Effects of Fermented Herbal Tea Residue on Serum Indices and Fecal Microorganisms of Chuanzhong Black Goats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silage Material and Preparation
2.2. Analyses of Fermentation Parameter and Chemical Composition
2.3. Statistical Analysis
3. Results
3.1. Serum Indices
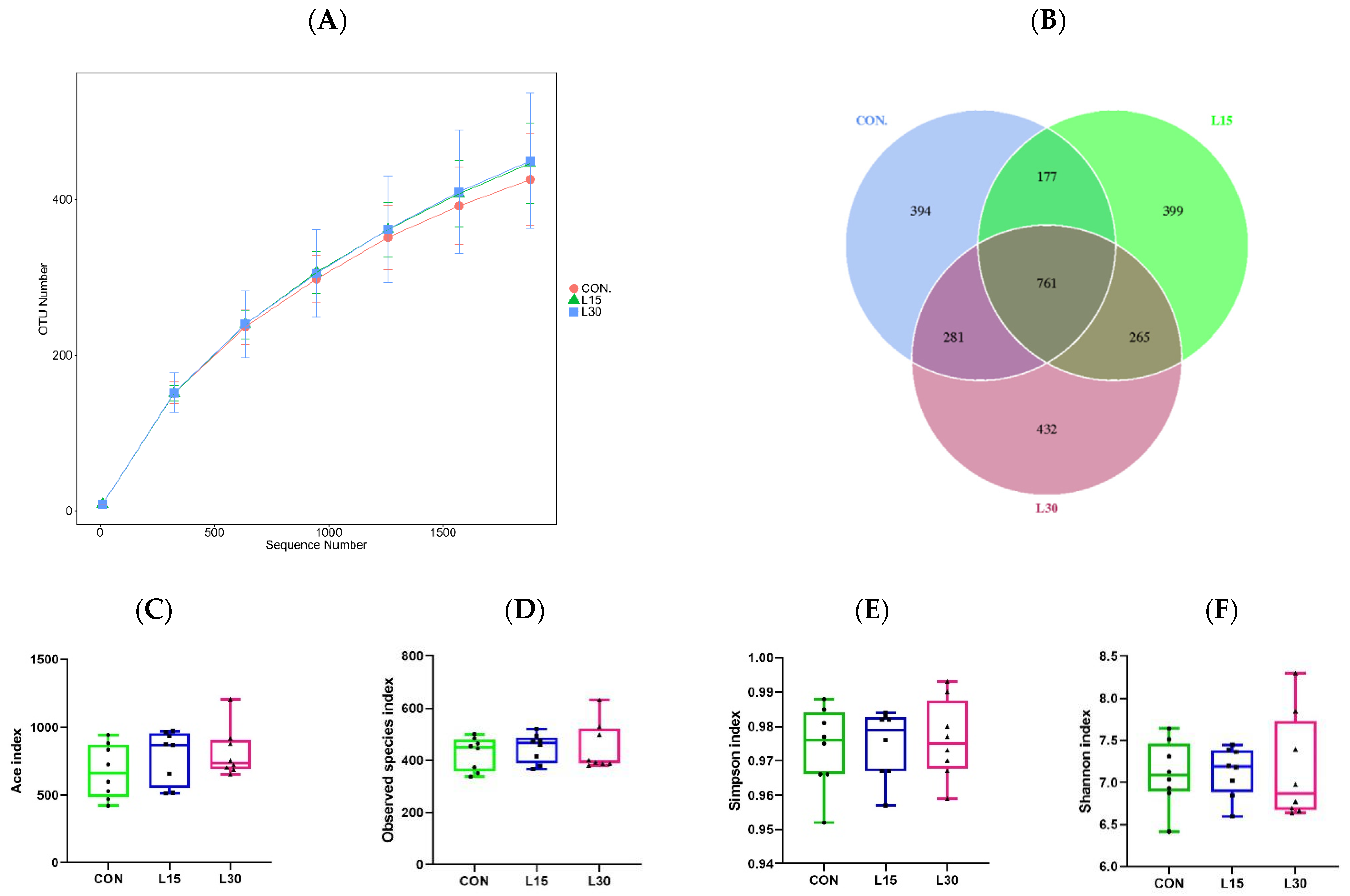
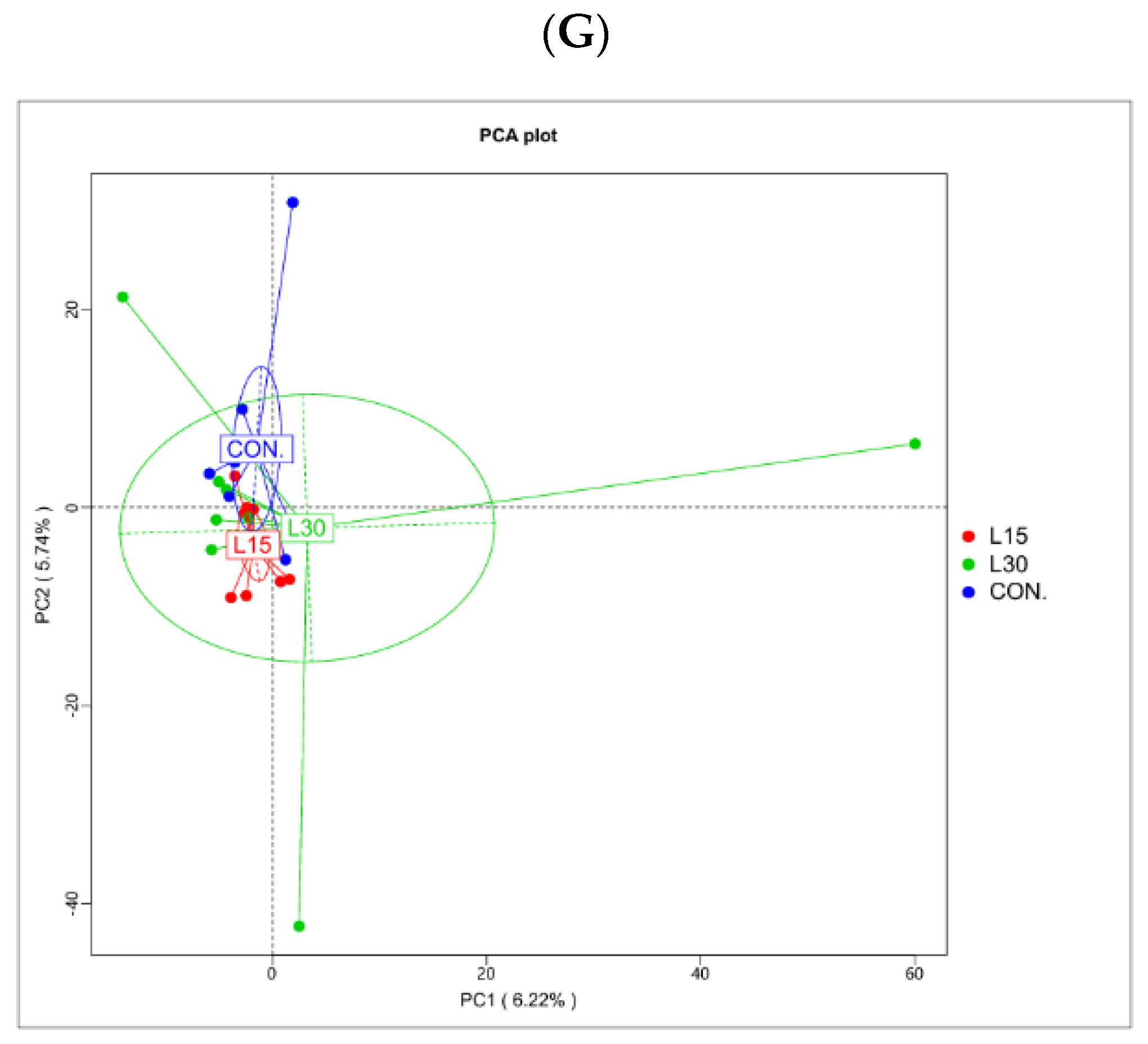
3.2. Sequencing Depth and Fecal Microbiota Diversity
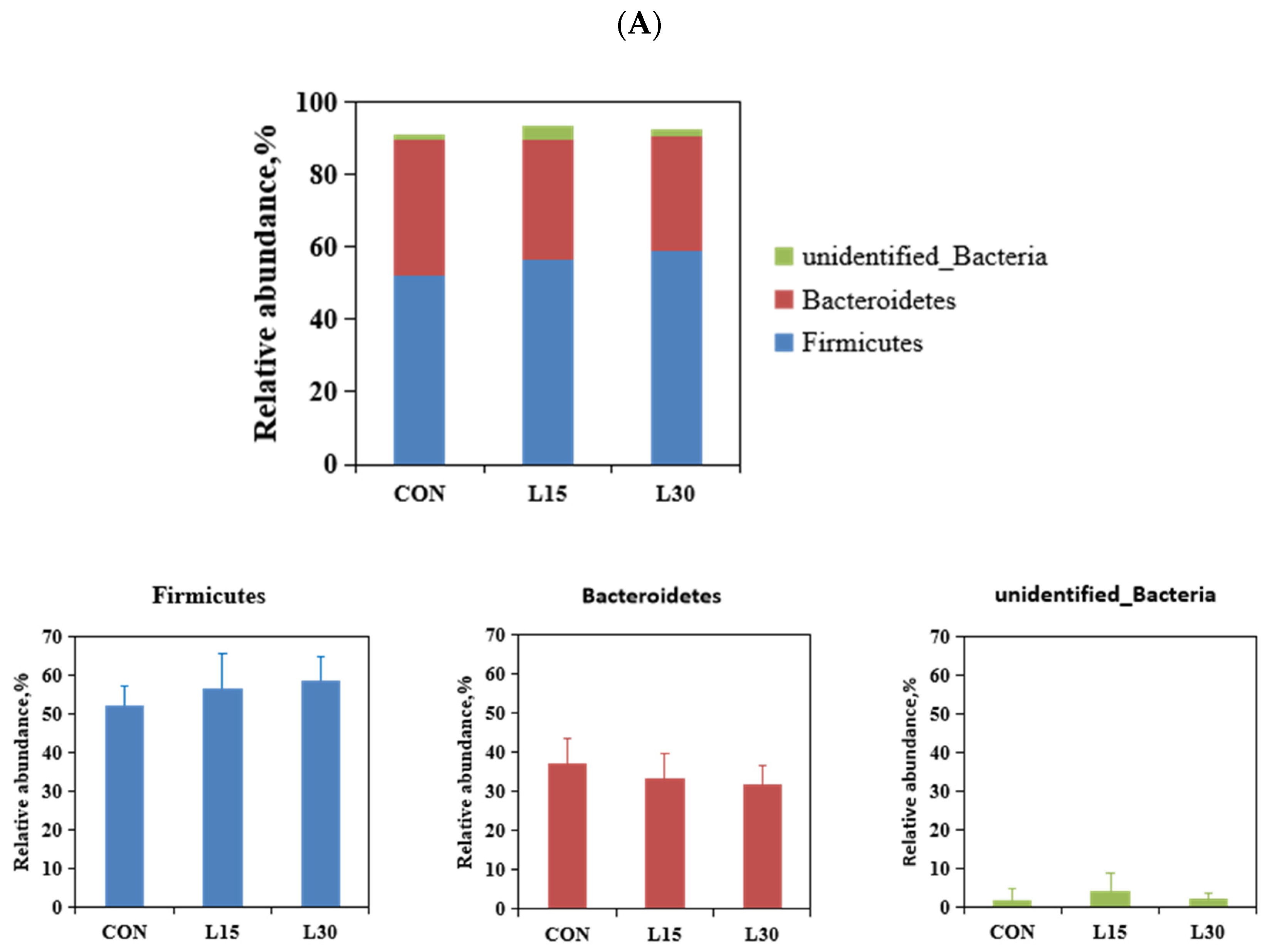
3.3. Composition of Fecal Microbiota at Various Taxonomic Levels
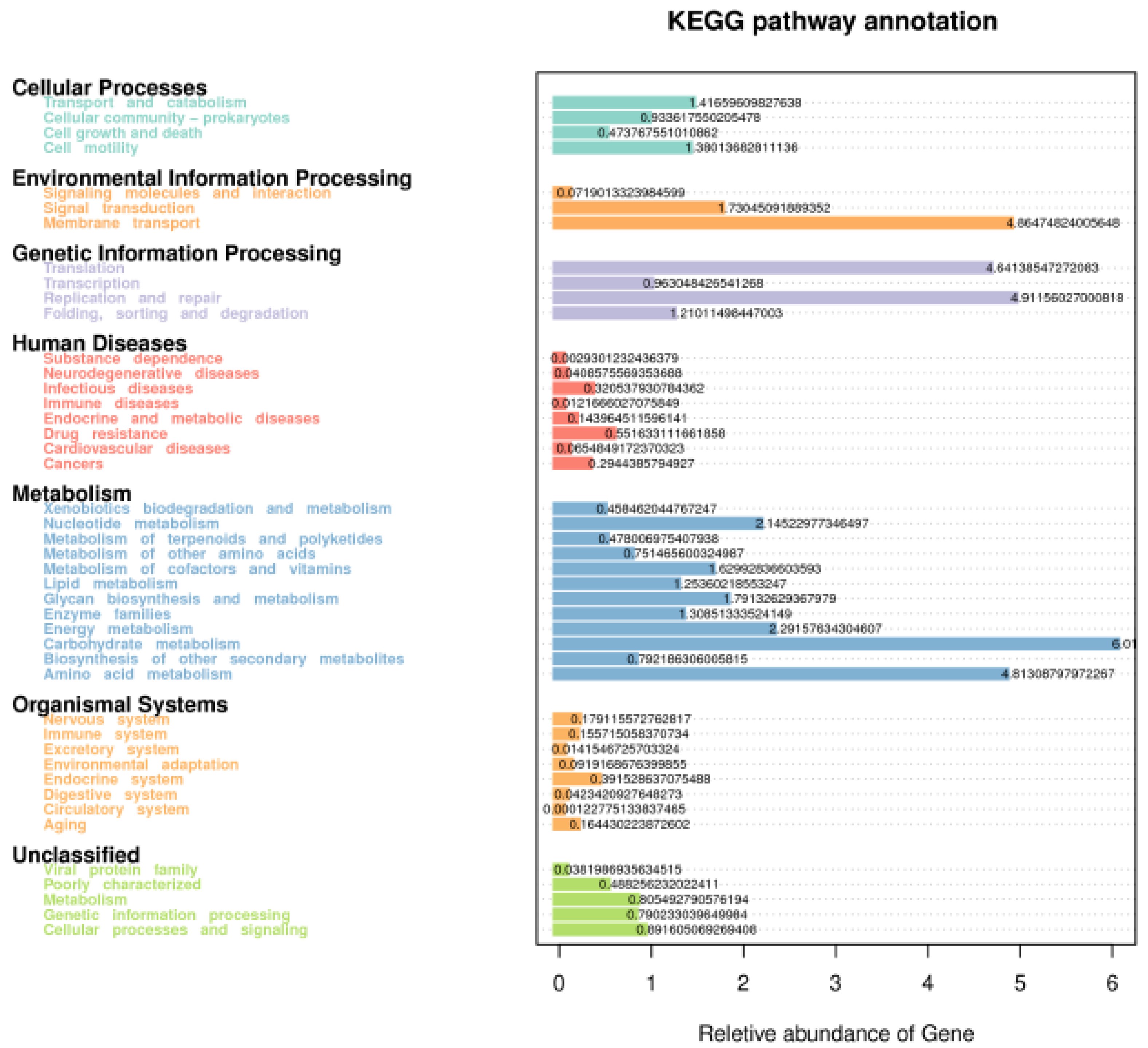
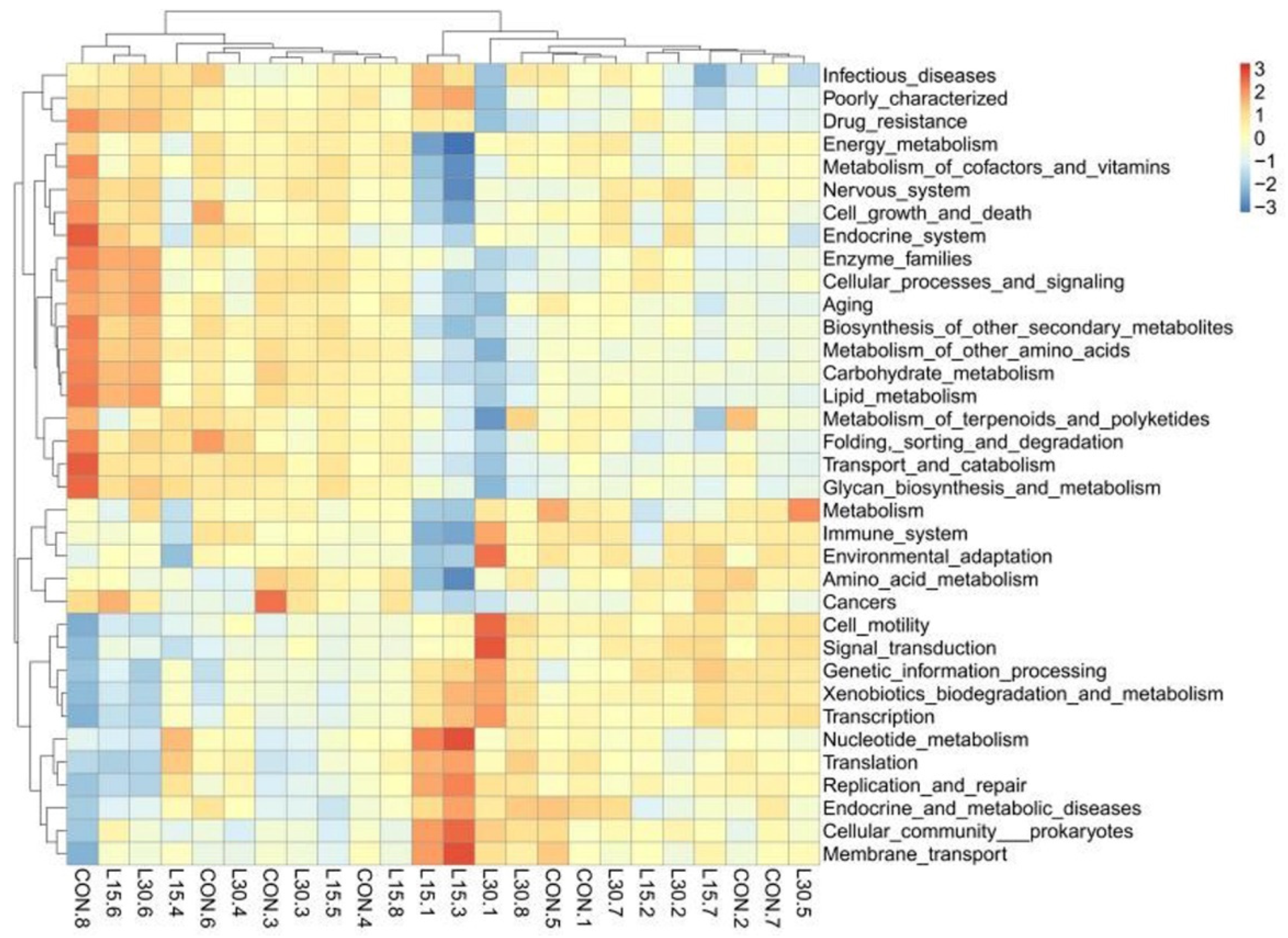
3.4. Function Prediction of Fecal Microbiota
4. Discussion
4.1. Serum Indices
4.2. Fecal Microbiota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, X.; Chen, Z.; Sun, X.; Li, F.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Fermentation quality of herbal tea residue and its application in fattening cattle under heat stress. BMC Vet. Res. 2021, 17, 348. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Daware, G.B.; Gogate, P.R. Removal of pyridine using ultrasound assisted and conventional batch adsorption based on tea waste residue as biosorbent. Environ. Technol. Innov. 2021, 21, 101292. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Fan, M.; Yuan, Y.; Sun, X.; Wang, D.; Xu, Y. High-efficiency adsorption of various heavy metals by tea residue biochar loaded with nanoscale zero-valent iron. Environ. Prog. Sustain. Energy 2021, 40, e13706. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Z.; Zhou, Y.; Jiang, X.; Cai, F.; Bai, Y.; Ning, H. Porous Co, N co-doped carbon derived from tea residue as efficient cathode catalyst in microbial fuel cells for swine wastewater treatment and the microbial community analysis. J. Water Process Eng. 2022, 45, 102471. [Google Scholar] [CrossRef]
