Targeted Analysis of the Gut Microbiome for Diagnosis, Prognosis and Treatment Individualization in Pediatric Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical, Endoscopic, Radiological and Laboratory Data
2.2. IBD Treatment
2.3. Microbiota Analysis
2.4. Statistical Analyses
3. Results
3.1. Phenotypes
3.2. Treatment of IBD Patients
3.3. Microbiota in IBD, Non-IBD and Healthy
3.4. Microbiota in IBD Patients and Association with Phenotypes and Treatment
3.5. Microbiota and Association with Fecal Calprotectin
3.6. Indexes: Diagnostic, Phenotype and Prognostic Index
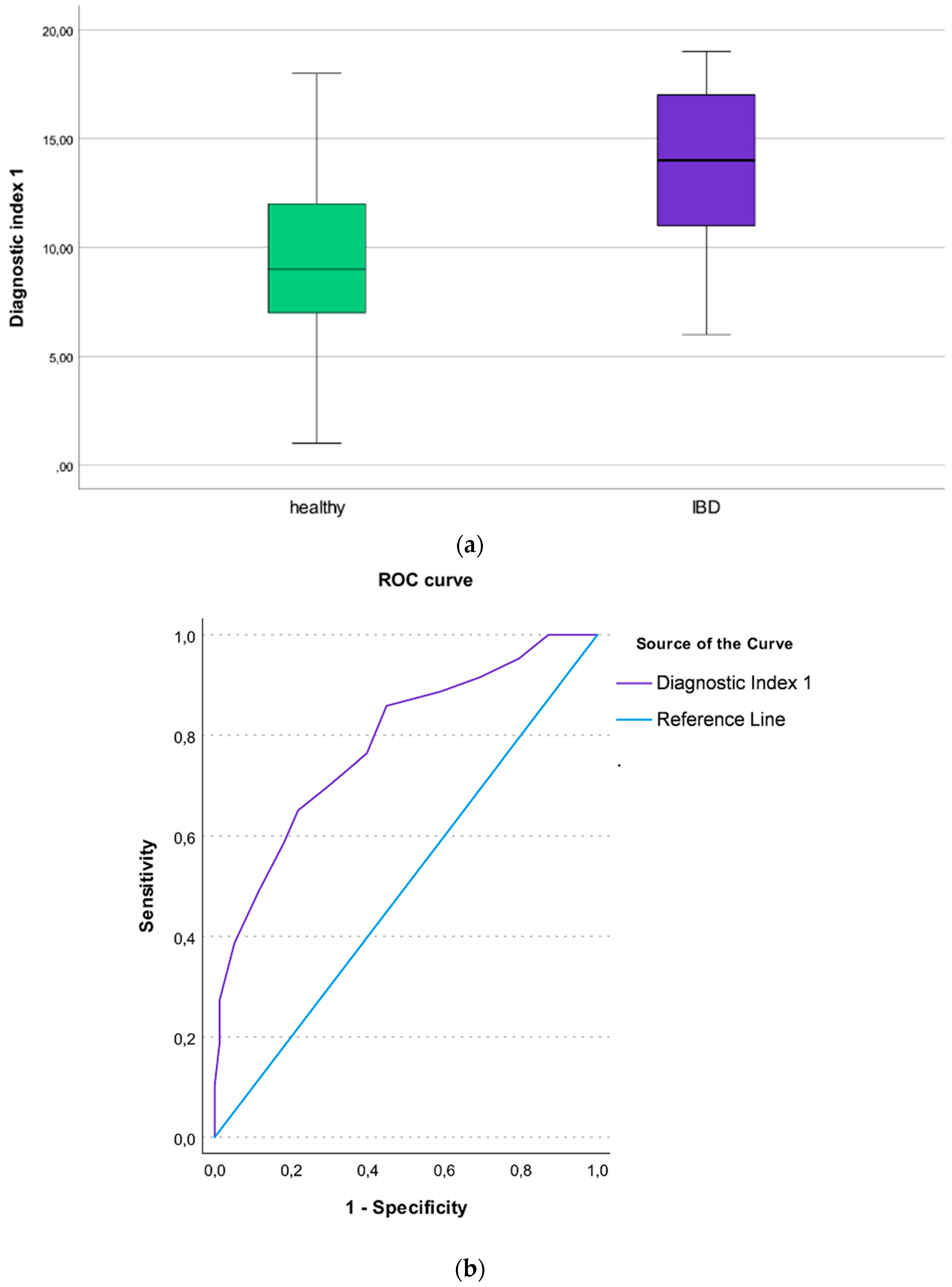
3.7. The Diagnostic Index 1 (IBD Patients vs. Healthy Individuals)
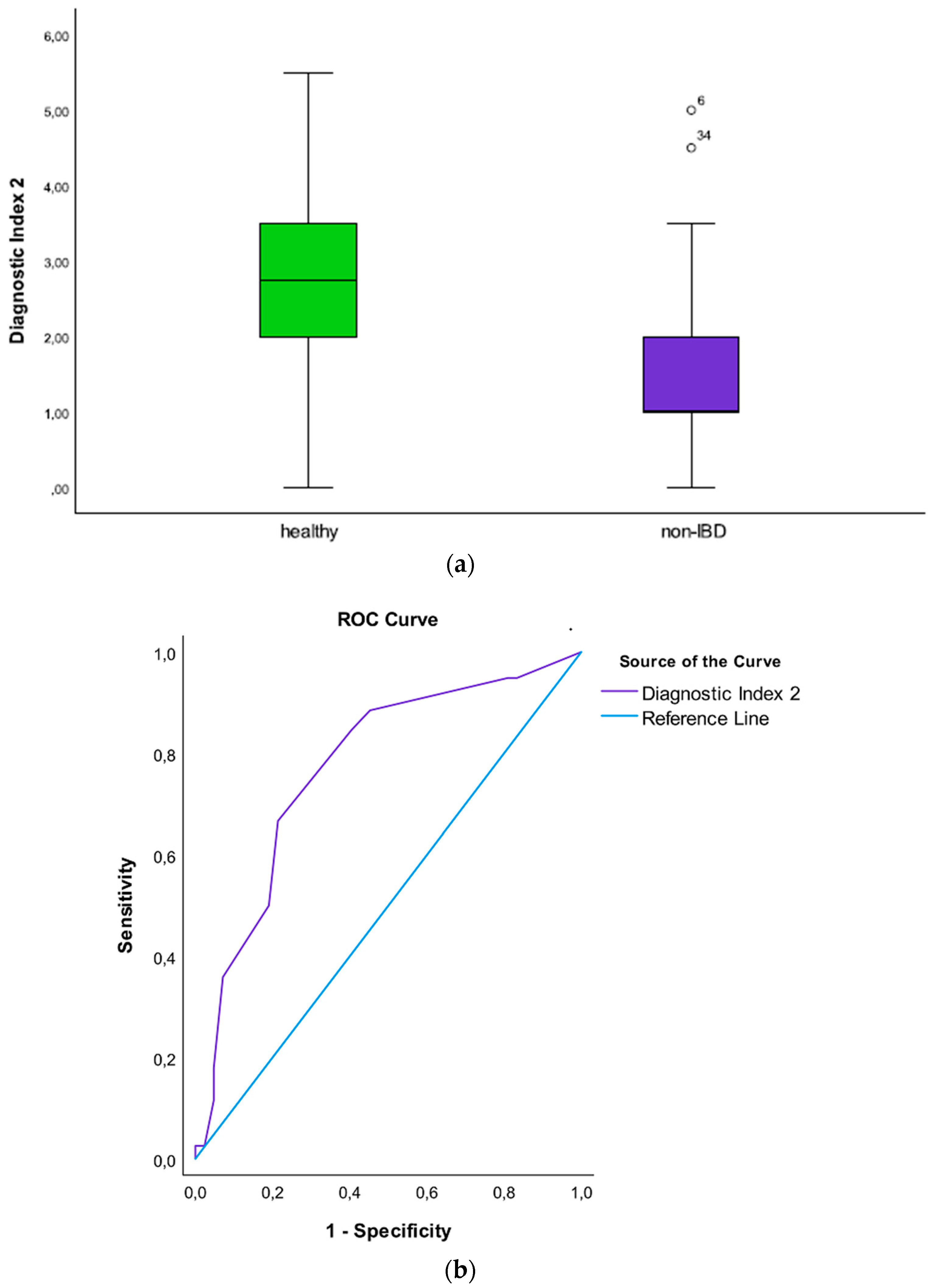
3.8. The Diagnostic Index 2 (Non-IBD Patients vs. Healthy Individuals)
3.9. The Diagnostic Index 3 (IBD Patients vs. Non-IBD Patients)
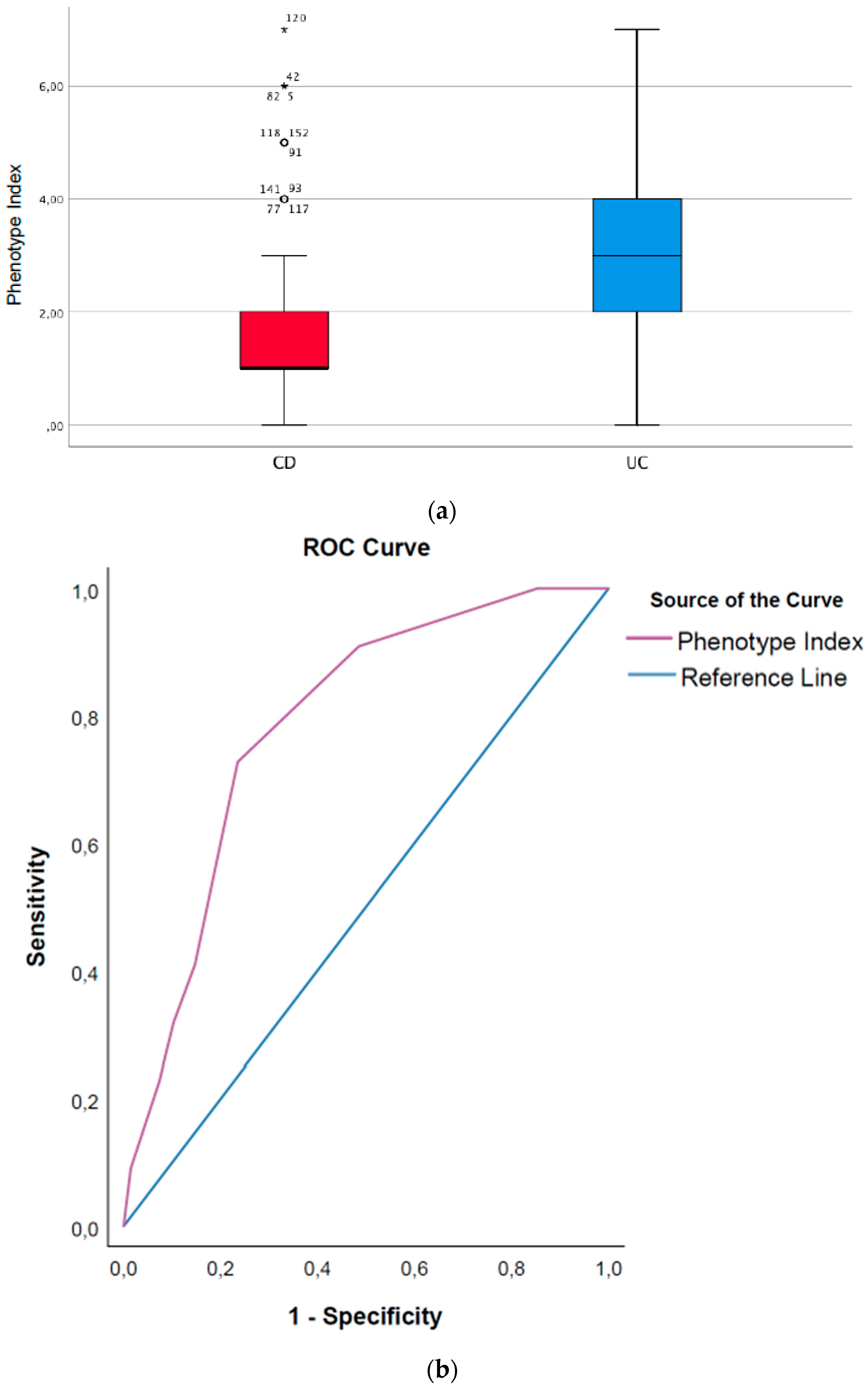
3.10. Phenotype Index (CD Patients vs. UC Patients)
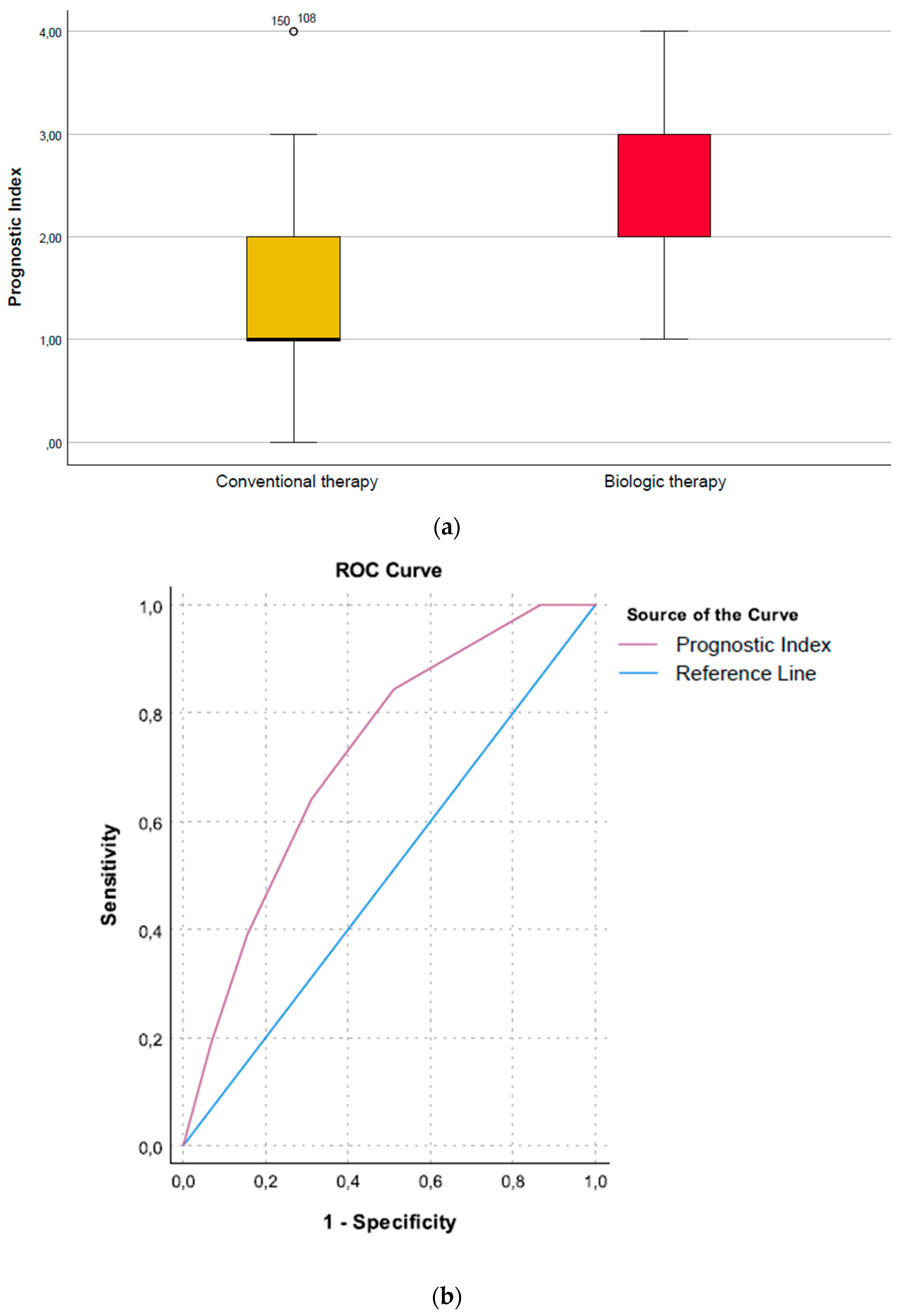
Prognostic Index (Need for Biologic Therapy vs. No Need)
4. Discussion
4.1. Diagnostic Potential of the Gut Microbiome
4.2. Loss of Beneficial Microbes
4.3. Prognostic Potential
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Khosravi, A.; Kusumawardhani, I.P.; Kwon, A.H.; Vasconcelos, A.C.; Cunha, L.D.; Mayer, A.E.; Shen, Y.; Wu, W.L.; Kambal, A.; et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016, 352, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339.E4. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, C.; Xu, J.; Xu, H.; Liu, L.; Zhao, H.; Wang, J.; Huang, W.; Peng, W.; Chen, Y.; et al. Gut Microbiota Is a Potential Biomarker in Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 818902. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Bennet, S.M.; Ohman, L.; Simren, M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver 2015, 9, 318–331. [Google Scholar] [CrossRef]
- Ricciuto, A.; Griffiths, A.M. Clinical value of fecal calprotectin. Crit. Rev. Clin. Lab. Sci. 2019, 56, 307–320. [Google Scholar] [CrossRef]
