Antiparasitic Drugs against SARS-CoV-2: A Comprehensive Literature Survey
Abstract
1. Introduction
2. Antimalarial Drugs
2.1. The Controversial Journey of 4-Aminoquinolines: Chloroquine (CQ) and Hydroxychloroquine (HCQ)
2.1.1. CQ and HCQ against SARS-CoV-2: In Silico and In Vitro Studies
2.1.2. Preclinical In Vivo Use of CQ and HCQ
2.1.3. CQ and HCQ in Clinical Trials
2.2. Quinine and other Aryl-Aminoalcohols: In Vitro and In Vivo Studies
2.3. Artemisinin and Its Derivatives
2.3.1. Artemisinin: In Vitro Studies
2.3.2. Artemisinin: Clinical Trials
2.3.3. Artemisinin Derivatives: Artefenomel (OZ439), Artemether, Artemisone, Artenimol (Dihydroartemisinin, DHA), AS
2.3.4. AS: Clinical Trials
2.3.5. Artemisinin and Its Derivatives in Combination with Other Molecules: In Vitro Studies
2.3.6. Artemisinin and Its Derivatives in Combination with Other Molecules: Clinical Trials
2.4. Other Antimalarial Drugs: Atovaquone
3. Other Antiprotozoan Drugs: K777
4. Anthelmintic Drugs against SARS-CoV-2
4.1. Niclosamide
4.1.1. Niclosamide: In Vitro and In Vivo Studies
4.1.2. Niclosamide: Clinical Trials
4.2. Nitazoxanide
4.3. Ivermectin
4.3.1. Ivermectin: In Silico and In Vitro Studies
4.3.2. Ivermectin: Preclinical Studies
4.3.3. Ivermectin: Retrospective, Observational Studies, and Clinical Trials
IVM Used as Monotherapy
IVM Treatment in Combination with Other Drugs
Prophylactic Use of IVM
Ivermectin: Summary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The Molecular Virology of Coronaviruses. J. Biol. Chem. 2020, 295, 12910. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 Genome, Structure, Evolution, Pathogenesis and Therapies: Structural Genomics Approach. Biochim. Biophys. Acta-Mol. Basis Disease 2020, 1866. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-Converting Enzyme 2 Is a Functional Receptor For the SARS Coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.; Masters, P.S. Functional Analysis of the Murine Coronavirus Genomic RNA Packaging Signal. J. Virol. 2013, 87, 5182–5192. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical Characteristics of 140 Patients Infected with SARS-CoV-2 in Wuhan, China. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef]
- Ge, H.; Wang, X.; Yuan, X.; Xiao, G.; Wang, C.; Deng, T.; Yuan, Q.; Xiao, X. The Epidemiology and Clinical Information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1011–1019. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The Cytokine Storm in COVID-19: An Overview of the Involvement of the Chemokine/Chemokine-Receptor System. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 7898, 2255–2273. [Google Scholar] [CrossRef]
- Wang, X.; Sacramento, C.Q.; Jockusch, S.; Chaves, O.A.; Tao, C.; Fintelman-Rodrigues, N.; Chien, M.; Temerozo, J.R.; Li, X.; Kumar, S.; et al. Combination of Antiviral Drugs Inhibits SARS-CoV-2 Polymerase and Exonuclease and Demonstrates COVID-19 Therapeutic Potential in Viral Cell Culture. Commun. Biol. 2022, 5, 154. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Takemae, H.; Sharma, I.; Furuya, T. Multipurpose Drugs Active Against Both Plasmodium Spp. and Microorganisms: Potential Application for New Drug Development. Front. Cell. Infect. Microbiol. 2021, 11, 797509. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Jane Escott, K.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Song, X.; Ma, T.; Pan, X.; Zhou, Y.; Hou, Y.; Zhang, Z.; Li, K.; Karypis, G.; Cheng, F. Repurpose Open Data to Discover Therapeutics for COVID-19 Using Deep Learning. J. Proteome Res. 2020, 19, 4624–4636. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Scaccabarozzi, D.; Signorini, L.; Perego, F.; Ilboudo, D.P.; Ferrante, P.; Delbue, S. The Use of Antimalarial Drugs against Viral Infection. Microorganisms 2020, 8, 85. [Google Scholar] [CrossRef]
- Dagen, M. History of Malaria and Its Treatment. Antimalarial Agents 2020, 1–48. [Google Scholar] [CrossRef]
- Christensen, S.B. Natural Products That Changed Society. Biomedicines 2021, 9, 472. [Google Scholar] [CrossRef]
- White, N.J. The Treatment of Malaria. N. Engl. J. Med. 1996, 335, 800–806. [Google Scholar] [CrossRef]
- McChesney, E.W. Animal Toxicity and Pharmacokinetics of Hydroxychloroquine Sulfate. Am. J. Med. 1983, 75, 11–18. [Google Scholar] [CrossRef]
- Al-Bari, M.A. Chloroquine Analogues in Drug Discovery: New Directions of Uses, Mechanisms of Actions and Toxic Manifestations from Malaria to Multifarious Diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef]
- Plantone, D.; Koudriavtseva, T. Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review. Clin. Drug Investig. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Landais, C.; Fenollar, F.; Thuny, F.; Raoult, D. From Acute Q Fever to Endocarditis: Serological Follow-Up Strategy. Clin. Infect. Dis. 2007, 44, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Emonet, S.; Wuillemin, T.; Harbarth, S.; Wassilew, N.; Cikirikcioglu, M.; Schrenzel, J.; Lagier, J.C.; Raoult, D.; van Delden, C. Relapse of Tropheryma Whipplei Endocarditis Treated by Trimethoprim/Sulfamethoxazole, Cured by Hydroxychloroquine plus Doxycycline. Int. J. Infect. Dis. 2015, 30, 17–19. [Google Scholar] [CrossRef]
- Taramelli, D.; Tognazioli, C.; Ravagnani, F.; Leopardi, O.; Giannulis, G.; Boelaert, J.R. Inhibition of Intramacrophage Growth of Penicillium Marneffei by 4-Aminoquinolines. Antimicrob. Agents Chemother. 2001, 45, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Henriet, S.S.; Jans, J.; Simonetti, E.; Kwon-Chung, K.J.; Rijs, A.J.; Hermans, P.W.; Holland, S.M.; de Jonge, M.I.; Warris, A. Chloroquine Modulates the Fungal Immune Response in Phagocytic Cells From Patients With Chronic Granulomatous Disease. J. Infect. Dis. 2013, 207, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Dias-Melicio, L.A.; Moreira, A.P.; Calvi, S.A.; de Campos Soares, A.M.V. Chloroquine Inhibits Paracoccidioides Brasiliensis Survival within Human Monocytes by Limiting the Availability of Intracellular Iron. Microbiol. Immunol. 2006, 50, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, M.A.A. Targeting Endosomal Acidification by Chloroquine Analogs as a Promising Strategy for the Treatment of Emerging Viral Diseases. Pharma Res. Per. 2017, 5, 293. [Google Scholar] [CrossRef]
- Daecke, J.; Fackler, O.T.; Dittmar, M.T.; Kräusslich, H.-G. Involvement of Clathrin-Mediated Endocytosis in Human Immunodeficiency Virus Type 1 Entry. J. Virol. 2005, 79, 1581–1594. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Piette, J.; Sperber, K. The Potential Place of Chloroquine in the Treatment of HIV-1-Infected Patients. J. Clin. Virol. 2001, 20, 137–140. [Google Scholar] [CrossRef]
- Baize, S.; Leroy, E.M.; Georges, A.J.; Georges-Courbot, M.-C.; Capron, M.; Bedjabaga, I.; Lansoud-Soukate, J.; Mavoungou, E. Inflammatory Responses in Ebola Virus-Infected Patients. Clin. Exp. Immunol. 2002, 128, 163–168. [Google Scholar] [CrossRef]
- Murray, S.M.; Down, C.M.; Boulware, D.R.; Stauffer, W.M.; Cavert, W.P.; Schacker, T.W.; Brenchley, J.M.; Douek, D.C. Reduction of Immune Activation with Chloroquine Therapy during Chronic HIV Infection. J. Virol. 2010, 84, 12082–12086. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, F.; Smith, K.M.; Hoven, A.D. Chloroquine and Hydroxychloroquine as Inhibitors of Human Immunodeficiency Virus (HIV-1) Activity. Curr. Pharm. Des. 2004, 10, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Juvenal, K.; Farias, S.; Renata, P.; Machado, L.; Pereira, J.A.; Muniz, C.; Amaral Imbeloni, A.; Antô, B.; Lopes Da Fonseca, N. Antiviral Activity of Chloroquine Against Dengue Virus Type 2 Replication in Aotus Monkeys. Viral Immunol. 2015, 28, 161–169. [Google Scholar] [CrossRef]
- Mizui, T.; Shunhei, A.E.; Ae, Y.; Tanida, I.; Yoshiyuki, A.E.; Ae, T.; Ae, T.U.; Sakamoto, N.; Kenichi, A.E.; Ae, I.; et al. Inhibition of Hepatitis C Virus Replication by Chloroquine Targeting Virus-Associated Autophagy. J. Gastroenterol. 2010, 45, 195–203. [Google Scholar] [CrossRef]
- Loo, Y.; Xu, Y.; Cheung, Y.B.; Ooi, E.E.; Paton, N.I.; Lee, L.; Xu, Y.; Eong Ooi, E.; Bun Cheung, Y.; Archuleta, S.; et al. Chloroquine for Influenza Prevention: A Randomised, Double-Blind, Placebo Controlled Trial. Lancet Infect. Dis. 2011, 11, 677–683. [Google Scholar] [CrossRef]
- Jiang, K.; Li, Y.; Zhu, Q.; Xu, J.; Wang, Y.; Deng, W.; Liu, Q.; Zhang, G.; Meng, S. Pharmacological Modulation of Autophagy Enhances Newcastle Disease Virus-Mediated Oncolysis in Drug-Resistant Lung Cancer Cells. BMC Cancer 2014, 14, 551. [Google Scholar] [CrossRef]
- Roques, P.; Thiberville, S.-D.; Dupuis-Maguiraga, L.; Lum, F.-M.; Labadie, K.; Martinon, F.; Gras, G.; Lebon, P.; P Ng, L.F.; de Lamballerie, X.; et al. Paradoxical Effect of Chloroquine Treatment in Enhancing Chikungunya Virus Infection. Viruses 2018, 10, 268. [Google Scholar] [CrossRef]
- Long, J.; Wright, E.; Molesti, E.; Temperton, N.; Barclay, W. Antiviral Therapies against Ebola and Other Emerging Viral Diseases Using Existing Medicines That Block Virus Entry. F1000Res 2015. [Google Scholar] [CrossRef]
- Falzarano, D.; Safronetz, D.; Prescott, J.; Marzi, A.; Feldmann, F.; Feldmann, H. Lack of Protection Against Ebola Virus from Chloroquine in Mice and Hamsters. Emerg. Infect. Dis. 2015, 21, 1065. [Google Scholar] [CrossRef]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine Is a Potent Inhibitor of SARS Coronavirus Infection and Spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef]
- De Wilde, A.H.; Jochmans, D.; Posthuma, C.C.; Zevenhoven-Dobbe, J.C.; van Nieuwkoop, S.; Bestebroer, T.M.; van den Hoogen, B.G.; Neyts, J.; Snijder, E.J. Screening of an FDA-Approved Compound Library Identifies Four Small-Molecule Inhibitors of Middle East Respiratory Syndrome Coronavirus Replication in Cell Culture. Antimicrob. Agents Chemother. 2014, 58, 4875. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-NCoV) in Vitro. Cell Res. 2020, 30, 269. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, Is Effective in Inhibiting SARS-CoV-2 Infection in Vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Hoffmann, M.; Mösbauer, K.; Hofmann-Winkler, H.; Kaul, A.; Kleine-Weber, H.; Krüger, N.; Gassen, N.C.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Chloroquine Does Not Inhibit Infection of Human Lung Cells with SARS-CoV-2. Nature 2020, 585, 588. [Google Scholar] [CrossRef]
- Schlesinger, P.H.; Krogstad, D.J.; Herwaldt, B.L. Antimalarial Agents: Mechanisms of Action. Antimicrob. Agents Chemother. 1988, 32, 793. [Google Scholar] [CrossRef]
- Di Trani, L.; Savarino, A.; Campitelli, L.; Norelli, S.; Puzelli, S.; Vignolo, E.; Donatelli, I.; Cassone, A. Different PH Requirements Are Associated with Divergent Inhibitory Effects of Chloroquine on Human and Avian Influenza A Viruses. Virol. J. 2007, 4, 39. [Google Scholar] [CrossRef]
- Shang, C.; Zhuang, X.; Zhang, H.; Li, Y.; Zhu, Y.; Lu, J.; Ge, C.; Cong, J.; Li, T.; Tian, M.; et al. Inhibitors of Endosomal Acidification Suppress SARS-CoV-2 Replication and Relieve Viral Pneumonia in HACE2 Transgenic Mice. Virol. J. 2021, 18, 46. [Google Scholar] [CrossRef]