- Khan, M.A.I.; Ueno, K.; Horimoto, S.; Komai, F.; Tanaka, K.; Ono, Y. Evaluation of the Physio-Chemical and Microbial Properties of Green Tea Waste-Rice Bran Compost and the Effect of the Compost on Spinach Production. Plant Prod. Sci. 2007, 10, 391–399. [Google Scholar] [CrossRef]
- Polo, A.; Cappello, C.; Carafa, I.; Da Ros, A.; Baccilieri, F.; Di Cagno, R.; Gobbetti, M. A novel functional herbal tea containing probiotic Bacillus coagulans GanedenBC30: An in vitro study using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME). J. Funct. Foods 2022, 88, 104873. [Google Scholar] [CrossRef]
- Zhou, X.; Qin, D.; Xiang, B.; Xi, J. Cyclodextrin-based liquid-phase pulsed discharge extraction of flavonoids from tangerine (Citrus reticulata) pericarp: Optimization, antioxidant activity and storage stability. Sep. Purif. Technol. 2022, 278, 119603. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Azzazy, H.M.E.-S.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef]
- Ding, X.; Li, H.; Wen, Z.; Hou, Y.; Wang, G.; Fan, J.; Qian, L. Effects of Fermented Tea Residue on Fattening Performance, Meat Quality, Digestive Performance, Serum Antioxidant Capacity, and Intestinal Morphology in Fatteners. Animals 2020, 10, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, R.; Tan, C.; Han, X.; Tang, S.; Tan, Z.; Zeng, B. Effect of dietary tea catechins supplementation in goats on the quality of meat kept under refrigeration. Small Rumin. Res. 2009, 87, 122–125. [Google Scholar] [CrossRef]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef]