- Ricciuto, A.; Fish, J.R.; Tomalty, D.E.; Carman, N.; Crowley, E.; Popalis, C.; Muise, A.; Walters, T.D.; Griffiths, A.M.; Church, P.C. Diagnostic delay in Canadian children with inflammatory bowel disease is more common in Crohn’s disease and associated with decreased height. Arch. Dis. Child. 2018, 103, 319–326. [Google Scholar] [CrossRef]
- De Bie, C.I.; Paerregaard, A.; Kolacek, S.; Ruemmele, F.M.; Koletzko, S.; Fell, J.M.; Escher, J.C. Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-year analyses of the EUROKIDS Registry. Inflamm. Bowel Dis. 2013, 19, 378–385. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Walters, T.D.; Mack, D.R.; Huynh, H.Q.; Jacobson, K.; Otley, A.R.; Debruyn, J.; El-Matary, W.; Deslandres, C.; Sherlock, M.E.; et al. Phenotypic Variation in Paediatric Inflammatory Bowel Disease by Age: A Multicentre Prospective Inception Cohort Study of the Canadian Children IBD Network. J. Crohn’s Colitis 2020, 14, 445–454. [Google Scholar] [CrossRef]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolacek, S.; et al. Nutrition in Paediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto IBD Group of ESPGHAN. J. Pediatric Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Garre, A.; Ricart, E.; Iglesias-Flores, E.; Taxonera, C.; Domenech, E.; Gisbert, J.P. Differences between childhood- and adulthood-onset inflammatory bowel disease: The CAROUSEL study from GETECCU. Aliment. Pharmacol. Ther. 2019, 49, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Olbjorn, C.; Nakstad, B.; Smastuen, M.C.; Thiis-Evensen, E.; Vatn, M.H.; Perminow, G. Early anti-TNF treatment in pediatric Crohn’s disease. Predictors of clinical outcome in a population-based cohort of newly diagnosed patients. Scand. J. Gastroenterol. 2014, 49, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatric Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Aldars-García, L.; Chaparro, M.; Gisbert, J.P. Systematic Review: The Gut Microbiome and Its Potential Clinical Application in Inflammatory Bowel Disease. Microorganisms 2021, 9, 977. [Google Scholar] [CrossRef]