- Prabhakara, C.; Godbole, R.; Sil, P.; Jahnavi, S.; Gulzar, S.J.; van Zanten, T.S.; Sheth, D.; Subhash, N.; Chandra, A.; Shivaraj, A.; et al. Strategies to Target SARS-CoV-2 Entry and Infection Using Dual Mechanisms of Inhibition by Acidification Inhibitors. PLoS Pathog. 2021, 17, e1009706. [Google Scholar] [CrossRef] [PubMed]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 Infects Cells after Viral Entry via Clathrin-Mediated Endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Mathiasen, S.; Bright, N.A.; Pierre, F.; Kelly, B.T.; Kladt, N.; Schauss, A.; Merrifield, C.J.; Stamou, D.; Höning, S.; et al. CALM Regulates Clathrin-Coated Vesicle Size and Maturation by Directly Sensing and Driving Membrane Curvature. Dev. Cell 2015, 33, 163. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.Y.; Frieman, M.; Wolfram, J. Insights from Nanomedicine into Chloroquine Efficacy against COVID-19. Nat. Nanotechnol. 2020, 15, 247. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.; Bertram, S.; Glowacka, I.; Steffen, I.; Chaipan, C.; Agudelo, J.; Lu, K.; Rennekamp, A.J.; Hofmann, H.; Bates, P.; et al. Different Host Cell Proteases Activate the SARS-Coronavirus Spike-Protein for Cell-Cell and Virus-Cell Fusion. Virology 2011, 413, 265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-M.; Yang, W.-L.; Yang, F.-Y.; Zhang, L.; Huang, W.-J.; Hou, W.; Fan, C.-F.; Jin, R.-H.; Feng, Y.-M.; Wang, Y.-C.; et al. Cathepsin L Plays a Key Role in SARS-CoV-2 Infection in Humans and Humanized Mice and Is a Promising Target for New Drug Development. Signal Transduct. Target. 2021, 6, 134. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE. Science 2020, 367, 1444. [Google Scholar] [CrossRef]
- Braz, H.L.B.; de Moraes Silveira, J.A.; Marinho, A.D.; de Moraes, M.E.A.; de Moraes Filho, M.O.; Monteiro, H.S.A.; Jorge, R.J.B. In Silico Study of Azithromycin, Chloroquine and Hydroxychloroquine and Their Potential Mechanisms of Action against SARS-CoV-2 Infection. Int. J. Antimicrob. Agents 2020, 56, 106119. [Google Scholar] [CrossRef]
- Amin, M.; Abbas, G. Docking Study of Chloroquine and Hydroxychloroquine Interaction with RNA Binding Domain of Nucleocapsid Phospho-Protein-an in Silico Insight into the Comparative Efficacy of Repurposing Antiviral Drugs. J. Biomol. Struct. Dyn. 2021, 39, 4243. [Google Scholar] [CrossRef]
- Matrosovich, M.; Herrler, G.; Klenk, H.D. Sialic Acid Receptors of Viruses. Top. Curr. Chem. 2015, 367, 1–28. [Google Scholar] [CrossRef]
- Fantini, J.; di Scala, C.; Chahinian, H.; Yahi, N. Structural and Molecular Modelling Studies Reveal a New Mechanism of Action of Chloroquine and Hydroxychloroquine against SARS-CoV-2 Infection. Int. J. Antimicrob. Agents 2020, 55, 105960. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Bai, Y.; Raha, A.; Su, Z.; Geng, F. Integrative In Silico Investigation Reveals the Host-Virus Interactions in Repurposed Drugs Against SARS-CoV-2. Front. Bioinform. 2022, 1. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. SARS-CoV-2 Takes Its Toll. Nat. Immunol. 2021, 22, 801. [Google Scholar] [CrossRef] [PubMed]
- Navya, V.B.; Hosur, M.V. A Computational Study on Hydroxychloroquine Binding to Target Proteins Related to SARS-COV-2 Infection. Inform. Med. Unlocked 2021, 26, 100714. [Google Scholar] [CrossRef]
- Doharey, P.K.; Singh, V.; Gedda, M.R.; Sahoo, A.K.; Varadwaj, P.K.; Sharma, B. In Silico Study Indicates Antimalarials as Direct Inhibitors of SARS-CoV-2-RNA Dependent RNA Polymerase. J. Biomol. Struct. Dyn. 2021, 1–18. [Google Scholar] [CrossRef]
- Lenzer, J. COVID-19: US Gives Emergency Approval to Hydroxychloroquine despite Lack of Evidence. BMJ 2020, m1335. [Google Scholar] [CrossRef]
- Park, S.-J.; Yu, K.-M.; Kim, Y.-I.; Kim, S.-M.; Kim, E.-H.; Kim, S.-G.; Kim, E.J.; Casel, M.A.B.; Rollon, R.; Jang, S.-G.; et al. Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets. mBio 2020, 11, 3. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Kim, S.-G.; Kim, S.-M.; Kim, E.-H.; Park, S.-J.; Yu, K.-M.; Chang, J.-H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709.e2. [Google Scholar] [CrossRef]
- Maisonnasse, P.; Guedj, J.; Contreras, V.; Behillil, S.; Solas, C.; Marlin, R.; Naninck, T.; Pizzorno, A.; Lemaitre, J.; Gonçalves, A.; et al. Hydroxychloroquine Use against SARS-CoV-2 Infection in Non-Human Primates. Nature 2020, 585, 584. [Google Scholar] [CrossRef]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, N.M.A.; et al. Comparative Pathogenesis of COVID-19, MERS, and SARS in a Nonhuman Primate Model. Science 2020, 368, 1012. [Google Scholar] [CrossRef]
- Rosenke, K.; Jarvis, M.A.; Feldmann, F.; Schwarz, B.; Okumura, A.; Lovaglio, J.; Saturday, G.; Hanley, P.W.; Meade-White, K.; Williamson, B.N.; et al. Hydroxychloroquine Prophylaxis and Treatment Is Ineffective in Macaque and Hamster SARS-CoV-2 Disease Models. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, S.J.F.; Jacobs, S.; Langendries, L.; Seldeslachts, L.; ter Horst, S.; Liesenborghs, L.; Hens, B.; Vergote, V.; Heylen, E.; Barthelemy, K.; et al. Favipiravir at High Doses Has Potent Antiviral Activity in SARS-CoV-2-Infected Hamsters, Whereas Hydroxychloroquine Lacks Activity. Proc. Natl. Acad. Sci. USA 2020, 117, 26955. [Google Scholar] [CrossRef] [PubMed]
- Cochin, M.; Touret, F.; Driouich, J.-S.; Moureau, G.; Petit, P.-R.; Laprie, C.; Solas, C.; de Lamballerie, X.; Nougairède, A. Hydroxychloroquine and Azithromycin Used Alone or Combined Are Not Effective against SARS-CoV-2 Ex Vivo and in a Hamster Model. Antivir. Res. 2022, 197, 105212. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, Y.; Qi, F.; et al. The Pathogenicity of SARS-CoV-2 in HACE2 Transgenic Mice. Nature 2020, 583. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine Phosphate Has Shown Apparent Efficacy in Treatment of COVID-19 Associated Pneumonia in Clinical Studies. BioSci. Trends 2020, 14, 72. [Google Scholar] [CrossRef]
- Borba, M.G.S.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Brito, M.; Mourao, M.P.G.; Brito-Sousa, J.D.; Baia-da-Silva, D.; Guerra, M.V.F.; et al. Effect of High vs. Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e208857. [Google Scholar] [CrossRef]
- Medical Association, A. Letters Internet Searches for Unproven COVID-19 Therapies in the United States. JAMA Intern. Med. 2020, 180, 1116. [Google Scholar] [CrossRef]
- Horby, P.; Mafham, M.; Linsell, L.; Phil, D.; Bell, J.L.; Staplin, N.; Emberson, J.-T.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020, 21, 2030. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Results of an Open-Label Non-Randomized Clinical Trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Boulware, D.R.; Pullen, M.F.; Bangdiwala, A.S.; Pastick, K.A.; Lofgren, S.M.; Okafor, E.C.; Skipper, C.P.; Nascene, A.A.; Nicol, M.R.; Abassi, M.; et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for COVID-19. N. Engl. J. Med. 2020, 383, 517. [Google Scholar] [CrossRef]
- Mitjà, O.; Corbacho-Monné, M.; Ubals, M.; Alemany, A.; Suñer, C.; Tebé, C.; Tobias, A.; Peñafiel, J.; Ballana, E.; Pérez, C.A.; et al. A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of COVID-19. N. Engl. J. Med. 2021, 384, 417. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Roman, Y.M.; Pasupuleti, V.; Barboza, J.J.; White, C.M. Hydroxychloroquine or Chloroquine for Treatment or Prophylaxis of COVID-19. Ann. Intern. Med. 2020, 173, 287. [Google Scholar] [CrossRef]
- Ip, A.; Ahn, J.; Zhou, Y.; Goy, A.H.; Hansen, E.; Pecora, A.L.; Sinclaire, B.A.; Bednarz, U.; Marafelias, M.; Sawczuk, I.S.; et al. Hydroxychloroquine in the Treatment of Outpatients with Mildly Symptomatic COVID-19: A Multi-Center Observational Study. BMC Infect. Dis 2021, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Peto, R.; Henao-Restrepo, A.; Preziosi, M.; Sathi-yamoorthy, V.; Abdool Karim, Q.; Ale-jandria, M.; Hernández García, C.; Kie-ny, M.; Malekzadeh, R.; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Brown, E.R.; Stewart, J.; Karita, H.C.S.; Kissinger, P.J.; Dwyer, J.; Hosek, S.; Oyedele, T.; Paasche-Orlow, M.K.; Paolino, K.; et al. Hydroxychloroquine with or without Azithromycin for Treatment of Early SARS-CoV-2 Infection among High-Risk Outpatient Adults: A Randomized Clinical Trial. EClinicalMedicine 2021, 33, 100773. [Google Scholar] [CrossRef]
- Reis, G.; dos Santos Moreira Silva, E.A.; Medeiros Silva, D.C.; Thabane, L.; Singh, G.; Park, J.J.H.; Forrest, J.I.; Harari, O.; Quirino Dos Santos, C.V.; Guimarães de Almeida, A.P.F.; et al. Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19. The TOGETHER Randomized Clinical Trial. JAMA Netw. Open 2021. [Google Scholar] [CrossRef]
- Rodrigues, C.; Freitas-Santos, R.S.; Levi, J.E.; Senerchia, A.A.; Lopes, A.T.A.; Santos, S.R.; Siciliano, R.F.; Pierrotti, L.C. Hydroxychloroquine plus Azithromycin Early Treatment of Mild COVID-19 in an Outpatient Setting: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial Evaluating Viral Clearance. Int. J. Antimicrob. Agent 2021, 58, 106428. [Google Scholar] [CrossRef]
- Dubée, V.; Roy, P.-M.; Vielle, B.; Parot-Schinkel, E.; Blanchet, O.; Darsonval, A.; Lefeuvre, C.; Abbara, C.; Boucher, S.; Devaud, E.; et al. Hydroxychloroquine in Mild-to-Moderate Coronavirus Disease 2019: A Placebo-Controlled Double Blind Trial. Clin. Microbiol. Infect. 2021, 27, 1124. [Google Scholar] [CrossRef]
- Avezum, Á.; Oliveira, G.B.F.; Oliveira, H.; Lucchetta, R.C.; Pereira, V.F.A.; Dabarian, A.L.; D O Vieira, R.; Silva, D.V.; Kormann, A.P.M.; Tognon, A.P.; et al. Hydroxychloroquine versus Placebo in the Treatment of Non-Hospitalised Patients with COVID-19 (COPE-Coalition V): A Double-Blind, Multicentre, Randomised, Controlled Trial. Lancet Reg. Health Am. 2022, 11, 100243. [Google Scholar] [CrossRef]
- Braga, C.B.; Martins, A.C.; Cayotopa, A.D.E.; Klein, W.W.; Schlosser, A.R.; da Silva, A.F.; de Souza, M.N.; Andrade, B.W.B.; Filgueira-Júnior, J.A.; de Pinto, W.J.; et al. Side Effects of Chloroquine and Primaquine and Symptom Reduction in Malaria Endemic Area (Mâncio Lima, Acre, Brazil). Interdiscip. Perspect. Infect. Dis. 2015, 2015, 346853. [Google Scholar] [CrossRef]
- Juurlink, D.N. Safety Considerations with Chloroquine, Hydroxychloroquine and Azithromycin in the Management of SARS-CoV-2 Infection. Can. Med. Assoc. J. 2020, 192, E450–E453. [Google Scholar] [CrossRef] [PubMed]
- Augustijns, P.; Geusens, P.; Verbeke, N. Chloroquine Levels in Blood during Chronic Treatment of Patients with Rheumatoid Arthritis. Eur. J. Clin. Pharmacol. 1992, 42, 429. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, J.; Farinotti, R. Clinical Pharmacokinetics and Metabolism of Chloroquine. Clin. Pharmacokinet. 1996, 31, 257. [Google Scholar] [CrossRef] [PubMed]
- Essien, E.E.; Ette, E.I. Effects of Chloroquine and Didesethylchloroquine on Rabbit Myocardium and Mitochondria. J. Pharm. Pharmacol. 1986, 38, 543. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Zheng, K.; Thai, S.; Simpson, R.J.; Kinlaw, A.C.; Xu, Y.; Wei, J.; Cui, X.; Buse, J.B.; et al. Serious Cardiovascular Adverse Events Associated with Hydroxychloroquine/Chloroquine Alone or with Azithromycin in Patients with COVID-19: A Pharmacovigilance Analysis of the FDA Adverse Event Reporting System (FAERS). Drugs Real World Outcomes 2022, 9, 231. [Google Scholar] [CrossRef]
- Conti, V.; Sellitto, C.; Torsiello, M.; Manzo, V.; de Bellis, E.; Stefanelli, B.; Bertini, N.; Costantino, M.; Maci, C.; Raschi, E.; et al. Identification of Drug Interaction Adverse Events in Patients With COVID-19. JAMA Netw. Open 2022, 5, e227970. [Google Scholar] [CrossRef]