- Tudisco, R.; Musco, N.; Pero, M.E.; Morittu, V.M.; Grossi, M.; Mastellone, V.; Cavaliere, G.; Wanapat, M.; Infascelli, F.; Lombardi, P. Influence of dietary hydrogenated palm oil supplementation on serum biochemistry and progesterone levels in dairy goats. Anim. Nutr. 2019, 5, 286–289. [Google Scholar] [CrossRef]
- Nauseef, W.M. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, J.; Zarzyńska, J.; Pławińska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- Forooshani, P.K.; Pinnaratip, R.; Polega, E.; Tyo, A.G.; Pearson, E.; Liu, B.; Folayan, T.-O.; Pan, L.; Rajachar, R.M.; Heldt, C.L.; et al. Hydroxyl Radical Generation through the Fenton-like Reaction of Hematin- and Catechol-Functionalized Microgels. Chem. Mater. 2020, 32, 8182–8194. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-H.; Li, L.; Xue, Y.-B.; Zhou, X.-X.; Tang, J.-H. Flavonoids from Epimedium pubescens: Extraction and mechanism, antioxidant capacity and effects on CAT and GSH-Px of Drosophila melanogaster. PeerJ 2020, 8, e8361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marković, R.; Ćirić, J.; Starčević, M.; Šefer, D.; Baltić, M. Effects of selenium source and level in diet on glutathione peroxidase activity, tissue selenium distribution, and growth performance in poultry. Anim. Health Res. Rev. 2018, 19, 166–176. [Google Scholar] [CrossRef]
- Hukerdi, Y.J.; Nasri, M.F.; Rashidi, L.; Ganjkhanlou, M.; Emami, A. Effects of dietary olive leaves on performance, carcass traits, meat stability and antioxidant status of fattening Mahabadi male kids. Meat Sci. 2019, 153, 2–8. [Google Scholar] [CrossRef]