- Perminow, G.; Brackmann, S.; Lyckander, L.G.; Franke, A.; Borthne, A.; Rydning, A.; Aamodt, G.; Schreiber, S.; Vatn, M.H. A characterization in childhood inflammatory bowel disease, a new population-based inception cohort from South-Eastern Norway, 2005–2007, showing increased incidence in Crohn’s disease. Scand. J. Gastroenterol. 2009, 44, 446–456. [Google Scholar] [CrossRef]
- Olbjorn, C.; Cvancarova Smastuen, M.; Thiis-Evensen, E.; Nakstad, B.; Vatn, M.H.; Perminow, G. Serological markers in diagnosis of pediatric inflammatory bowel disease and as predictors for early tumor necrosis factor blocker therapy. Scand. J. Gastroenterol. 2017, 52, 414–419. [Google Scholar] [CrossRef][Green Version]
- Olbjorn, C.; Cvancarova Smastuen, M.; Thiis-Evensen, E.; Nakstad, B.; Vatn, M.H.; Jahnsen, J.; Ricanek, P.; Vatn, S.; Moen, A.E.F.; Tannaes, T.M.; et al. Fecal microbiota profiles in treatment-naive pediatric inflammatory bowel disease—Associations with disease phenotype, treatment, and outcome. Clin. Exp. Gastroenterol. 2019, 12, 37–49. [Google Scholar] [CrossRef]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Casen, C.; Vebo, H.C.; Sekelja, M.; Hegge, F.T.; Karlsson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Froyland, C.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef]
- Farup, P.G.; Maseng, M.G. Are Faecal Microbiota Analyses on Species-Level Suitable Clinical Biomarkers? A Pilot Study in Subjects with Morbid Obesity. Microorganisms 2021, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Duplaga, K.; Gosiewski, T.; Kapusta, P.; Sroka-Oleksiak, A.; Wędrychowicz, A.; Pieczarkowski, S.; Ludwig-Słomczyńska, A.H.; Wołkow, P.P.; Fyderek, K. Differences in the intestinal microbiome of healthy children and patients with newly diagnosed Crohn’s disease. Sci. Rep. 2019, 9, 18880. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, M. Special issues in pediatric inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 413–420. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Putignani, L.; Oliva, S.; Isoldi, S.; Del Chierico, F.; Carissimi, C.; Laudadio, I.; Cucchiara, S.; Stronati, L. Fecal and mucosal microbiota profiling in pediatric inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, A.; Zorzi, F.; Del Chierico, F.; Altomare, A.; Cocca, S.; Avola, A.; De Biasio, F.; Russo, A.; Cella, E.; Reddel, S.; et al. Fecal and Mucosal Microbiota Profiling in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Front. Microbiol. 2019, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- De Meij, T.G.J.; de Groot, E.F.J.; Peeters, C.F.W.; de Boer, N.K.H.; Kneepkens, C.M.F.; Eck, A.; Benninga, M.A.; Savelkoul, P.H.M.; van Bodegraven, A.A.; Budding, A.E. Variability of core microbiota in newly diagnosed treatment-naive paediatric inflammatory bowel disease patients. PLoS ONE 2018, 13, e0197649. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T.Y. Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. Int. J. Mol. Sci. 2021, 22, 8070. [Google Scholar] [CrossRef]
- Duranti, S.; Gaiani, F.; Mancabelli, L.; Milani, C.; Grandi, A.; Bolchi, A.; Santoni, A.; Lugli, G.A.; Ferrario, C.; Mangifesta, M.; et al. Elucidating the gut microbiome of ulcerative colitis: Bifidobacteria as novel microbial biomarkers. FEMS Microbiol. Ecol. 2016, 92, fiw191. [Google Scholar] [CrossRef]
- Maukonen, J.; Kolho, K.L.; Paasela, M.; Honkanen, J.; Klemetti, P.; Vaarala, O.; Saarela, M. Altered Fecal Microbiota in Paediatric Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 9, 1088–1095. [Google Scholar] [CrossRef]
- Fitzgerald, R.S.; Sanderson, I.R.; Claesson, M.J. Paediatric Inflammatory Bowel Disease and its Relationship with the Microbiome. Microb. Ecol. 2021, 82, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients with vs without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.E931. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- El Mouzan, M.I.; Winter, H.S.; Assiri, A.A.; Korolev, K.S.; Al Sarkhy, A.A.; Dowd, S.E.; Al Mofarreh, M.A.; Menon, R. Microbiota profile in new-onset pediatric Crohn’s disease: Data from a non-Western population. Gut Pathog. 