- Treon, S.P.; Castillo, J.J.; Skarbnik, A.P.; Soumerai, J.D.; Ghobrial, I.M.; Guerrera, M.L.; Meid, K.; Yang, G. The BTK Inhibitor Ibrutinib May Protect against Pulmonary Injury in COVID-19–Infected Patients. Blood 2020, 135, 1912. [Google Scholar] [CrossRef]
- Miranda, V.; Fede, A.; Nobuo, M.; Ayres, V.; Giglio, A.; Miranda, M.; Riechelmann, R.P. Adverse Drug Reactions and Drug Interactions as Causes of Hospital Admission in Oncology. J. Pain Symptom Manag. 2011, 42, 342. [Google Scholar] [CrossRef]
- Réa-Neto, Á.; Stradiotto Bernardelli, R.; Martins, B.; Câmara, D.; Reese, F.B.; Vinicius, M.; Queiroga, O.; Oliveira, M.C. An Open-Label Randomized Controlled Trial Evaluating the Efficacy of Chloroquine/Hydroxychloroquine in Severe COVID-19 Patients. Sci. Rep. 2021, 11, 9023. [Google Scholar] [CrossRef]
- Self, W.H.; Semler, M.W.; Leither, L.M.; Casey, J.D.; Angus, D.C.; Brower, R.G.; Chang, S.Y.; Collins, S.P.; Eppensteiner, J.C.; Filbin, M.R.; et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19. JAMA 2020, 324, 2165. [Google Scholar] [CrossRef]
- Furtado, R.H.M.; Berwanger, O.; Fonseca, H.A.; Corrêa, T.D.; Ferraz, L.R.; Lapa, M.G.; Zampieri, F.G.; Veiga, V.C.; Azevedo, L.C.P.; Rosa, R.G.; et al. Azithromycin in Addition to Standard of Care versus Standard of Care Alone in the Treatment of Patients Admitted to the Hospital with Severe COVID-19 in Brazil (COALITION II): A Randomised Clinical Trial. Lancet 2020, 396, 959. [Google Scholar] [CrossRef]
- Gendrot, M.; Andreani, J.; Boxberger, M.; Jardot, P.; Fonta, I.; le Bideau, M.; Duflot, I.; Mosnier, J.; Rolland, C.; Bogreau, H.; et al. Antimalarial Drugs Inhibit the Replication of SARS-CoV-2: An in Vitro Evaluation. Travel. Med. Infect. Dis. 2020, 37, 101873. [Google Scholar] [CrossRef] [PubMed]
- Latarissa, I.R.; Barliana, M.I.; Meiliana, A.; Lestari, K. Potential of Quinine Sulfate for COVID-19 Treatment and Its Safety Profile: Review. Clin. Pharmacol. Adv. Appl. 2021, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Lestari, K.; Sitorus, T.; Megantara, S.; Levita, J. Molecular Docking of Quinine, Chloroquine and Hydroxychloroquine to Angiotensin Converting Enzyme 2 (ACE2) Receptor for Discovering New Potential COVID-19 Antidote. J. Adv. Pharm. Educ. Res. 2020, 10, 1–4. [Google Scholar]
- Sumitha, A.; Devi, P.B.; Hari, S.; Dhanasekaran, R. COVID-19—In Silico Structure Prediction and Molecular Docking Studies with Doxycycline and Quinine. Biomed. Pharmacol. J. 2020, 13, 1185. [Google Scholar] [CrossRef]
- Srimathi, R.; Mohan Maruga Raja, M.K.; Kathiravan, M.K. In Silico Screening of Traditional Herbal Medicine Derived Chemical Constituents for Possible Potential Inhibition against Sars-Cov-2. J. Nat. Remedies 2020, 20, 79. [Google Scholar] [CrossRef]
- Große, M.; Ruetalo, N.; Layer, M.; Hu, D.; Businger, R.; Rheber, S.; Setz, C.; Rauch, P.; Auth, J.; Fröba, M.; et al. Quinine Inhibits Infection of Human Cell Lines with Sars-Cov-2. Viruses 2021, 13, 647. [Google Scholar] [CrossRef]
- Persoons, L.; Vanderlinden, E.; Vangeel, L.; Wang, X.; Do, N.D.T.; Foo, S.Y.C.; Leyssen, P.; Neyts, J.; Jochmans, D.; Schols, D.; et al. Broad Spectrum Anti-Coronavirus Activity of a Series of Anti-Malaria Quinoline Analogues. Antivir. Res. 2021, 193. [Google Scholar] [CrossRef]
- Roy Chattopadhyay, N.; Chatterjee, K.; Banerjee, A.; Choudhuri, T. Combinatorial Therapeutic Trial Plans for COVID-19 Treatment Armed up with Antiviral, Antiparasitic, Cell-Entry Inhibitor, and Immune-Boosters. VirusDisease 2020, 31, 479. [Google Scholar] [CrossRef]
- Sachdeva, C.; Wadhwa, A.; Kumari, A.; Hussain, F.; Jha, P.; Kaushik, N.K. In Silico Potential of Approved Antimalarial Drugs for Repurposing against COVID-19. OMICS A J. Integr. Biol. 2020, 24, 568. [Google Scholar] [CrossRef]
- Gendrot, M.; Jardot, P.; Delandre, O.; Boxberger, M.; Andreani, J.; Duflot, I.; le Bideau, M.; Mosnier, J.; Fonta, I.; Hutter, S.; et al. In Vitro Evaluation of the Antiviral Activity of Methylene Blue Alone or in Combination against SARS-CoV-2. J. Clin. Med. 2021, 10, 3007. [Google Scholar] [CrossRef]
- Shionoya, K.; Yamasaki, M.; Iwanami, S.; Ito, Y.; Fukushi, S.; Ohashi, H.; Saso, W.; Tanaka, T.; Aoki, S.; Kuramochi, K.; et al. Mefloquine, a Potent Anti-Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Drug as an Entry Inhibitor In Vitro. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob. Agents Chemother. 2020, 64, e00819-20. [Google Scholar] [CrossRef] [PubMed]
- Weston, S.; Coleman, C.M.; Haupt, R.; Logue, J.; Matthews, K.; Li, Y.; Reyes, H.M.; Weiss, S.R.; Frieman, M.B. Broad Anti-Coronavirus Activity of Food and Drug Administration-Approved Drugs against SARS-CoV-2 In Vitro and SARS-CoV In Vivo. J. Virol. 2020, 94, e01218-20. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.-T.; Cheng, T.-J.R.; Juang, Y.-P.; Ma, H.-H.; Wu, Y.-T.; Yang, W.-B.; Cheng, C.-W.; Chen, X.; Chou, T.-H.; Shie, J.-J.; et al. Identification of Existing Pharmaceuticals and Herbal Medicines as Inhibitors of SARS-CoV-2 Infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2021579118. [Google Scholar] [CrossRef]
- Ellinger, B.; Bojkova, D.; Zaliani, A.; Cinatl, J.; Claussen, C.; Westhaus, S.; Keminer, O.; Reinshagen, J.; Kuzikov, M.; Wolf, M.; et al. A SARS-CoV-2 Cytopathicity Dataset Generated by High-Content Screening of a Large Drug Repurposing Collection. Sci. Data 2021, 8. [Google Scholar] [CrossRef]
- Gendrot, M.; Duflot, I.; Boxberger, M.; Delandre, O.; Jardot, P.; le Bideau, M.; Andreani, J.; Fonta, I.; Mosnier, J.; Rolland, C.; et al. Antimalarial Artemisinin-Based Combination Therapies (ACT) and COVID-19 in Africa: In Vitro Inhibition of SARS-CoV-2 Replication by Mefloquine-Artesunate. Int. J. Infect. Dis 2020, 99, 437. [Google Scholar] [CrossRef]
- Postasman, I.; Beny, A.; Seligmann, H. Neuropsychiatric Problems in 2500 Long-Term Young Travelers to the Tropics. J. Travel Med. 2000, 7, 5. [Google Scholar] [CrossRef]
- Croft, A.M.; Herxheimer, A. Adverse Effects of the Antimalaria Drug, Mefloquine: Due to Primary Liver Damage with Secondary Thyroid Involvement? BMC Public Health 2002, 2, 6. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The Birth of Artemisinin. Pharmacol. Ther. 2020, 216, 107658. [Google Scholar] [CrossRef]
- Qiu, F.; Liu, J.; Mo, X.; Liu, H.; Chen, Y.; Dai, Z. Immunoregulation by Artemisinin and Its Derivatives: A New Role for Old Antimalarial Drugs. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Kiani, B.H.; Kayani, W.K.; Khayam, A.U.; Dilshad, E.; Ismail, H.; Mirza, B. Artemisinin and Its Derivatives: A Promising Cancer Therapy. Mol. Biol. Rep. 2020, 47, 6321. [Google Scholar] [CrossRef] [PubMed]
- Cheong, D.H.J.; Tan, D.W.S.; Wong, F.W.S.; Tran, T. Anti-Malarial Drug, Artemisinin and Its Derivatives for the Treatment of Respiratory Diseases. Pharmacol. Res. 2020, 158, 104901. [Google Scholar] [CrossRef] [PubMed]
- Rolta, R.; Salaria, D.; Sharma, P.P.; Sharma, B.; Kumar, V.; Rathi, B.; Verma, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. Phytocompounds of Rheum Emodi, Thymus Serpyllum, and Artemisia Annua Inhibit Spike Protein of SARS-CoV-2 Binding to ACE2 Receptor: In Silico Approach. Curr. Pharmacol. Rep. 2021, 7, 135. [Google Scholar] [CrossRef]
- Ribaudo, G.; Coghi, P.; Yang, L.J.; Ng, J.P.L.; Mastinu, A.; Memo, M.; Wong, V.K.W.; Gianoncelli, A. Computational and Experimental Insights on the Interaction of Artemisinin, Dihydroartemisinin and Chloroquine with SARS-CoV-2 Spike Protein Receptor-Binding Domain (RBD). Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef]
- Prashantha, C.N.; Gouthami, K.; Lavanya, L.; Bhavanam, S.; Jakhar, A.; Shakthiraju, R.G.; Suraj, V.; Sahana, K.V.; Sujana, H.S.; Guruprasad, N.M.; et al. Molecular Screening of Antimalarial, Antiviral, Anti-Inflammatory and HIV Protease Inhibitors against Spike Glycoprotein of Coronavirus. J. Mol. Graph. Model. 2021, 102. [Google Scholar] [CrossRef]
- Pojskic, L. Screening of Preferential Binding Affinity of Selected Natural Compounds to SARS-CoV-2 Proteins Using in Silico Methods. EJMO 2020, 4, 319. [Google Scholar] [CrossRef]
- Badraoui, R.; Saoudi, M.; Hamadou, W.S.; Elkahoui, S.; Siddiqui, A.J.; Alam, J.M.; Jamal, A.; Adnan, M.; Suliemen, A.M.E.; Alreshidi, M.M.; et al. Antiviral Effects of Artemisinin and Its Derivatives against SARS-CoV-2 Main Protease: Computational Evidences and Interactions with ACE2 Allelic Variants. Pharmaceuticals 2022, 15, 129. [Google Scholar] [CrossRef]
- Belhassan, A.; Zaki, H.; Chtita, S.; Alaqarbeh, M.; Alsakhen, N.; Benlyas, M.; Lakhlifi, T.; Bouachrine, M. Camphor, Artemisinin and Sumac Phytochemicals as Inhibitors against COVID-19: Computational Approach. Comput. Biol. Med. 2021, 136. [Google Scholar] [CrossRef]
- Dogan, K.; Erol, E.; Didem Orhan, M.; Degirmenci, Z.; Kan, T.; Gungor, A.; Yasa, B.; Avsar, T.; Cetin, Y.; Durdagi, S.; et al. Instant Determination of the Artemisinin from Various Artemisia Annua L. Extracts by LC-ESI-MS/MS and Their In-Silico Modelling and In Vitro Antiviral Activity Studies against SARS-CoV-2. Phytochem. Anal. 2021. [Google Scholar] [CrossRef]
- Hasan, A.; Jannat, K.; Bondhon, T.A.; Jahan, R.; Hossan, M.S.; de Lourdes Pereira, M.; Nissapatorn, V.; Wiart, C.; Rahmatullah, M. Can Antimalarial Phytochemicals Be a Possible Cure for COVID-19? Molecular Docking Studies of Some Phytochemicals to SARS-CoV-2 3C-like Protease. Infect. Disord. Drug Targets 2021, 21. [Google Scholar] [CrossRef]
- Sudeep, H. Molecular Docking Analysis of Withaferin A from Withania Somnifera with the Glucose Regulated Protein 78 (GRP78) in Comparison with the COVID-19 Main Protease. Bioinformation 2020, 16, 411. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.M.; da Rocha, M.N.; Magalhães, E.P.; da Silva Mendes, F.R.; Marinho, M.M.; de Menezes, R.R.P.P.B.; Sampaio, T.L.; dos Santos, H.S.; Martins, A.M.C.; Marinho, E.S. Computational Approach towards the Design of Artemisinin–Thymoquinone Hybrids against Main Protease of SARS-COV-2. Future J. Pharm. Sci. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Gurung, A.B.; Ali, M.A.; Lee, J.; Farah, M.A.; Al-Anazi, K.M.; Al-Hemaid, F. Artesunate Induces Substantial Topological Alterations in the SARS-CoV-2 Nsp1 Protein Structure. J. King Saud Univ. Sci. 2022, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnøe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; et al. In Vitro Efficacy of Artemisinin-Based Treatments against SARS-CoV-2. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Trieu, V. Targeting TGF-β Pathway with COVID-19 Drug Candidate ARTIVeda/PulmoHeal Accelerates Recovery from Mild-Moderate COVID-19. 2021. medRxiv 2021. [Google Scholar] [CrossRef]
- Sehailia, M.; Chemat, S. Antimalarial-Agent Artemisinin and Derivatives Portray More Potent Binding to Lys353 and Lys31-Binding Hotspots of SARS-CoV-2 Spike Protein than Hydroxychloroquine: Potential Repurposing of Artenimol for COVID-19. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef]
- Sharma, S.; Deep, S. In-Silico Drug Repurposing for Targeting SARS-CoV-2 Main Protease (Mpro). J. Biomol. Struct. Dyn. 2022, 40, 3003. [Google Scholar] [CrossRef]
- Dey, D.; Borkotoky, S.; Banerjee, M. In Silico Identification of Tretinoin as a SARS-CoV-2 Envelope (E) Protein Ion Channel Inhibitor. Comput. Biol. Med. 2020, 127. [Google Scholar] [CrossRef]