- Lin, C.-Z.; Zhang, R.-J.; Yao, Y.-F.; Huang, X.-D.; Zheng, R.-B.; Wu, B.-J.; Zhu, C.-C. Qualitative and Quantitative Analysis of the Major Constituents in WLJ Herbal Tea Using Multiple Chromatographic Techniques. Molecules 2018, 23, 2623. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar] [CrossRef]
- Guo, J.; Li, P.; Zhang, K.; Zhang, L.; Wang, X.; Li, L.; Zhang, H. Distinct Stage Changes in Early-Life Colonization and Acquisition of the Gut Microbiota and Its Correlations with Volatile Fatty Acids in Goat Kids. Front. Microbiol. 2020, 11, 584742. [Google Scholar] [CrossRef]
- Ma, Z.; Cheng, Y.; Wang, S.; Ge, J.; Shi, H.; Kou, J. Positive effects of dietary supplementation of three probiotics on milk yield, milk composition and intestinal flora in Sannan dairy goats varied in kind of probiotics. J. Anim. Physiol. Anim. Nutr. 2020, 104, 44–55. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Leslie, J.; Kitamoto, S.; Jin, C.; Thomsson, K.A.; Iii, M.G.G.; Kuffa, P.; Goto, Y.; Jenq, R.R.; Ishii, C.; et al. Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 2020, 26, 608–617. [Google Scholar] [CrossRef]
- Wu, D.-T.; Fu, Y.; Guo, H.; Yuan, Q.; Nie, X.-R.; Wang, S.-P.; Gan, R.-Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2020, 168, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ding, S.; Zheng, P. Central metabolism of anammox bacteria—A review. Acta Microbiol. Sin. 2011, 51, 1014–1022. [Google Scholar]