2018, 10, 49. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Rocha, C.; Correia, L.; Lago, P.; Ministro, P.; Portela, F.; Trindade, E.; Afonso, J.; Peyrin-Biroulet, L.; Magro, F. Features of Fecal and Colon Microbiomes Associate with Responses to Biologic Therapies for Inflammatory Bowel Diseases: A Systematic Review. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 1054–1069. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Luo, C.; Yajnik, V.; Khalili, H.; Garber, J.J.; Stevens, B.W.; Cleland, T.; Xavier, R.J. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe 2017, 21, 603–610.E603. [Google Scholar] [CrossRef]
- Kolho, K.L.; Korpela, K.; Jaakkola, T.; Pichai, M.V.; Zoetendal, E.G.; Salonen, A.; de Vos, W.M. Fecal Microbiota in Pediatric Inflammatory Bowel Disease and Its Relation to Inflammation. Am. J. Gastroenterol. 2015, 110, 921–930. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Franzosa, E.A.; Lloyd-Price, J.; McIver, L.J.; Schwager, R.; Poon, T.W.; Ananthakrishnan, A.N.; Andrews, E.; Barron, G.; Lake, K.; et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat. Microbiol. 2018, 3, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, 8914. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Jones, C.M.A.; Connors, J.; Dunn, K.A.; Bielawski, J.P.; Comeau, A.M.; Langille, M.G.I.; Van Limbergen, J. Bacterial Taxa and Functions Are Predictive of Sustained Remission Following Exclusive Enteral Nutrition in Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2020, 26, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Zahraee, M.; Ye, Z.; Xi, L.; Baniecki, M.L.; Li, X.; Hyde, C.L.; Zhang, J.; Raha, N.; Karlsson, F.; Quan, J.; et al. Antitumor Necrosis Factor-like Ligand 1A Therapy Targets Tissue Inflammation and Fibrosis Pathways and Reduces Gut Pathobionts in Ulcerative Colitis. Inflamm. Bowel Dis. 2022, 28, 434–446. [Google Scholar] [CrossRef]