- Cao, R.; Hu, H.; Li, Y.; Wang, X.; Xu, M.; Liu, J.; Zhang, H.; Yan, Y.; Zhao, L.; Li, W.; et al. Anti-SARS-CoV-2 Potential of Artemisinins in Vitro. ACS Infect. Dis. 2020, 6, 2524. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, M.; Qin, H.; Lin, H.; An, X.; Shi, Z.; Song, L.; Yang, X.; Fan, H.; Tong, Y. Artemether, Artesunate, Arteannuin B, Echinatin, Licochalcone B and Andrographolide Effectively Inhibit SARS-CoV-2 and Related Viruses In Vitro. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Miller, H.; Knox, K.; Kundu, M.; Henrickson, K.J.; Arav-Boger, R. Inhibition of Human Coronaviruses by Antimalarial Peroxides. ACS Infect. Dis. 2021, 7, 1985. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial Pharmacology and Therapeutics of Atovaquone. J. Antimicrob. Chemother. 2013, 68, 977. [Google Scholar] [CrossRef] [PubMed]
- Weyant, R.B.; Kabbani, D.; Doucette, K.; Lau, C.; Cervera, C. Pneumocystis Jirovecii: A Review with a Focus on Prevention and Treatment. Expert Opin. Pharmacother. 2021, 22, 1579. [Google Scholar] [CrossRef] [PubMed]
- Florez, H.; Singh, S. Coronavirus Disease 2019 Drug Discovery through Molecular Docking. F1000Res 2020, 9. [Google Scholar] [CrossRef]
- Lizbeth Ramírez-Salinas, G.; Martínez-Archundia, M.; Correa-Basurto, J.; García-Machorro, J. Repositioning of Ligands That Target the Spike Glycoprotein as Potential Drugs for SARS-CoV-2 in an In Silico Study. Molecules 2020, 25, 5615. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, S.Y.; Kim, D.; Kim, M.; Baek, K.; Kang, M.; An, S.; Gong, J.; Park, S.; Kandeel, M.; et al. Abiraterone Acetate Attenuates SARS-CoV-2 Replication by Interfering with the Structural Nucleocapsid Protein. Biomol. Ther. 2022. [Google Scholar] [CrossRef]
- Marak, B.N.; Dowarah, J.; Khiangte, L.; Singh, V.P. Step toward Repurposing Drug Discovery for COVID-19 Therapeutics through in Silico Approach. Drug Dev. Res. 2020, 82, 374. [Google Scholar] [CrossRef]
- Carter-Timofte, M.E.; Arulanandam, R.; Kurmasheva, N.; Fu, K.; Laroche, G.; Taha, Z.; van der Horst, D.; Cassin, L.; van der Sluis, R.M.; Palermo, E.; et al. Antiviral Potential of the Antimicrobial Drug Atovaquone against SARS-CoV-2 and Emerging Variants of Concern. ACS Infect. Dis. 2021, 7, 3034. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, B.A.; Noval, M.G.; Kaczmarek, M.E.; Jang, K.K.; Thannickal, S.A.; Kottkamp, A.C.; Brown, R.S.; Kielian, M.; Cadwell, K.; Stapleford, K.A.; et al. Atovaquone and Berberine Chloride Reduce SARS-CoV-2 Replication In Vitro. Viruses 2021, 13, 2437. [Google Scholar] [CrossRef]
- Pickardid, A.; Calverleyid, B.C.; Changid, J.; Garvaid, R.; Gago, S.; Lu, Y.; Kadlerid, K.E. Discovery of Re-Purposed Drugs That Slow SARS-CoV-2 Replication in Human Cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mellott, D.M.; Tseng, C.-T.; Drelich, A.; Fajtová, P.; Chenna, B.C.; Kostomiris, D.H.; Hsu, J.; Zhu, J.; Taylor, Z.W.; Kocurek, K.I.; et al. A Clinical-Stage Cysteine Protease Inhibitor Blocks SARS-CoV-2 Infection of Human and Monkey Cells. ACS Chem. Biol. 2021, 16, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.C.; Doyle, P.S.; Hsieh, I.; McKerrow, J.H. Cysteine Protease Inhibitors Cure an Experimental Trypanosoma Cruzi Infection. J. Exp. Med. 1998, 188, 725. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.C.; Warner, K.L.; Kornreic, B.G.; Piscitelli, J.; Wolfe, A.; Benet, L.; McKerrow, J.H. A Cysteine Protease Inhibitor Protects Dogs from Cardiac Damage during Infection by Trypanosoma Cruzi. Antimicrob. Agents Chemother. 2005, 49, 5160. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, M.N.; Juliano, L.; Carmona, E.; Silva, S.G.; Costa, E.M.; Murta, A.C.; Scharfstein, J. Inhibitors of the Major Cysteinyl Proteinase (GP57/51) Impair Host Cell Invasion and Arrest the Intracellular Development of Trypanosoma Cruzi in Vitro. Mol. Biochem. Parasitol. 1992, 52, 175. [Google Scholar] [CrossRef]
- Franke de Cazzulo, B.M.; Martínez, J.; North, M.J.; Coombs, G.H.; Cazzulo, J.J. Effects of Proteinase Inhibitors on the Growth and Differentiation of Trypanosoma Cruzi. FEMS Microbiol. Lett. 1994, 124, 81. [Google Scholar] [CrossRef][Green Version]
- Otta, D.A.; de Araújo, F.F.; de Rezende, V.B.; Souza-Fagundes, E.M.; Elói-Santos, S.M.; Costa-Silva, M.F.; Santos, R.A.; Costa, H.A.; Siqueira-Neto, J.L.; Martins-Filho, O.A.; et al. Identification of Anti-Trypanosoma Cruzi Lead Compounds with Putative Immunomodulatory Activity. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R.; Nunneley, J.W.; Barnard, D.; Pöhlmann, S.; McKerrow, J.H.; Renslo, A.R.; et al. Protease Inhibitors Targeting Coronavirus and Filovirus Entry. Antivir. Res. 2015, 116, 76. [Google Scholar] [CrossRef]
- Chen, W.; Mook, R.A.; Premont, R.T.; Wang, J. Niclosamide: Beyond an Antihelminthic Drug. Cell. Signal. 2018, 41, 89. [Google Scholar] [CrossRef]
- Kadri, H.; Lambourne, L.A.; Mehellou, Y. Niclosamide, ADrug with Many (Re)Purposes. Chem. Med. Chem. 2018, 13, 1088. [Google Scholar] [CrossRef]
- Jung, E.; Nam, S.; Oh, H.; Jun, S.; Ro, H.J.; Kim, B.; Kim, M.; Go, Y.Y. Neutralization of Acidic Intracellular Vesicles by Niclosamide Inhibits Multiple Steps of the Dengue Virus Life Cycle In Vitro. Sci Rep. 2019, 9, 8682. [Google Scholar] [CrossRef]
- Xu, J.; Shi, P.Y.; Li, H.; Zhou, J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 2020, 6, 909. [Google Scholar] [CrossRef]
- Pindiprolu, S.K.S.S.; Pindiprolu, S.H. Plausible Mechanisms of Niclosamide as an Antiviral Agent against COVID-19. Med. Hypotheses 2020, 140. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Kandeil, A.; Elshaier, Y.A.M.M.; Kutkat, O.; Moatasim, Y.; Rashad, A.A.; Shehata, M.; Gomaa, M.R.; Mahrous, N.; Mahmoud, S.H.; et al. Fda-Approved Drugs with Potent in Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceuticals 2020, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeño, J.M.; Ortega-Prieto, A.M.; Bussani, R.; et al. Drugs That Inhibit TMEM16 Proteins Block SARS-CoV-2 Spike-Induced Syncytia. Nature 2021, 594, 88. [Google Scholar] [CrossRef]
- Yu, S.; Piao, H.; Sanoj Rejinold, N.; Jin, G.; Choi, G.; Choy, J.H. Niclosamide–Clay Intercalate Coated with Nonionic Polymer for Enhanced Bioavailability toward COVID-19 Treatment. Polymers 2021, 13, 1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gaikwad, H.; McCarthy, M.; Gonzalez-Juarrero, M.; Li, Y.; Armstrong, M.; Reisdorph, N.; Morrison, T.; Simberg, D. Lipid Nanoparticle Formulation of Niclosamide (Nano NCM) Effectively Inhibits SARS-CoV-2 Replication in Vitro. Precis. Nanomed. 2021, 4, 724. [Google Scholar] [CrossRef]
- Needham, D. The PH Dependence of Niclosamide Solubility, Dissolution, and Morphology: Motivation for Potentially Universal Mucin-Penetrating Nasal and Throat Sprays for COVID19, Its Variants and Other Viral Infections. Pharm. Res. 2021. [Google Scholar] [CrossRef]
- Weiss, A.; Touret, F.; Baronti, C.; Gilles, M.; Hoen, B.; Nougairède, A.; de Lamballerie, X.; Sommer, M.O.A. Niclosamide Shows Strong Antiviral Activity in a Human Airway Model of SARS-CoV-2 Infection and a Conserved Potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta Variant (B.1.617.2). PLoS ONE 2021, 16. [Google Scholar] [CrossRef]
- Cairns, D.M.; Dulko, D.; Griffiths, J.K.; Golan, Y.; Cohen, T.; Trinquart, L.; Price, L.L.; Beaulac, K.R.; Selker, H.P. Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19. JAMA Netw. Open 2022, 5, e2144942. [Google Scholar] [CrossRef]
- Rossignol, J.-F. Nitazoxanide: A First-in-Class Broad-Spectrum Antiviral Agent. Antivir. Res. 2014, 110, 94. [Google Scholar] [CrossRef]
- Rossignol, J.-F.A.; Ayoub, A.; Ayers, M.S. Treatment of Diarrhea Caused by Cryptosporidium Parvum: A Prospective Randomized, Double-Blind, Placebo-Controlled Study of Nitazoxanide. J. Infect. Dis. 2001, 184, 103. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.J.; Ayoub, A.; Gargalaà, G.; Chegne, N.L.; Favennecà, L. Randomized Clinical Study of Nitazoxanide Compared to Metronidazole in the Treatment of Symptomatic Giardiasis in Children from Northern Peru. Aliment. Pharm. 2001, 15, 1409. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, L.; Houcke, I.; Mouton, Y.; Rossignol, J.F. In Vitro Evaluation of Activities of Nitazoxanide and Tizoxanide against Anaerobes and Aerobic Organisms. Antimicrob. Agents Chemother. 1996, 40, 2266. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.P.S.; Lin, G.; Jiang, X.; Nathan, C. Nitazoxanide Kills Replicating and Nonreplicating Mycobacterium Tuberculosis and Evades Resistance. J. Med. Chem. 2009, 52, 5789. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.P.S.; Darby, C.M.; Rhee, K.Y.; Nathan, C. Nitazoxanide Disrupts Membrane Potential and Intrabacterial PH Homeostasis of Mycobacterium Tuberculosis. ACS Med. Chem. Lett. 2011, 2, 849. [Google Scholar] [CrossRef]
- Rossignol, J.-F. Nitazoxanide, a New Drug Candidate for the Treatment of Middle East Respiratory Syndrome Coronavirus. J. Inf Pub. Health 2016, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Driouich, J.-S.; Cochin, M.; Touret, F.; Petit, P.-R.; Gilles, M.; Moureau, G.; Barthélémy, K.; Laprie, C.; Wattanakul, T.; Chotsiri, P.; et al. Pre-Clinical Evaluation of Antiviral Activity of Nitazoxanide against Sars-CoV-2. bioRxiv 2021. [Google Scholar] [CrossRef]
- Pepperrell, T.; Pilkington, V.; Owen, A.; Wang, J.; Hill, A.M. Review of Safety and Minimum Pricing of Nitazoxanide for Potential Treatment of COVID-19. J. Virus Erad. 2020, 6, 52. [Google Scholar] [CrossRef]
- Kelleni, M.T. Nitazoxanide/Azithromycin Combination for COVID-19: A Suggested New Protocol for Early Management. Pharm. Res. 2020, 157, 104874. [Google Scholar] [CrossRef]
- Sayed, A.M.; Khalaf, A.M.; Mohamed, I.; Abdelrahim, E.A.; Marwa, I.; Elgendy, O. Repurposing of Some Anti-Infective Drugs for COVID-19 Treatment: A Surveillance Study Supported by an in Silico Investigation. Int. J. Clin. Pract. 2021, 75, 13877. [Google Scholar] [CrossRef]
- Brosey, C.A.; Houl, J.H.; Katsonis, P.; Balapiti-Modarage, L.P.F.; Bommagani, S.; Arvai, A.; Moiani, D.; Bacolla, A.; Link, T.; Warden, L.S.; et al. Targeting SARS-CoV-2 Nsp3 Macrodomain Structure with Insights from Human Poly(ADP-Ribose) Glycohydrolase (PARG) Structures with Inhibitors. Prog. Biophys. Mol. Biol. 2021, 163, 171. [Google Scholar] [CrossRef] [PubMed]
- Meneses Calderón, J.; Figueroa Flores, M.D.R.; Paniagua Coria, L.; Briones Garduño, J.C.; Meneses Figueroa, J.; Vargas Contretas, M.J.; de la Cruz Ávila, L.; Díaz Meza, S.; Ramírez Chacón, R.; Padmanabhan, S.; et al. Nitazoxanide against COVID-19 in Three Explorative Scenarios. J. Infect. Dev. Ctries 2020, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Enríquez López, K.Y.; Meneses Calderón, J.; de la Cruz Ávila, L.; López Esquivel, M.Á.; Meneses Figueroa, J.; Vargas Contreras, M.J.; Anaya Herrera, J.; Sotelo Martínez, O.R.; Mendoza López, J.A.; Mendieta Zerón, H. Evolution of COVID-19 Pregnancies Treated With Nitazoxanide in a Third-Level Hospital. Cureus 2021, 13, e15002. [Google Scholar] [CrossRef] [PubMed]
- Rocco, P.R.; Silva, P.L.; Cruz, F.F.; Antonio Melo-Junior, M.C.; FGMM Tierno, P.; Moura, M.A.; Frederico De Oliveira, L.G.; Lima, C.C.; dos Santos, E.A.; Junior, W.F.; et al. Early Use of Nitazoxanide in Mild COVID-19 Disease: Randomised, Placebo-Controlled Trial on Behalf of the SARITA-2 Investigators. Eur. Respir J. 2021, 58. [Google Scholar] [CrossRef] [PubMed]
