africa. J. Food Prot. 2018, 81, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Guidelines for Environmental Health Officers on the Interpretation of Microbiological Analysis Data of Food; Department of Health, Directorate Food Control: Pretoria, South Africa, 2000. [Google Scholar]
- Huang, E.; Gurzau, A.E.; Hanson, B.M.; Kates, A.E.; Smith, T.C.; Pettigrew, M.M.; Spinu, M.; Rabinowitz, P.M. Detection of livestock-associated methicillin-resistant Staphylococcus aureus among swine workers in Romania. J. Infect. Public Health 2014, 7, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Loncaric, I.; Kuebber-Heiss, A.; Posautz, A.; Ruppitsch, W.; Lepuschitz, S.; Schauer, B.; Fessler, A.T.; Krametter-Froetscher, R.; Harrison, E.M.; Holmes, M.A. Characterization of mecC gene-carrying coagulase-negative Staphylococcus spp. isolated from various animals. J. Vet. Microbiol. 2019, 230, 138–144. [Google Scholar] [CrossRef]
- Abolghait, S.K.; Fathi, A.G.; Youssef, F.M.; Algammal, A.M. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from chicken meat and giblets often produces staphylococcal enterotoxin B (SEB) in non-refrigerated raw chicken livers. Int. J. Food Microbiol. 2020, 328, 108669. [Google Scholar] [CrossRef] [PubMed]
- Normanno, G.; La Salandra, G.; Dambrosio, A.; Quaglia, N.; Corrente, M.; Parisi, A.; Santagada, G.; Firinu, A.; Crisetti, E.; Celano, G.V. Occurrence, characterization and antimicrobial resistance of enterotoxigenic Staphylococcus aureus isolated from meat and dairy products. Int. J. Food Microbiol. 2007, 115, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Nworie, A.; Onyema, A.S.; Okekpa, S.I.; Elom, M.O.; Umoh, N.O.; Usanga, V.U.; Ibiam, G.A.; Ukwah, B.N.; Nwadi, L.C.; Ezeruigbo, C.; et al. A novel Methicillin-Resistant Staphylococcus aureus t11469 and a poultry endemic strain t002 (ST5) are present in chicken in Ebonyi State, Nigeria. BioMed Res. Int. 2017, 2017, 2936461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupa, P.; Bystroń, J.; Bania, J.; Podkowik, M.; Empel, J.; Mroczkowska, A. Genotypes and oxacillin resistance of Staphylococcus aureus from chicken and chicken meat in Poland. Poult. Sci. 2014, 93, 3179–3186. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Papadopoulos, T.; Angelidis, A.S.; Boukouvala, E.; Zdragas, A.; Papa, A.; Hadjichristodoulou, C.; Sergelidis, D. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus (MRSA) along the production chain of dairy products in north-western Greece. Food Microbiol. 2018, 69, 43–50. [Google Scholar] [CrossRef]
- Bergdoll, M.S. Enterotoxins. In Staphylococci and Staphylococcal Infections; Easmon, C.S.F., Adlam, C., Eds.; Academic Press, Ltd.: London, UK, 1983; pp. 559–598. [Google Scholar]
- Ikeda, T.; Tamate, N.; Yamaguchi, K.; Makino, S. Mass outbreak of food poisoning disease caused by small amounts of staphylococcal enterotoxins A and H. Appl. Environ. Microbiol. 2005, 71, 2793–2795. [Google Scholar] [CrossRef] [Green Version]
- Evenson, M.L.; Hinds, M.W.; Bernstein, R.S.; Bergdoll, M.S. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Inter. J. Food Microbiol. 1988, 7, 311–316. [Google Scholar] [CrossRef]
- Fiebelkorn, K.R.; Crawford, S.A.; McElmeel, M.L.; Jorgensen, J.H. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J. Clin. Microbiol. 2003, 41, 4740–4744. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.Y.; Kim, S.H.; Jang, E.J.; Kwon, N.H.; Park, Y.K.; Koo, H.C.; Jung, W.K.; Kim, J.M.; Park, Y.H. Novel multiplex PCR for the detection of the Staphylococcus aureus superantigen and its application to raw meat isolates in Korea. Int. J. Food Microbiol. 2007, 117, 99–105. [Google Scholar] [CrossRef]
- McLauchlin, J.; Narayanan, G.; Mithani, V.; O’neill, G. The detection of enterotoxins and toxic shock syndrome toxin genes in Staphylococcus aureus by polymerase chain reaction. J. Food Prot. 2000, 63, 479–488. [Google Scholar] [CrossRef]
- Nashev, D.; Toshkova, K.; Bizeva, L.; Akineden, Ö.; Lämmler, C.; Zschöck, M. Distribution of enterotoxin genes among carriage-and infection-associated isolates of Staphylococcus aureus. Lett. Appl. Microbiol. 2007, 45, 681–685. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).