- Schirmer, M.; Denson, L.; Vlamakis, H.; Franzosa, E.A.; Thomas, S.; Gotman, N.M.; Rufo, P.; Baker, S.S.; Sauer, C.; Markowitz, J.; et al. Compositional and Temporal Changes in the Gut Microbiome of Pediatric Ulcerative Colitis Patients Are Linked to Disease Course. Cell Host Microbe 2018, 24, 600–610.e604. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Cook, G.W.; Benton, M.G.; Akerley, W.; Mayhew, G.F.; Moehlenkamp, C.; Raterman, D.; Burgess, D.L.; Rowell, W.J.; Lambert, C.; Eng, K.; et al. Structural variation and its potential impact on genome instability: Novel discoveries in the EGFR landscape by long-read sequencing. PLoS ONE 2020, 15, e0226340. [Google Scholar] [CrossRef]

| Demographics | CD | UC | Non-IBD | Healthy |
|---|---|---|---|---|
| Patients, n (%) | 80 (100) | 27 (100) | 50 (100) | 75 (100) |
| Age, median (range) | 13 (0.74–17.9) | 11.5 (4–17) | 12 (3.7–18) | 10 (2–17.9) |
| Sex (male), n (%) | 43 (54) | 11 (41) | 18 (36) | 34 (45) |
| Fecal calprotectin > 1000 mg/kg, n (%) | 31 (39) | 12 (48) | 2 (4) | 0 |
| Bacteria | IBD vs. Healthy, (p-Value) a | Non-IBD vs. Healthy, (p-Value) a | IBD vs. Non-IBD, (p-Value) a |
|---|---|---|---|
| Actinomycetota | |||
| Paraprevotella clara | ↓ 0.025 | ||
| Bifidobacterium adolescentis | ↓ 0.003 | ||
| Bifidobacterium angulatum | ↓ 0.003 | ↓ 0.032 | ↓ 0.004 |
| Bifidobacterium bifidum | ↓ 0.009 | ↓ 0.004 | |
| Bifidobacterium catenulatum | ↓ 0.000 | ||
| Bifidobacterium longum | ↓ 0.022 | ||
| Bifidobacterium pseudocatenulatum | ↓ 0.004 | ||
| Bacillota | |||
| Christensenella minuta | ↓ 0.000 | ↓ 0.018 | |
| Clostridium leptum | ↓ 0.000 | ↓ 0.025 | |
| Clostridium scindens | ↓ 0.042 | ||
| Coprococcus comes | ↓ 0.042 | ||
| Desulfovibrio piger | ↓ 0.027 | ||
| Eubacterium eligens | ↓ 0.000 | ↓ 0.000 | |
| Eubacterium rectale | ↓ 0.001 | ↓ 0.016 | |
| Eubacterium siraeum | ↓ 0.009 | ↓ 0.045 | |
| Eubacterium ventriosum | ↓ 0.000 | ↓ 0.001 | |
| Lactobacillus acidophilus | ↓ 0.038 | ↓ 0.009 | |
| Lacticaseibacillus paracasei | ↓ 0.001 | ↓ 0.031 | |
| Methano smithii | ↓ 0.007 | ||