- Van Niftrik, L.; Geerts, W.J.C.; Van Donselaar, E.G.; Humbel, B.M.; Webb, R.I.; Harhangi, H.R.; Camp, H.J.M.O.D.; Fuerst, J.A.; Verkleij, A.J.; Jetten, M.S.M.; et al. Cell division ring, a new cell division protein and vertical inheritance of a bacterial organelle in anammox planctomycetes. Mol. Microbiol. 2009, 73, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Radha, V.; Jagadeeshwari, U.; Sasikala, C.; Ramana, C.V. Bacterial communities of sponges from the wetland ecosystem of Little Rann of Kutch, India with particular reference to Planctomycetes. 3 Biotech 2020, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S.; Jogler, M.; Boedeker, C.; Pinto, D.; Vollmers, J.; Rivas-Marín, E.; Kohn, T.; Peeters, S.H.; Heuer, A.; Rast, P.; et al. Cultivation and functional characterization of 79 planctomycetes uncovers their unique biology. Nat. Microbiol. 2020, 5, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Gordon, A.W.; O’Connell, N.E.; Yan, T. Nitrogen utilization efficiency and prediction of nitrogen excretion in sheep offered fresh perennial ryegrass (Lolium perenne). J. Anim. Sci. 2016, 94, 5321–5331. [Google Scholar] [CrossRef]
- Scott, K.A.; Penner, G.B.; Mutsvangwa, T. Influence of forage level and corn grain processing on whole-body urea kinetics, and serosal-to-mucosal urea flux and expression of urea transporters and aquaporins in the ovine ruminal, duodenal, and cecal epithelia. J. Anim. Sci. 2020, 98, skaa098. [Google Scholar] [CrossRef]
- De Seram, E.; Penner, G.; Mutsvangwa, T. Nitrogen utilization, whole-body urea-nitrogen kinetics, omasal nutrient flow, and production performance in dairy cows fed lactose as a partial replacement for barley starch. J. Dairy Sci. 2019, 102, 6088–6108. [Google Scholar] [CrossRef]