- Blum, V.F.; Cimerman, S.; Hunter, J.R.; Tierno, P.; Lacerda, A.; Soeiro, A.; Cardoso, F.; Bellei, N.C.; Maricato, J.; Mantovani, N.; et al. Nitazoxanide Superiority to Placebo to Treat Moderate COVID-19—A Pilot Prove of Concept Randomized Double-Blind Clinical Trial. EClinicalMedicine 2021, 37, 100981. [Google Scholar] [CrossRef]
- Elalfy, H.; Besheer, T.; El-Mesery, A.; El-Gilany, A.H.; Soliman, M.A.A.; Alhawarey, A.; Alegezy, M.; Elhadidy, T.; Hewidy, A.A.; Zaghloul, H.; et al. Effect of a Combination of Nitazoxanide, Ribavirin, and Ivermectin plus Zinc Supplement (MANS.NRIZ Study) on the Clearance of Mild COVID-19. J. Med. Virol. 2021, 93, 3176. [Google Scholar] [CrossRef]
- Rossignol, J.-F.; Bardin, M.C.; Fulgencio, J.; Mogelnicki, D.; Br Echot, C. A Randomized Double-Blind Placebo-Controlled Clinical Trial of Nitazoxanide for Treatment of Mild or Moderate COVID-19. eClinicalMedicine 2022, 45, 101310. [Google Scholar] [CrossRef]
- Riccio, A.; Santopolo, S.; Rossi, A.; Piacentini, S.; Rossignol, J.-F.; Santoro, M. Gabriella Impairment of SARS-CoV-2 Spike Glycoprotein Maturation and Fusion Activity by Nitazoxanide: An Effect Independent of Spike Variants Emergence. Cell. Mol. Life Sci. 2022, 79, 227. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, H.J.; Song, C.S.; Choi, I.S.; Lee, J.B.; Park, S.Y. Nitazoxanide Suppresses IL-6 Production in LPS-Stimulated Mouse Macrophages and TG-Injected Mice. Int. Immunopharmacol. 2012, 13, 23. [Google Scholar] [CrossRef]
- Clerici, M.; Trabattoni, D.; Pacei, M.; Biasin, M.; Rossignol, J. The Anti-Infective Nitazoxanide Shows Strong Immumodulating Effects (155.21). J. Immunol. 2011, 186, 155. [Google Scholar]
- Riberoid, M.S.; Jouvenet, N.; Dreuxid, M.; Bastien Nisoleid, S. Interplay between SARS-CoV-2 and the Type I Interferon Response. PLoS Pathog. 2020, 16, e1008737. [Google Scholar] [CrossRef]
- Miner, K.; Labitzke, K.; Liu, B.; Wang, P.; Henckels, K.; Gaida, K.; Elliott, R.; Chen, J.J.; Liu, L.; Leith, A.; et al. Drug Repurposing: The Anthelmintics Niclosamide and Nitazoxanide Are Potent TMEM16A Antagonists That Fully Bronchodilate Airways. Front. Pharm. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.; Gillan, V.; Devaney, E. Ivermectin–Old Drug, New Tricks? Trends Parasitol. 2017, 33, 463. [Google Scholar] [CrossRef] [PubMed]
- Canga, A.G.; Prieto, A.M.S.; Diez Liébana, M.J.; Martínez, N.F.; Sierra Vega, M.; García Vieitez, J.J. The Pharmacokinetics and Interactions of Ivermectin in Humans—A Mini-Review. AAPS J. 2008, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Salas-Coronas, J.; Muñoz, J.; Maruri, B.T.; Rodari, P.; Castelli, F.; Zammarchi, L.; Bianchi, L.; Gobbi, F.; Cabezas-Fernández, T.; et al. Multiple-Dose versus Single-Dose Ivermectin for Strongyloides Stercoralis Infection (Strong Treat 1 to 4): A Multicentre, Open-Label, Phase 3, Randomised Controlled Superiority Trial. Lancet Infect. Dis. 2019, 19, 1181. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin Is a Specific Inhibitor of Importin α/β-Mediated Nuclear Import Able to Inhibit Replication of HIV-1 and Dengue Virus. Biochem. J. 2012, 443, 851. [Google Scholar] [CrossRef]
- Oka, M.; Yoneda, Y. Importin α: Functions as a Nuclear Transport Factor and Beyond. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 259. [Google Scholar] [CrossRef]
- Jans, D.A.; Wagstaff, K.M. The Broad Spectrum Host-Directed Agent Ivermectin as an Antiviral for SARS-CoV-2? Biochem. Biophys. Res. Commun. 2021, 538, 163. [Google Scholar] [CrossRef]
- Martin, A.J.; Jans, D.A. Antivirals That Target the Host IMPα/Β1-Virus Interface. Biochem. Soc. Trans. 2021, 49, 281. [Google Scholar] [CrossRef]
- Rowland, R.R.R.; Chauhan, V.; Fang, Y.; Pekosz, A.; Kerrigan, M.; Burton, M.D. Intracellular Localization of the Severe Acute Respiratory Syndrome Coronavirus Nucleocapsid Protein: Absence of Nucleolar Accumulation during Infection and after Expression as a Recombinant Protein in Vero Cells. J. Virol. 2005, 79, 11507. [Google Scholar] [CrossRef]
- Timani, K.A.; Liao, Q.; Ye, L.; Zeng, Y.; Liu, J.; Zheng, Y.; Ye, L.; Yang, X.; Lingbao, K.; Gao, J.; et al. Nuclear/Nucleolar Localization Properties of C-Terminal Nucleocapsid Protein of SARS Coronavirus. Virus Res. 2005, 114, 23. [Google Scholar] [CrossRef] [PubMed]
- Wulan, W.N.; Heydet, D.; Walker, E.J.; Gahan, M.E.; Ghildyal, R. Nucleocytoplasmic Transport of Nucleocapsid Proteins of Enveloped RNA Viruses. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 in Vitro. Antivir. Res. 2020, 178, 3. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Ivermectin Docks to the SARS-CoV-2 Spike Receptor-Binding Domain Attached to ACE2. In Vivo 2020, 34, 3023. [Google Scholar] [CrossRef] [PubMed]
- Francés-Monerris, A.; García-Iriepa, C.; Iriepa, I.; Hognon, C.; Miclot, T.; Barone, G.; Monari, A.; Marazzi, M. Microscopic Interactions between Ivermectin and Key Human and Viral Proteins Involved in SARS-CoV-2 Infection. Phys. Chem. Chem. Phys. 2021, 23, 22957. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, L.; Zhan, X. Quantitative Proteomics Reveals a Broad-Spectrum Antiviral Property of Ivermectin, Benefiting for COVID-19 Treatment. J. Cell Phys. 2021, 236, 2959. [Google Scholar] [CrossRef]
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.C.C.J.C.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of 3-Chymotrypsin like Protease (3CLPro) Inhibitors as Potential Anti-SARS-CoV-2 Agents. Commun. Biol. 2021, 4. [Google Scholar] [CrossRef] [PubMed]
- Udofia, I.A.; Gbayo, K.O.; Oloba-Whenu, O.A.; Ogunbayo, T.B.; Isanbor, C. In Silico Studies of Selected Multi-Drug Targeting against 3CLpro and Nsp12 RNA-Dependent RNA-Polymerase Proteins of SARS-CoV-2 and SARS-CoV. Netw. Model. Anal. Health Inform. Bioinform. 2021, 10. [Google Scholar] [CrossRef]
- Bello, M. Elucidation of the Inhibitory Activity of Ivermectin with Host Nuclear Importin α and Several SARS-CoV-2 Targets. J. Biomol. Struct. Dyn. 2021, 1–9. [Google Scholar] [CrossRef]
- Qureshi, U.; Mir, S.; Naz, S.; Nur-e-Alam, M.; Ahmed, S.; Ul-Haq, Z. Mechanistic Insights into the Inhibitory Activity of FDA Approved Ivermectin against SARS-CoV-2: Old Drug with New Implications. J. Biomol. Struct. Dyn. 2021, 1–12. [Google Scholar] [CrossRef]
- Kern, C.; Schöning, V.; Chaccour, C.; Hammann, F. Modeling of SARS-CoV-2 Treatment Effects for Informed Drug Repurposing. Front. Pharmacol. 2021, 12, 1. [Google Scholar] [CrossRef]
- Dinesh Kumar, N.; ter Ellen, B.M.; Bouma, E.M.; Troost, B.; van de Pol, D.P.I.; van der Ende-Metselaar, H.H.; van Gosliga, D.; Apperloo, L.; Carpaij, O.A.; van den Berge, M.; et al. Moxidectin and Ivermectin Inhibit Sars-Cov-2 Replication in Vero E6 Cells But Not in Human Primary Airway Epithelium Cells. Antimicrob. Agent Chemother. 2021, 66, e0154321. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Das, N.C.; Patra, R.; Bhattacharya, M.; Ghosh, P.; Patra, B.C.; Mukherjee, S. Exploring the Binding Efficacy of Ivermectin against the Key Proteins of SARS-CoV-2 Pathogenesis: An Approach. Future Virol. 2021, 16, 277. [Google Scholar] [CrossRef]
- Muthusamy, S.; Gopal, H.; Manivarma, T. Virtual Screening Reveals Potential Anti-Parasitic Drugs Inhibiting the Receptor Binding Domain of SARS-CoV-2 Spike Protein. J. Virol. Antivir. Res. 2021, 110. [Google Scholar] [CrossRef]
- González-paz, L.; Hurtado-león, M.L.; Lossada, C.; Fernández-materán, F.V. Structural Deformability Induced in Proteins of Potential Interest Associated with COVID-19 by Binding of Homologues Present in Ivermectin: Comparative Study Based in Elastic Networks Models. J. Mol. Liq. 2021, 340, 117284. [Google Scholar] [CrossRef]
- González-Paz, L.; Hurtado-León, M.L.; Lossada, C.; Fernández-Materán, F.V.; Vera-Villalobos, J.; Loroño, M.; Paz, J.L.; Jeffreys, L.; Alvarado, Y.J. Comparative Study of the Interaction of Ivermectin with Proteins of Interest Associated with SARS-CoV-2: A Computational and Biophysical Approach. Biophys. Chem. 2021, 278, 106677. [Google Scholar] [CrossRef]
- Jeffreys, L.N.; Pennington, S.H.; Duggan, J.; Caygill, C.H.; Lopeman, R.C.; Breen, A.F.; Jinks, J.B.; Ardrey, A.; Donnellan, S.; Patterson, E.I.; et al. Remdesivir-Ivermectin Combination Displays Synergistic Interaction with Improved in Vitro Activity against SARS-CoV-2. Int. J. Antimicrob. Agents 2022, 59, 106542. [Google Scholar] [CrossRef]
- Eweas, A.F.; Alhossary, A.A.; Abdel-Moneim, A.S. Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2. Front. Microbiol. 2021, 11, 592908. [Google Scholar] [CrossRef]
- Segatori, V.I.; Garona, J.; Caligiuri, L.G.; Bizzotto, J.; Lavignolle, R.; Toro, A.; Sanchis, P.; Spitzer, E.; Krolewiecki, A.; Gueron, G.; et al. Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of Sars-cov-2- Positive Patients. Viruses 2021, 13, 2084. [Google Scholar] [CrossRef]
- Arévalo, A.P.; Pagotto, R.; Pórfido, J.L.; Daghero, H.; Segovia, M.; Yamasaki, K.; Varela, B.; Hill, M.; Verdes, J.M.; Duhalde Vega, M.; et al. Ivermectin Reduces in Vivo Coronavirus Infection in a Mouse Experimental Model. Sci. Rep. 2021, 11, 7132. [Google Scholar] [CrossRef]
- Melo, G.D.; Lazarini, F.; Larrous, F.; Feige, L.; Kornobis, E.; Levallois, S.; Marchio, A.; Kergoat, L.; Hardy, D.; Cokelaer, T.; et al. Attenuation of Clinical and Immunological Outcomes during SARS-CoV-2 Infection by Ivermectin. EMBO Mol. Med. 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, C.A.; Furtek, C.I.; Porras, A.G. Safety, Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects. J. Clin. Pharmacol. 2002, 42, 1122. [Google Scholar] [CrossRef] [PubMed]
- Ndyomugyenyi, R.; Kabatereine, N.; Olsen, A.; Magnussen, P. Efficacy of Ivermectin and Albendazole Alone and in Combination for Treatment of Soil-Transmitted Helminths in Pregnancy and Adverse Events: A Randomized Open Label Controlled Intervention Trial in Masindi District, Western Uganda. Am. J. Trop. Med. Hyg. 2009, 79, 856. [Google Scholar] [CrossRef]
- Wise, L.D.; Scialli, A.R. Ivermectin for COVID-19: Concerns during Pregnancy. Reprod. Toxicol. 2022, 107, 43. [Google Scholar] [CrossRef]
- Taylor, M.M.; Kobeissi, L.; Kim, C.; Amin, A.; Thorson, A.E.; Bellare, N.B.; Brizuela, V.; Bonet, M.; Kara, E.; Thwin, S.S.; et al. Inclusion of Pregnant Women in COVID-19 Treatment Trials: A Review and Global Call to Action. Lancet Glob. Health 2021, 9, e366. [Google Scholar] [CrossRef]
- Arshad, U.; Pertinez, H.; Box, H.; Tatham, L.; Rajoli, R.K.R.; Curley, P.; Neary, M.; Sharp, J.; Liptrott, N.J.; Valentijn, A.; et al. Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from Their Established Human Pharmacokinetics. Clin. Pharmacol. Ther. 2020, 108, 775. [Google Scholar] [CrossRef]
- Ahmed, S.; Mahbubul, M.; Ross, A.G.; Sharif, M.; Clemens, J.D.; Kibtiya, M.; Swe, C.; Zaman, K.; Somani, J.; Yasmin, R.; et al. A Five-Day Course of Ivermectin for the Treatment of COVID-19 May Reduce the Duration of Illness. Biomed. Biotechnol. Res. J. 2020, 21, 214–216. [Google Scholar] [CrossRef]
- Babalola, O.E.; Bode, C.O.; Ajayi, A.A.; Alakaloko, F.M.; Akase, I.E.; Otrofanowei, E.; Salu, O.B.; Adeyemo, W.L.; Ademuyiwa, A.O.; Omilabu, S. Ivermectin Shows Clinical Benefits in Mild to Moderate COVID19: A Randomized Controlled Double-Blind, Dose-Response Study in Lagos. QJM Int. J. Med. 2022, 114, 780. [Google Scholar] [CrossRef]