| Parapravotella clara | ↓ 0.003 | ↓ 0.025 | |
| Roseburia intestinalis | ↓ 0.009 | ↓ 0.002 | |
| Roseburia inulinivorans | ↓ 0.000 | ↓ 0.005 | |
| Roseburia hominis | ↓ 0.001 | ↓ 0.034 | |
| Ruminococcus bromii | ↓ 0.000 | ↓ 0.014 | ↓ 0.005 |
| Streptococcus sanguinis | ↓ 0.031 | ||
| Streptococcus thermophilus | ↓ 0.000 | ↓ 0.013 | |
| Bacteroidota | |||
| Alistipes finegoldii | ↓ 0.002 | ↓ 0.002 | |
| Alistipes shahii | ↓ 0.006 | ↓ 0.006 | |
| Alistipes onkerdonkii | ↓ 0.001 | ||
| Anaerostipes hadrus | ↓ 0.001 | ||
| Bacteroides caccae | ↓ 0.032 | ↓ 0.032 | |
| Bacteroides dorei | ↓ 0.038 | ↓ 0.043 | |
| Bacteroides plebeius | ↓ 0.028 | ||
| Bacteroides stercoris | ↓ 0.038 | ||
| Barnesiella intestinihominis | ↓ 0.004 | ||
| Firmicutes | |||
| Intestinibacter bartlettii | ↓ 0.017 | ||
| Enterococcus faecium | ↑ 0.05 | ||
| Citrobacter koseri | ↑ 0.05 | ||
| Pseudomonadota | |||
| Haemophilus parainfluenzae | ↑ 0.009 | ||
| Verrucomicrobiota | |||
| Akkermansia muciniphila | ↓ 0.000 | ↓ 0.032 | |
| Bacteria | Biological vs. Conventional Therapy, (p-Value) a | Calprotectin Levels > 1000 mg/kg vs. < 1000 mg/kg, (p-Value) a |
|---|---|---|
| Actinomycetota | ||
| Bifidobacterium adolescentis | ↓ (0.032) | |
| Bifidobacterium bifidum | ↓ (0.044) | |
| Bacillota | ||
| Eubacterium rectale | ↓ (0.018) | |
| Roseburia hominis | ↓ (0.012) | ↓ (0.000) |
| Roseburia inulinivorans | ↓ (0.011) | ↓ (0.028) |
| Roseburia intestinales | ↓ (0.001) | |
| Ruminococcus gnavus | ↑ (0.029) | |
| Ruminococcus bromii | ↓ (0.040) | |
| Bacteroidota | ||
| Alistipes finegoldii | ↓ (0.006) | |
| Bacteroides finegoldii | ↓ (0.016) | |
| Bacteroides intestinalis | ↓ (0.039) | |
| Bacteroides vulgatus | ↑ (0.017) | |
| Bacteroides cellulosilytius | ↓ (0.035) | |
| Barnesiella intestinihominis | ↓ (0.028) | |
| Paraprevotella clara | ↓ (0.017) |
| Bacteria | Diagnostic Index 1 a,b | Diagnostic Index 2 a,b | Diagnostic Index 3 a,b | Phenotype Index a,b | Prognostic Index a,b |
|---|---|---|---|---|---|
| Actinomycetota | |||||