- Tsushima, I.; Ogasawara, Y.; Kindaichi, T.; Satoh, H.; Okabe, S. Development of high-rate anaerobic ammonium-oxidizing (anammox) biofilm reactors. Water Res. 2007, 41, 1623–1634. [Google Scholar] [CrossRef] [Green Version]
- Kuenen, J.G. Anammox bacteria: From discovery to application. Nat. Rev. Microbiol. 2008, 6, 320–326. [Google Scholar] [CrossRef]
- Nishimura, F.; Hidaka, T.; Nakagawa, A.; Yorozu, H.; Tsuno, H. Removal of high concentration ammonia from wastewater by a combination of partial nitrification and anammox treatment. Environ. Technol. 2012, 33, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Jenni, S.; Vlaeminck, S.; Morgenroth, E.; Udert, K.M. Successful application of nitritation/anammox to wastewater with elevated organic carbon to ammonia ratios. Water Res. 2014, 49, 316–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cocker, P.; Bessiere, Y.; Hernandez-Raquet, G.; Dubos, S.; Mozo, I.; Gaval, G.; Caligaris, M.; Barillon, B.; Vlaeminck, S.; Sperandio, M. Enrichment and adaptation yield high anammox conversion rates under low temperatures. Bioresour. Technol. 2018, 250, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, X.; Yuan, S.; Wang, Y.; Liao, X.; Zhong, M.; He, Q.; Shen, H.; Liao, W.; Shen, J. Flammulina velutipes polysaccharide improves C57BL/6 mice gut health through regulation of intestine microbial metabolic activity. Int. J. Biol. Macromol. 2020, 167, 1308–1318. [Google Scholar] [CrossRef] [PubMed]


| Items | Dietary Treatment | ||
|---|---|---|---|
| CON | L15 | L30 | |
| Ingredient, % | |||
| Whole plant corn silage | 76.2 | 64.8 | 53.3 |
| Fermented herbal tea residue | 0 | 11.4 | 22.9 |
| Peanut seedling feed | 19 | 19 | 19 |
| Soya bean meal | 1.2 | 1.2 | 1.2 |