- Pott-Junior, H.; Bastos Paoliello, M.M.; de Miguel, A.Q.C.; da Cunha, A.F.; de Melo Freire, C.C.; Neves, F.F.; da Silva de Avó, L.R.; Roscani, M.G.; dos Santos, S.D.S.; Chachá, S.G.F. Use of Ivermectin in the Treatment of COVID-19: A Pilot Trial. Toxicol. Rep. 2021, 8, 505. [Google Scholar] [CrossRef]
- Aref, Z.F.; Bazeed, S.E.E.S.; Hassan, M.H.; Hassan, A.S.; Rashad, A.; Hassan, R.G.; Abdelmaksoud, A.A. Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19. Int. J. Nanomed. 2021, 16, 4063. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Goren, A.; Wambier, C.G.; McCoy, J. Early COVID-19 Therapy with Azithromycin plus Nitazoxanide, Ivermectin or Hydroxychloroquine in Outpatient Settings Significantly Improved COVID-19 Outcomes Compared to Known Outcomes in Untreated Patients. New Microbes New Infect. 2021, 43, 100915. [Google Scholar] [CrossRef]
- Krolewiecki, A.; Lifschitz, A.; Moragas, M.; Travacio, M.; Valentini, R.; Alonso, D.F.; Solari, R.; Tinelli, M.A.; Cimino, R.O.; Álvarez, L.; et al. Antiviral Effect of High-Dose Ivermectin in Adults with COVID-19: A Proof-of-Concept Randomized Trial. EClinicalMedicine 2021, 37. [Google Scholar] [CrossRef] [PubMed]
- Chaccour, C.; Casellas, A.; Blanco-Di Matteo, A.; Pineda, I.; Fernandez-Montero, A.; Ruiz-Castillo, P.; Richardson, M.A.; Rodríguez-Mateos, M.; Jordán-Iborra, C.; Brew, J.; et al. The Effect of Early Treatment with Ivermectin on Viral Load, Symptoms and Humoral Response in Patients with Non-Severe COVID-19: A Pilot, Double-Blind, Placebo-Controlled, Randomized Clinical Trial. EClinicalMedicine 2021, 32. [Google Scholar] [CrossRef] [PubMed]
- Ravikirti; Roy, R.; Pattadar, C.; Raj, R.; Agarwal, N.; Biswas, B.; Manjhi, P.K.; Rai, D.K.; Shyama; Kumar, A.; et al. Evaluation of Ivermectin as a Potential Treatment for Mild to Moderate Covid-19: A Double-Blind Randomized Placebo Controlled Trial in Eastern India. J. Pharm. Pharm. Sci. 2021, 24, 343. [Google Scholar] [CrossRef]
- Mohan, A.; Tiwari, P.; Menon Suri, T.; Mittal, S.; Patel, A.; Jain, A.; Velpandian, T.; Das, S.; Boppana, K.; Pandey, R.M.; et al. Single-Dose Oral Ivermectin in Mild and Moderate COVID-19 (RIVET-COV): A Single-Centre Randomized, Placebo-Controlled Trial. J. Infect. Chemother. 2021, 27, 1341. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Chesini, F.; Martini, D.; Roncaglioni, M.C.; Ojeda Fernandez, M.L.; Alvisi, M.F.; de Simone, I.; Rulli, E.; Nobili, A.; Casalini, G.; et al. High-Dose Ivermectin for Early Treatment of COVID-19 (COVER Study): A Randomised, Double-Blind, Multicentre, Phase II, Dose-Finding, Proof-of-Concept Clinical Trial. Int. Antimicrob. Agents 2022, 19, 106516. [Google Scholar] [CrossRef]
- Podder, C.S.; Chowdhury, N.; Sina, M.I.; Haque, W.M.M.U. Outcome of Ivermectin Treated Mild to Moderate COVID-19 Cases: A Single-Centre, Open-Label, Randomised Controlled Study. IMC J. Med. Sci. 2021, 14, 11. [Google Scholar] [CrossRef]
- Zeeshan Khan Chachar, A.; Ahmad Khan, K.; Asif, M.; Tanveer, K.; Khaqan, A.; Basri, R. Effectiveness of Ivermectin in SARS-CoV-2/COVID-19 Patients. Int. J. Sci. 2020, 9, 31. [Google Scholar] [CrossRef]
- Lim, S.C.L.; Hor, C.P.; Tay, K.H.; Mat Jelani, A.; Tan, W.H.; Ker, H.B.; Chow, T.S.; Zaid, M.; Cheah, W.K.; Lim, H.H.; et al. Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 426. [Google Scholar] [CrossRef]
- Galan, L.E.B.; Santos, N.M.D.; Asato, M.S.; Araújo, J.V.; de Lima Moreira, A.; Araújo, A.M.M.; Paiva, A.D.P.; Portella, D.G.S.; Marques, F.S.S.; Silva, G.M.A.; et al. Phase 2 Randomized Study on Chloroquine, Hydroxychloroquine or Ivermectin in Hospitalized Patients with Severe Manifestations of SARS-CoV-2 Infection. Pathog. Glob. Health 2021, 115, 235. [Google Scholar] [CrossRef]
- Shahbaznejad, L.; Davoudi, A.; Eslami, G.; Markowitz, J.S.; Navaeifar, M.R.; Hosseinzadeh, F.; Movahedi, F.S.; Rezai, M.S. Effects of Ivermectin in Patients With COVID-19: A Multicenter, Double-Blind, Randomized, Controlled Clinical Trial. Clin. Ther. 2021, 43, 1007. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Hirata, H.; Kabata, D.; Tokuhira, N.; Koide, M.; Ueda, A.; Tachino, J.; Shintani, A.; Uchiyama, A.; Fujino, Y.; et al. Ivermectin Administration Is Associated with Lower Gastrointestinal Complications and Greater Ventilator-Free Days in Ventilated Patients with COVID-19: A Propensity Score Analysis. J. Infect. Chemother. 2022, 28, 548. [Google Scholar] [CrossRef] [PubMed]
- Camprubí, D.; Almuedo-Riera, A.; Martí-Soler, H.I.; Soriano, A.; Hurtado, J.C.; Subirà, C.; Grau-Pujol, B.; Krolewiecki, A.; Muñoz, J. Lack of Efficacy of Standard Doses of Ivermectin in Severe COVID-19 Patients. PLoS ONE 2020, 15, e0242184. [Google Scholar] [CrossRef]
- Abd-Elsalam, S.; Noor, R.A.; Badawi, R.; Khalaf, M.; Esmail, E.S.; Soliman, S.; Abd El Ghafar, M.S.; Elbahnasawy, M.; Moustafa, E.F.; Hassany, S.M.; et al. Clinical Study Evaluating the Efficacy of Ivermectin in COVID-19 Treatment: A Randomized Controlled Study. J. Med. Virol. 2021, 93, 5833. [Google Scholar] [CrossRef] [PubMed]
- Szente Fonseca, S.N.; de Queiroz Sousa, A.; Wolkoff, A.G.; Moreira, M.S.; Pinto, B.C.; Valente Takeda, C.F.; Rebouças, E.; Vasconcellos Abdon, A.P.; Nascimento, A.L.A.; Risch, H.A. Risk of Hospitalization for COVID-19 Outpatients Treated with Various Drug Regimens in Brazil: Comparative Analysis. Travel Med. Infect. Dis. 2020, 38, 101906. [Google Scholar] [CrossRef]
- Vallejos, J.; Zoni, R.; Bangher, M.; Villamandos, S.; Bobadilla, A.; Plano, F.; Campias, C.; Chaparro Campias, E.; Achinelli, F.; Guglielmone, H.A.; et al. Ivermectin to Prevent Hospitalizations in Patients with COVID-19 (IVERCOR-COVID19): A Structured Summary of a Study Protocol for a Randomized Controlled Trial. Trials 2020, 21, 10. [Google Scholar] [CrossRef]
- Franco Haroldo, D.; Hector, C.; Roberto, H. Ivermectin in Long-Covid Patients: A Retrospective Study. J. Biomed. Res. Clin. Investig. 2021, 2, 1008. [Google Scholar] [CrossRef]
- Rajter, J.C.; Sherman, M.S.; Fatteh, N.; Vogel, F.; Sacks, J.; Rajter, J.J. Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study. Chest 2021, 159, 85. [Google Scholar] [CrossRef]
- Shakhsi Niaee, M.; Namdar, P.; Allami, A.; Zolghadr, L.; Javadi, A.; Karampour, A.; Varnaseri, M.; Bijani, B.; Cheraghi, F.; Naderi, Y.; et al. Ivermectin as an Adjunct Treatment for Hospitalized Adult COVID-19 Patients: A Randomized Multi-Center Clinical Trial. Asian Pac. J. Trop. Med. 2021, 14, 266. [Google Scholar] [CrossRef]
- Rezk, N.; Elsayed Sileem, A.; Gad, D.; Khalil, A.O. MiRNA-223-3p, MiRNA-2909 and Cytokines Expression in COVID-19 Patients Treated with Ivermectin. Zagazig Univ. Med. J. 2021. [Google Scholar] [CrossRef]
- Zubair, S.M.; Chaudhry, M.W.; Zubairi, A.B.S.; Shahzad, T.; Zahid, A.; Khan, I.A.; Khan, J.A.; Irfan, M. The Effect of Ivermectin on Non-Severe and Severe COVID-19 Disease and Gender-Based Difference of Its Effectiveness. Monaldi Arch. Chest Dis. 2022. [Google Scholar] [CrossRef]
- Abbas, K.U.; Muhammad, S.; Ding, S.F. The Effect of Ivermectin on Reducing Viral Symptoms in Patients with Mild COVID-19. Indian J. Pharm. Sci. 2020, 82. [Google Scholar] [CrossRef]
- Ozer, M.; Goksu, S.Y.; Conception, R.; Ulker, E.; Balderas, R.M.; Mahdi, M.; Manning, Z.; To, K.; Effendi, M.; Anandakrishnan, R.; et al. Effectiveness and Safety of Ivermectin in COVID-19 Patients: A Prospective Study at a Safety-Net Hospital. J. Med. Virol. 2021, 2, 1473. [Google Scholar] [CrossRef]
- Chowdhury, A.T.M.M.; Shahbaz, M.; Karim, M.R.; Islam, J.; Dan, G.; He, S. A Comparative Study on Ivermectin-Doxycycline and Hydroxychloro-quine-Azithromycin Therapy on COVID-19 Patients. Eurasian J. Med. Oncol. 2021, 5, 63. [Google Scholar] [CrossRef]
- Rahman, M.A.; Iqbal, S.A.; Islam, M.A.; Niaz, M.K.; Hussain, T.; Siddiquee, T.H. Comparison of Viral Clearance between Ivermectin with Doxycycline and Hy-droxychloroquine with Azithromycin in COVID-19 Patients. J. Bangladesh Coll. Phys. Surg. 2020, 5. [Google Scholar] [CrossRef]
- Alam, M.T.; Murshed, R.; Bhiuyan, E.; Saber, S.; Alam, R.F.; Robin, R.C. A Case Series of 100 COVID-19 Positive Patients Treated with Combination of Ivermectin and Doxycycline. J. Bangladesh Coll. Phys. Surg. 2020, 38, 10. [Google Scholar] [CrossRef]
- Mahmud, R.; Rahman, M.M.; Alam, I.; Ahmed, K.G.U.; Kabir, A.K.M.H.; Sayeed, S.K.J.B.; Rassel, M.A.; Monayem, F.B.; Islam, M.S.; Islam, M.M.; et al. Iver-mectin in Combination with Doxycycline for Treating COVID-19 Symptoms: A Randomized Trial. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, T.; Rani, B.; Siddiqui, R.; D‘Souza, G.; Memon, R.; Lutfi, I.; Hasan, O.I.; Javed, R.; Khan, F.; Hassan, M. Clinical Variants, Characteristics, and Outcomes Among COVID-19 Patients: A Case Series Analysis at a Tertiary Care Hospital in Karachi, Pakistan. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Ray, I.; Mukherjee, R.; Chowdhury, S.; Kulasreshtha, M.; Ghosh, R. Observational Study on Clinical Features, Treatment and Outcome of Covid 19 in a Tertiary Care Centre in India—A Retrospective Case Series. Int. J. Sci. Res. 2020, 1. [Google Scholar] [CrossRef]
- Faisal, R.; Shah, S.F.A.; Hussain, M. Potential Use of Azithromycin Alone and in Combination with Ivermectin in Fighting against the Symptoms of COVID-19. Prof. Med. J. 2021, 28, 737. [Google Scholar] [CrossRef]
- Espitia-Hernandez, G.; Munguia, L.; Diaz-Chiguer, D.; Lopez-Elizalde, R.; Jimenez-Ponce, F. Effects of Ivermectin-Azithromycin-Cholecalciferol Combined Therapy on COVID-19 Infected Patients: A Proof of Concept Study. Biomed. Res. 2020, 31, 129. [Google Scholar]
- Lima-morales, R.; Méndez-hernández, P.; Flores, Y.N.; Cuecuecha-rugerio, E.; Nava-zamora, A.; Hernández-galdamez, D.R.; Romo-dueñas, D.K.; Salmerón, J. Effectiveness of a Multidrug Therapy Consisting of Ivermectin, Azithromycin, Mon-telukast, and Acetylsalicylic Acid to Prevent Hospitalization and Death among Ambulatory COVID-19 Cases in Tlaxcala, Mexico. Int. J. Infect. Dis 2021, 105, 598. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Ascencio-Montiel, I.; Tomás-López, J.C.; Álvarez-Medina, V.; Gil-Velázquez, L.E.; Vega-Vega, H.; Vargas-Sánchez, H.R.; Cervantes-Ocampo, M.; Villasís-Keever, M.Á.; González-Bonilla, C.R.; Duque-Molina, C. A Multimodal Strategy to Reduce the Risk of Hospitalization/Death in Ambulatory Patients with COVID-19. Arch. Med. Res. 2022, 1. [Google Scholar] [CrossRef]
- Kishoria, N.; Mathur, S.L.; Parmar, V.; Kaur, R.J.; Agarwal, H.; Parihar, B.S.; Verma, S. Ivermectin as adjuvant to hydroxycholoroquine in patients resistant to standard treatment for SARS-CoV-2: Results of an open-label randomized clinical study. Paripex-Indian J. Res. 2020, 50. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Beranger, R.W.; Sampaio, P.P.N.; Filho, J.M.; Lima, R.A.C. Out-comes Associated with Hydroxychloroquine and Ivermectin in Hospitalized Pa-tients with COVID-19: A Single-Center Experience. Rev. Assoc. Med. Bras. 2021, 67, 1466. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, M.; Ajay Singh, T.; Deepti Singh, H.; Vibhu Sagar, K.; Yash, S. Comparative Analytical Study of Two Different Drug Regimens in Treatment of Covid-19 Positive Patients in Index Medical College Hospital and Research Center, Indore, India. Available online: https://ijhcr.com/index.php/ijhcr/article/view/1263/1064 (accessed on 19 February 2022).