| Bifidobacterium adolescentis | 1*x | 2*x | 3*x | ||
| Bifidobacterium angulatum | 1*x | 1*x | 1*x | ||
| Bifidobacterium bifidum | 1*x | 3*x | 1*x | 1*x | |
| Bacillota | |||||
| Clostridium leptum | 1*x | 1*x | |||
| Coprococcus comes | 1*x | 1*x | |||
| Eubacterium eligens | 1*x | 1*x | 1*x | ||
| Eubacterium rectale | 2*x | 1*x | |||
| Eubacterium ventriosum | 1*x | 1*x | 1*x | ||
| Parapravotella clara | 1*x | 1*x | |||
| Roseburia intestinalis | 1*x | 1*x | 1*x | ||
| Roseburia inulinivorans | 1*x | 1*x | |||
| Roseburia hominis | 2*x | 1*x | 1*x | ||
| Ruminococcus bromii | 1*x | 1*x | |||
| Bacteroidota | |||||
| Alistipes onkerdonkii | 1*x | 3*x | |||
| Alistipes shahii | 1*x | 1*x | 1*x | ||
| Alistipes finegoldii | 1*x | 1*x | 1*x | ||
| Anaerostipes hadrus | 1*x | 1*x | |||
| Verrucomicrobiota | |||||
| Akkermansia muciniphila | 2*x | 1*x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olbjørn, C.; Småstuen, M.C.; Moen, A.E.F. Targeted Analysis of the Gut Microbiome for Diagnosis, Prognosis and Treatment Individualization in Pediatric Inflammatory Bowel Disease. Microorganisms 2022, 10, 1273. https://doi.org/10.3390/microorganisms10071273
Olbjørn C, Småstuen MC, Moen AEF. Targeted Analysis of the Gut Microbiome for Diagnosis, Prognosis and Treatment Individualization in Pediatric Inflammatory Bowel Disease. Microorganisms. 2022; 10(7):1273. https://doi.org/10.3390/microorganisms10071273
Chicago/Turabian StyleOlbjørn, Christine, Milada Cvancarova Småstuen, and Aina Elisabeth Fossum Moen. 2022. "Targeted Analysis of the Gut Microbiome for Diagnosis, Prognosis and Treatment Individualization in Pediatric Inflammatory Bowel Disease" Microorganisms 10, no. 7: 1273. https://doi.org/10.3390/microorganisms10071273
APA StyleOlbjørn, C., Småstuen, M. C., & Moen, A. E. F. (2022). Targeted Analysis of the Gut Microbiome for Diagnosis, Prognosis and Treatment Individualization in Pediatric Inflammatory Bowel Disease. Microorganisms, 10(7), 1273. https://doi.org/10.3390/microorganisms10071273