| Alfalfa | 0.47 | 0.47 | 0.47 |
| Mountain flour | 0.1 | 0.1 | 0.1 |
| Salt | 0.05 | 0.05 | 0.05 |
| Nutritional ingredient, % | |||
| DM | 30.84 | 33.45 | 31.33 |
| CP | 11.68 | 12.13 | 11.79 |
| EE | 0.01 | 0.01 | 0.02 |
| Ash | 0.13 | 0.12 | 0.12 |
| NDF | 69.23 | 69.93 | 68.76 |
| ADF | 52.21 | 53.83 | 51.49 |
| Ca | 0.9 | 0.91 | 1.04 |
| P | 0.44 | 0.44 | 0.41 |
| Items | Diet | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | L15 | L30 | Line | Quad | ||
| Firmicutes | 52.12 | 56.42 | 58.67 | 1.589 | 0.100 | 0.760 |
| Bacteroidetes | 37.27 | 33.17 | 31.67 | 1.326 | 0.090 | 0.639 |
| unidentified_Bacteria | 1.66 | 3.88 | 1.92 | 0.746 | 0.889 | 0.202 |
| Spirochaetes | 2.44 | 1.70 | 2.77 | 0.703 | 0.852 | 0.564 |
| Tenericutes | 2.72 | 2.02 | 2.36 | 0.274 | 0.600 | 0.390 |
| Proteobacteria | 0.88 | 0.46 | 0.57 | 0.155 | 0.438 | 0.436 |
| Melainabacteria | 1.18 | 1.17 | 1.01 | 0.142 | 0.637 | 0.801 |
| Deferribacteres | 0.44 | 0.15 | 0.19 | 0.098 | 0.311 | 0.439 |
| Elusimicrobia | 0.03 | 0.13 | 0.05 | 0.038 | 0.835 | 0.252 |
| Lentisphaerae | 0.40 | 0.19 | 0.22 | 0.052 | 0.163 | 0.292 |
| Planctomycetes | 0.28 | 0.23 | 0.14 | 0.030 | 0.060 | 0.788 |
| Items | Diet | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | L15 | L30 | Line | Quad | ||
| Alistipes | 2.09 | 2.23 | 2.40 | 0.225 | 0.592 | 0.974 |
| Bacteroides | 2.44 | 1.70 | 1.95 | 0.220 | 0.373 | 0.298 |
| Campylobacter | 1.45 | 3.80 | 1.90 | 0.751 | 0.812 | 0.199 |
| Roseburia | 1.00 | 0.64 | 1.85 | 0.277 | 0.210 | 0.183 |
| Anaerovibrio | 0.10 | 0.56 | 0.98 | 0.237 | 0.140 | 0.963 |
| unidentified_Ruminococcaceae | 1.55 | 1.85 | 2.30 | 0.179 | 0.095 | 0.853 |