- Sathi, S.; Garg, A.K.; Mittal, A.; Verma, S.; Saini, V.S.; Singh, M.K.; Vohra, D.; Kumar, S. Clinical Effect of the Combination Therapy of Hydroxychloroquine, Azithromycin and Ivermectin in Patients with COVID-19. J. Cardiovasc. Dis. Res. 2021, 12, 61. [Google Scholar] [CrossRef]
- Okumuş, N.; Demirtürk, N.; Çetinkaya, R.A.; Güner, R.; Avcı, İ.Y.; Orhan, S.; Konya, P.; Şaylan, B.; Karalezli, A.; Yamanel, L.; et al. Evaluation of the Effectiveness and Safety of Adding Ivermectin to Treatment in Severe COVID-19 Patients. BMC Infect. Dis. 2021, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Hector, C.; Roberto, H.; Protocol, C.-I.D.E.A. Safety and Efficacy of the Combined Use of Ivermectin, Dexamethasone, Enoxaparin and Aspirina against COVID-19 the I.D.E.A. Protocol. J. Clin. Trials 2021, 11, 459. [Google Scholar]
- Shoumann, W.M.; Hegazy, A.A.; Nafae, R.M.; Ragab, M.I.; Samra, S.R.; Ibrahim, D.A.; Al-Mahrouky, T.H.; Sileem, A.E. Use of Ivermectin as a Potential Chemopro-phylaxis for COVID-19 in Egypt: A Randomised Clinical Trial. J. Clin. Diagn. Res. 2021, 27. [Google Scholar] [CrossRef]
- Behera, P.; Patro, B.K.; Singh, A.K.; Chandanshive, P.D.; Ravikumar, S.R.; Pra-dhan, S.K.; Pentapati, S.S.K.; Batmanabane, G.; Mohapatra, P.R.; Padhy, B.M.; et al. Role of Ivermectin in the Prevention of SARS-CoV-2 Infection among Healthcare Workers in India: A Matched Case-Control Study. PLoS ONE 2021, 16, e0247163. [Google Scholar] [CrossRef]
- Morgenstern, J.; Redondo, J.N.; Olavarria, A.; Rondon, I.; Roca, S.; De Leon, A.; Canela, J.; Tavares, J.; Minaya, M.; Lopez, O.; et al. Ivermectin as a SARS-CoV-2 Pre-Exposure Prophylaxis Method in Healthcare Workers: A Propensity Score-Matched Retrospective Cohort Study. Cureus 2021, 13, e17455. [Google Scholar] [CrossRef]
- Alam, M.T.; Murshed, R.; Gomes, P.F.; Masud, Z.M.; Saber, S.; Chaklader, M.A.; Khanam, F.; Hossain, M.; Momen, A.B.I.M.; Yasmin, N.; et al. Ivermectin as Pre-Exposure Prophylaxis for COVID-19 among Healthcare Providers in a Selected Tertiary Hospital in Dhaka—An Observational Study. Eur. J. Med. Health Sci. 2020, 2. [Google Scholar] [CrossRef]
- Behera, P.; Patro, B.K.; Padhy, B.M.; Mohapatra, P.R.; Bal, S.K.; Chandanshive, P.D.; Mohanty, R.R.; Ravikumar, S.R.; Singh, A.; Singh, S.R.; et al. Prophylactic Role of Ivermectin in Severe Acute Respiratory Syndrome Coronavirus 2 Infection Among Healthcare Workers. Cureus 2021, 5, e16897. [Google Scholar] [CrossRef] [PubMed]
- Carvallo, H.; Hirsch, R.; Psaltis, A.; Contreras, V. Study of the Efficacy and Safety of Topical Ivermectin + Iota-Carrageenan in the Prophylaxis against COVID-19 in Health Personnel. J. Biomed. Res. Clin. Investig. 2020, 2, 1007. [Google Scholar] [CrossRef]
- Chahla, R.E.; Ruiz, L.M.; Ortega, E.S.; Morales, M.F.; Barreiro, F.; George, A.; Mancilla, C.; D’Amato, S.; Barrenechea, G.; Goroso, D.G.; et al. Intensive Treatment with Ivermectin and Iota-Carrageenan as Pre-Exposure Prophylaxis for COVID-19 in Health Care Workers from Tucuman, Argentina. Am. J. Ther. 2021, 28, E601. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Quek, A.M.L.; Ooi, D.S.Q.; Sengupta, S.; Lakshminarasappa, S.R.; Koo, C.Y.; So, J.B.Y.; Goh, B.C.; Loh, K.S.; Fisher, D.; et al. Positive Impact of Oral Hydroxychloroquine and Povidone-Iodine Throat Spray for COVID-19 Prophylaxis: An Open-Label Randomized Trial. International. J. Infect. Dis. 2021, 106, 314. [Google Scholar] [CrossRef]
- Guerrero, R.; Bravo, L.E.; Muñoz, E.; Ardila, E.K.G.; Guerrero, E. COVID-19: The Ivermectin African Enigma. Colomb. Med. 2020, 51, 1–8. [Google Scholar] [CrossRef]
- Hellwig, M.D.; Maia, A. A COVID-19 Prophylaxis? Lower Incidence Associated with Prophylactic Administration of Ivermectin. Int. J. Antimicrob. Agents 2021, 57, 106248. [Google Scholar] [CrossRef]
- Available online: https://C19adoption.Com/ (accessed on 3 May 2022).
- Available online: https://www.Covid19treatmentguidelines.Nih.Gov/Therapies/Antiviral-Therapy/Ivermectin/ (accessed on 3 May 2022).
- Available online: https://www.Fda.Gov (accessed on 3 May 2022).
- López-Medina, E.; López, P.; Hurtado, I.C.; Dávalos, D.M.; Ramirez, O.; Mar-tínez, E.; Díazgranados, J.A.; Oñate, J.M.; Chavarriaga, H.; Herrera, S.; et al. Effect of Ivermectin on Time to Resolution of Symptoms among Adults with Mild COVID-19: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2021, 325, 1426–1435. [Google Scholar] [CrossRef]
- Sarhan, R.M.; Harb, H.S.; Abou, A.E.; Sa-lem-bekhit, M.M. Efficacy of the Early Treatment with Tocili-zumab-Hydroxychloroquine and Tocilizumab-Remdesivir in Severe COVID-19 Patients. J. Infect. Public Health. 2022, 15, 116. [Google Scholar] [CrossRef]
- Hazan, S.; Dolai, S.; Clancy, R.L.; Borody, T.J. Effectiveness of Ivermec-tin-Based Multidrug Therapy in Severely Hypoxic, Ambulatory COVID-19 Patients. Future Med. 2022, 17, 339. [Google Scholar] [CrossRef]
- Manivannan, E.; Karthikeyan, C.; Moorthy, N.S.H.N.; Chaturvedi, S.C. The Rise and Fall of Chloroquine/Hydroxychloroquine as Compassionate Therapy of COVID-19. Front. Pharmacol. 2021, 12, 584940. [Google Scholar] [CrossRef] [PubMed]
- Abubaker Bagabir, S.; Ibrahim, N.K.; Abubaker Bagabir, H.; Hashem Ateeq, R. COVID-19 and Artificial Intelligence: Genome Sequencing, Drug Development and Vaccine Discovery. J. Infect. Public Health 2022, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Shin, B.; Choi, Y.; Park, S.; Kang, K. Predicting Commercially Avail-able Antiviral Drugs That May Act on the Novel Coronavirus (SARS-CoV-2) through a Drug-Target Interaction Deep Learning Model. Comput. Struct. Biotechnol. J. 2020, 18, 784. [Google Scholar] [CrossRef]
- Aghdam, R.; Habibi, M.; Taheri, G. Using Informative Features in Machine Learning Based Method for COVID-19 Drug Repurposing. J. Cheminform. 2021, 13, 70. [Google Scholar] [CrossRef]
- Ho, W.S.; Zhang, R.; Tan, Y.L.; Chai, C.L.L. COVID-19 and the Promise of Small Molecule Therapeutics: Are There Lessons to Be Learnt? Pharmacol. Res. 2022, 179, 106201. [Google Scholar] [CrossRef] [PubMed]
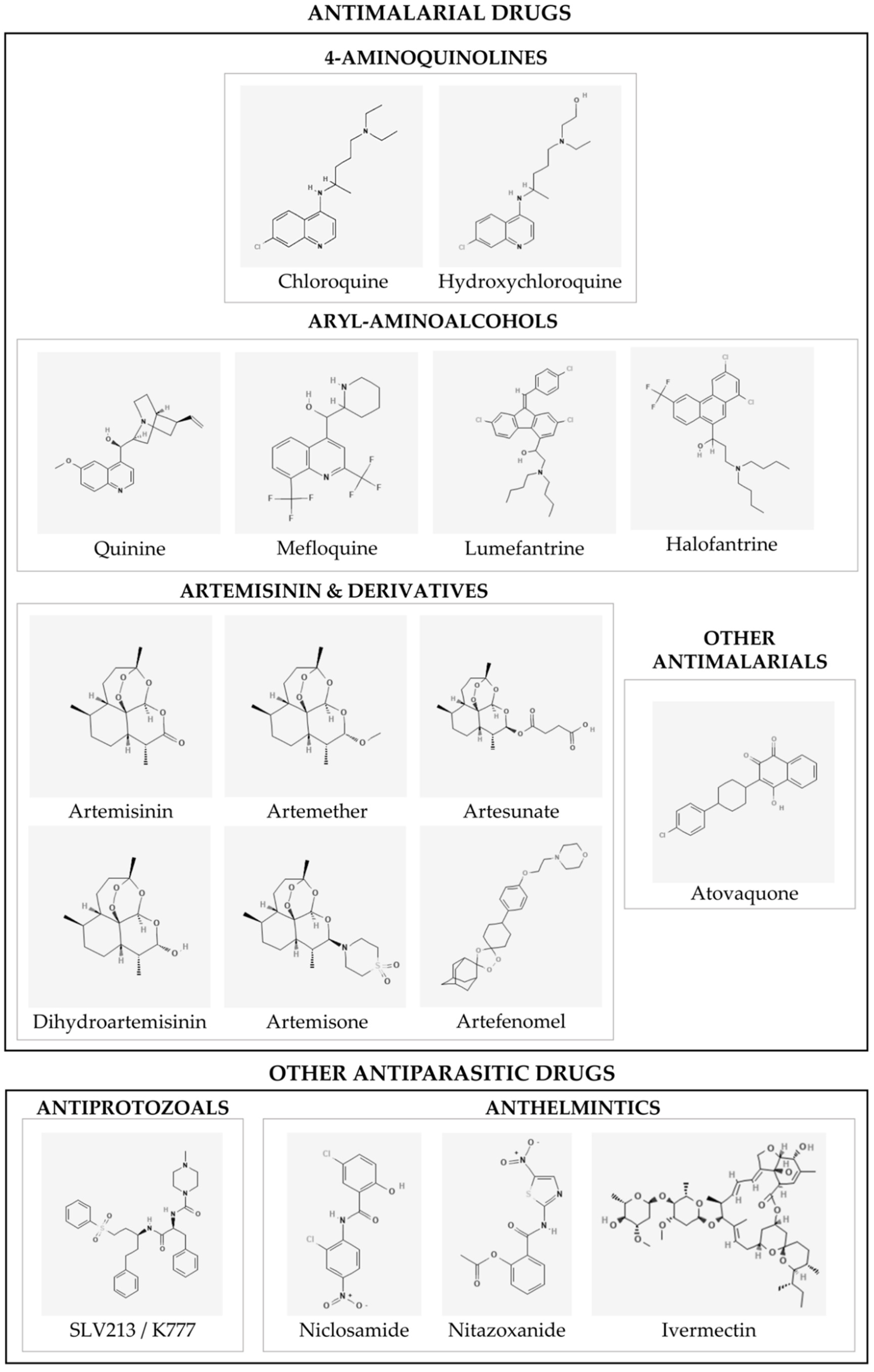
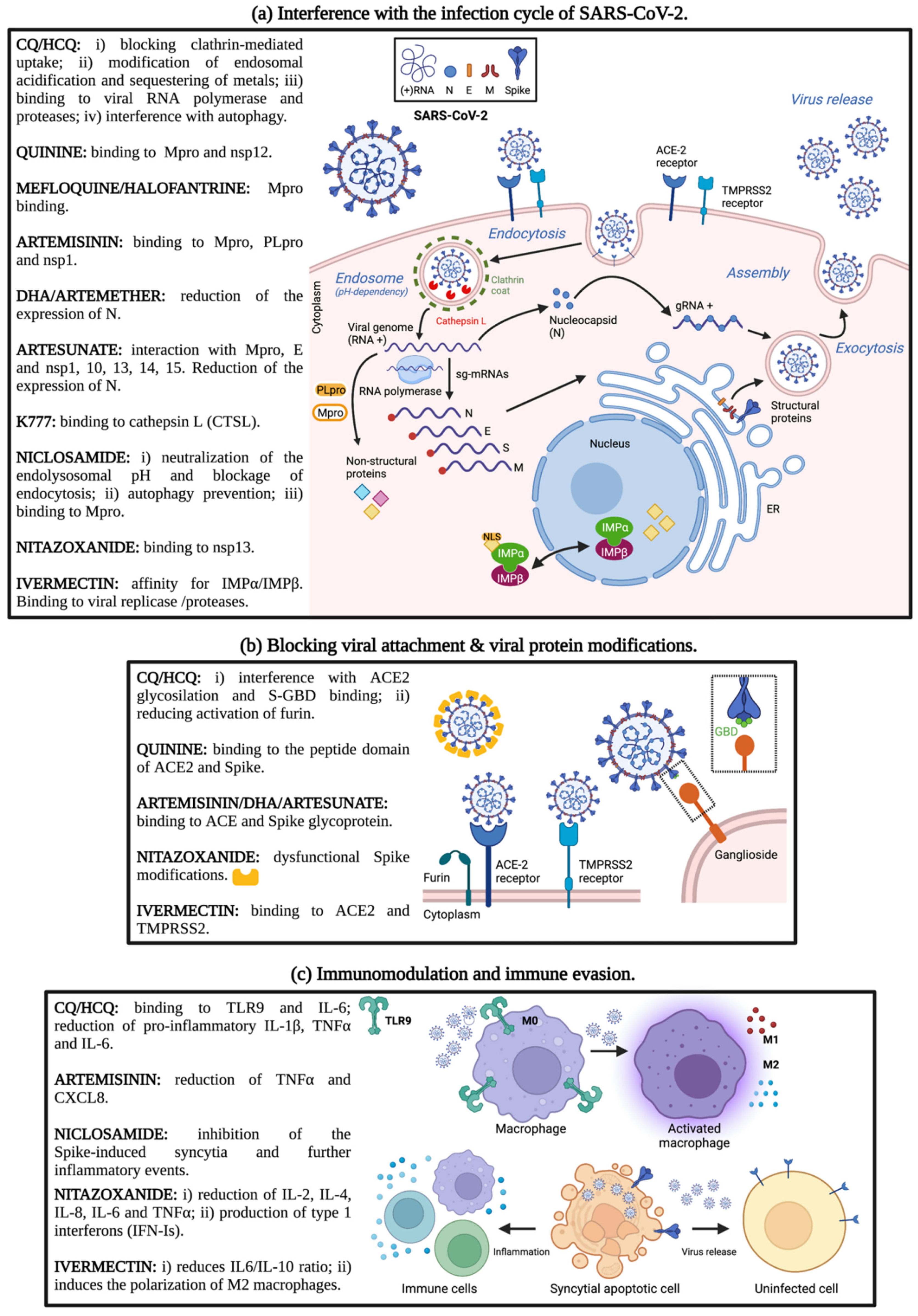
| Chloroquine (CQ) | ||||
|---|---|---|---|---|
| Trial No. | Phase | Drugs | No. Participants | Status & Results |
| NCT04323527 | Phase 2 | CQ diphosphate: high dosage (600 mg twice daily for 10 days) vs. low-dosage (450 mg twice on day 1 and once daily for 4 days) | 278 | Completed. Higher CQ dosage should not be recommended for critically ill patients with COVID-19 because of its potential safety hazards, especially when taken concurrently with azithromycin and oseltamivir [76]. |
| NCT04420247 | Phase 3 | CQ/HCQ added to standard of care (SoC) | 142 | Completed. The trial was stopped before reaching the planned sample size due to harmful effects. In patients with severe COVID-19, the use of CQ/HCQ added to SoC resulted in a significant worsening of clinical status, an increased risk of renal dysfunction and an increased need for invasive mechanical ventilation [99]. |
| Hydroxychloroquine (HCQ) | ||||
| Trial No. | Phase | Drugs | No. Participants | Status & Results |
| NCT04381936 “RECOVERY Trial” | Phase 2/Phase 3 | HCQ vs. SoC | 4716 | Completed (HCQ arm). COVID-19 patients receiving HCQ did not have a lower incidence of death at 28 days than those who received usual care [78]. |
| NCT04315948 “SOLIDARITY Trial” | Phase 3 | HCQ vs. Remdesivir vs. Lopinavir/ritonavir vs. Interferon Beta vs. SoC vs. AZD7442 vs. Placebo | 2416 | Recruiting. HCQ has little or no effect on hospitalized COVID-19 patients according to mortality, initiation of ventilation and duration of hospital stay [84]. |