| Phascolarctobacterium | 1.70 a | 2.24 b | 1.57 a | 0.145 | 0.697 | 0.048 |
| Mucispirillum | 0.44 | 0.15 | 0.19 | 0.098 | 0.311 | 0.439 |
| Tyzzerella | 0.25 | 0.37 | 0.47 | 0.072 | 0.216 | 0.932 |
| unidentified_Clostridiales | 0.57 | 0.35 | 0.51 | 0.065 | 0.709 | 0.167 |
| Anaerosporobacter | 0.44 | 0.4 | 0.31 | 0.073 | 0.480 | 0.870 |
| Candidatus_Soleaferrea | 0.61 | 0.56 | 0.62 | 0.040 | 0.948 | 0.523 |
| Anaerovorax | 0.27 | 0.23 | 0.21 | 0.027 | 0.381 | 0.955 |
| Turicibacter | 0.19 | 0.27 | 0.18 | 0.035 | 0.881 | 0.307 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Wu, L.; Zhao, W.; Chen, Y.; Deng, M.; Liu, G.; Guo, Y.; Sun, B. Effects of Fermented Herbal Tea Residue on Serum Indices and Fecal Microorganisms of Chuanzhong Black Goats. Microorganisms 2022, 10, 1228. https://doi.org/10.3390/microorganisms10061228
Gao C, Wu L, Zhao W, Chen Y, Deng M, Liu G, Guo Y, Sun B. Effects of Fermented Herbal Tea Residue on Serum Indices and Fecal Microorganisms of Chuanzhong Black Goats. Microorganisms. 2022; 10(6):1228. https://doi.org/10.3390/microorganisms10061228
Chicago/Turabian StyleGao, Chongya, Longfei Wu, Weiran Zhao, Yiye Chen, Ming Deng, Guangbin Liu, Yongqing Guo, and Baoli Sun. 2022. "Effects of Fermented Herbal Tea Residue on Serum Indices and Fecal Microorganisms of Chuanzhong Black Goats" Microorganisms 10, no. 6: 1228. https://doi.org/10.3390/microorganisms10061228
APA StyleGao, C., Wu, L., Zhao, W., Chen, Y., Deng, M., Liu, G., Guo, Y., & Sun, B. (2022). Effects of Fermented Herbal Tea Residue on Serum Indices and Fecal Microorganisms of Chuanzhong Black Goats. Microorganisms, 10(6), 1228. https://doi.org/10.3390/microorganisms10061228