| NCT04308668 | Phase 3 | HCQ vs. Placebo | 1312 | Completed. HCQ failed to prevent illness compatible with COVID-19 or confirmed infection when used as high-risk or moderate-risk postexposure prophylaxis within 4 days after exposure [80]. |
| NCT04304053 | Phase 3 | HCQ as prophylactic treatment | 2300 | Completed. Postexposure therapy with HCQ did not prevent SARS-CoV-2 infection or symptomatic COVID-19 in healthy persons exposed to a PCR-positive case patient [81]. |
| NCT04332991 | Phase 3 | HCQ vs. Placebo | 479 | Completed. Among adults hospitalized with respiratory illness from COVID-19, treatment with HCQ did not significantly improve clinical status at day 14 [100]. |
| NCT04466540 | Phase 4 | HCQ vs. Placebo | 1372 | Completed. HCQ did not reduce the risk of hospitalization in outpatients with mild or moderate forms of COVID-19 [89]. |
| NCT04321278 | Phase 3 | HCQ vs. HCQ + azithromycin | 447 | Completed. In patients with severe COVID-19, the use of HCQ + azithromycin did not improve clinical outcomes [101]. |
| NCT04325893 | Phase 3 | HCQ vs. Placebo | 259 | Terminated (decrease in number of eligible patients). Trial involving mainly older patients with mild to moderate COVID-19. HCQ treatment did not result in better clinical or virological outcomes [88]. |
| NCT04403100 | Phase 3 | HCQ Sulfate Tablets vs. Lopinavir/Ritonavir Oral Tablet vs. HCQ Sulfate Tablets +Lopinavir/Ritonavir Oral vs. Placebo | 1968 | Recruiting. Neither HCQ nor lopinavir-ritonavir showed any significant benefit for decreasing COVID-19–associated hospitalization or other secondary clinical outcomes [86]. |
| NCT04354428 | Phase 2/Phase 3 | HCQ sulfate vs. HCQ+AZ | 300 | Active, not recruiting. HCQ and HCQ + AZ do not affect the clinical course of COVID-19 among outpatients and should not be used to treat SARS-CoV-2 infection [85]. |
| Trial No. | Phase | Drugs | No. Participants | Status & Results |
| NCT05004753 | Phase 4 | ARTIVeda™ (Artemisinin) +/− SoC | 120 | Completed. ARTIVeda™ provides a faster recovery of patients with mild-moderate COVID-19 (preliminary data on 60 patients) [136] |
| Trial No. | Phase | Drugs | No. Participants | Status & Results |
|---|---|---|---|---|
| NCT04399356 | Phase 2 | Niclosamide vs. Placebo | 73 | Completed. No significant difference in oropharyngeal clearance of SARS-CoV-2 at day 3 between placebo and niclosamide-treated groups [170]. |
| Observational Studies | ||||
|---|---|---|---|---|
| Trial No. | Phase | Drugs | No. Participants | Status & Results |
| NCT04434144 | NA | IVM + Doxycycline/HCQ + AZ | 116 | Completed. Faster negative conversion of PCR and significantly faster symptom resolution in IVM-Doxycycline [255]. |
| NCT04425863 | NA | IVM + Aspirin/ IVM + Dexamethasone injection + Aspirin/ IVM + Dexamethasone injection + Enoxaparin injection | 167 | Completed. A positive role of IVM + aspirin + dexamethasone + enoxaparin therapy. No hospitalization of mild cases and lower mortality rate compared to national rate [270]. |
| Clinical Trials | ||||
| Trial No. | Phase | Drugs | No. Participants | Status & Results |
| NCT04381884 | 2 | IVM and SoC vs. SoC | 45 | Completed. No differences between the two groups in viral load and in clinical evolution of the patients. Significant difference between patients that showed high IVM levels in plasma samples vs. untreated patients: correlation of IVM level with decrease of the viral load. High dose of IVM did not show toxicity [233]. |
| NCT04591600 | 1/2 | IVM + Doxycycline/SoC | 140 | Completed. Therapy improvement by IVM + Doxycycline. |
| NCT04343092 | 1 | IVM vs. IVM + HCQ + AZT | 16 | Completed. Better efficacy of combination of IVM, HCQ and AZT; shorter hospitalization, and safety. |
| NCT04405843 | 2/3 | IVM vs. Placebo | 476 | Completed. No significant resolution of symptoms [284]. |
| NCT04529525 | 2/3 | IVM vs. Placebo | 501 | Completed. No effect on preventing hospitalization of COVID-19 patients [247]. |
| NCT04391127 | 3 | IVM vs. Placebo vs. HCQ | 108 | Completed. No efficacy of IVM or HCQ in decreasing hospitalization days, respiratory problems or deaths. |
| NCT04523831 | 3 | IVM + Doxycycline vs. SoC | 400 | Completed. Improvements in earlier recovery, prevention to progress to more serious disease (mortality) and increased likeliness to be COVID-19 negative by RT-PCR [258]. |
| NCT04784481 | 1/2 | IVM | 254 | Completed. Reduction of the number of symptoms and improvement of clinical state. |
| NCT04701710 | 1/2 | IVM + Iota-carrageenan | 300 | Completed. Reduction of number of infected health workers with preventive treatment with IVM and Iotacarrigean. Prevention of severe disease. |
| NCT04390022 | 1/2 | IVM vs. Placebo | 24 | Completed. No difference between treated and untreated patients in the decrease of viral load but early recovery of anosmia and hyposmia in IVM-treated patients [234]. |
| NCT04422561 | 2/3 | IVM chemoprophylaxis vs. no treatment | 304 | Completed. Significant differences among the two groups: 7.4% SARS-CoV-2 positive in IVM-treated vs. 58.4% of untreated subjects. Protective role of IVM [271]. |
| NCT04446104 | 3 | IVM vs. HCQ vs. Zinc vs. Povidone-Iodine vs. Vitamin C | 4257 | Completed. No efficacy of IVM [278]. |
| NCT04438850 | 2 | IVM vs. Placebo | 93 | Completed. Incidence dramatically dropped and is lack of eligible patients. |
| NCT04602507 | 2 | IVM vs. Placebo | 75 | Completed. Lack of severe COVID-19 cases in the place of study. |
| NCT04374019 | 2 | IVM vs. Camostat Mesilate vs. Artemesia annua vs. AS | 13 | Completed. Slow accrual. |
| NCT04431466 | 2 | IVM vs. SoC | 32 | Completed. IVM for SARS-CoV-2 treatment is safe. IVM antiviral effect is dose-dependent [230]. |
| NCT04716569 | 2/3 | Intranasal IVM spray | 150 | Completed. Reduction of anosmia and rapid viral clearance in treated patients [231]. |
| NCT04779047 | 4 | IVM vs. HCQ vs. Remdesivir vs. Tocilizumab vs. Lopinavir/Ritonavir 150 | 150 | Completed. No positive effect of IVM treatment [285]. |
| NCT04482686 | 1 | IVM + Doxycycline + Zinc + Vitamin D3 + Vitamin C | 31 | Completed. The combination of drugs is safe and effective [286]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Alvarez, E.; Dolci, M.; Perego, F.; Signorini, L.; Parapini, S.; D’Alessandro, S.; Denti, L.; Basilico, N.; Taramelli, D.; Ferrante, P.; et al. Antiparasitic Drugs against SARS-CoV-2: A Comprehensive Literature Survey. Microorganisms 2022, 10, 1284. https://doi.org/10.3390/microorganisms10071284
Calvo-Alvarez E, Dolci M, Perego F, Signorini L, Parapini S, D’Alessandro S, Denti L, Basilico N, Taramelli D, Ferrante P, et al. Antiparasitic Drugs against SARS-CoV-2: A Comprehensive Literature Survey. Microorganisms. 2022; 10(7):1284. https://doi.org/10.3390/microorganisms10071284
Chicago/Turabian StyleCalvo-Alvarez, Estefanía, Maria Dolci, Federica Perego, Lucia Signorini, Silvia Parapini, Sarah D’Alessandro, Luca Denti, Nicoletta Basilico, Donatella Taramelli, Pasquale Ferrante, and et al. 2022. "Antiparasitic Drugs against SARS-CoV-2: A Comprehensive Literature Survey" Microorganisms 10, no. 7: 1284. https://doi.org/10.3390/microorganisms10071284
APA StyleCalvo-Alvarez, E., Dolci, M., Perego, F., Signorini, L., Parapini, S., D’Alessandro, S., Denti, L., Basilico, N., Taramelli, D., Ferrante, P., & Delbue, S. (2022). Antiparasitic Drugs against SARS-CoV-2: A Comprehensive Literature Survey. Microorganisms, 10(7), 1284. https://doi.org/10.3390/microorganisms10071284








