Effects of Nano-Aerators on Microbial Communities and Functions in the Water, Sediment, and Shrimp Intestine in Litopenaeus vannamei Aquaculture Ponds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Description and Experimental Design
2.2. Sample Collection
2.3. DNA Extraction
2.4. 16S rRNA Gene Amplicon Sequencing
2.5. Bioinformatics and Statistical Analysis
3. Results
3.1. Sample Bacterial Sequencing Results
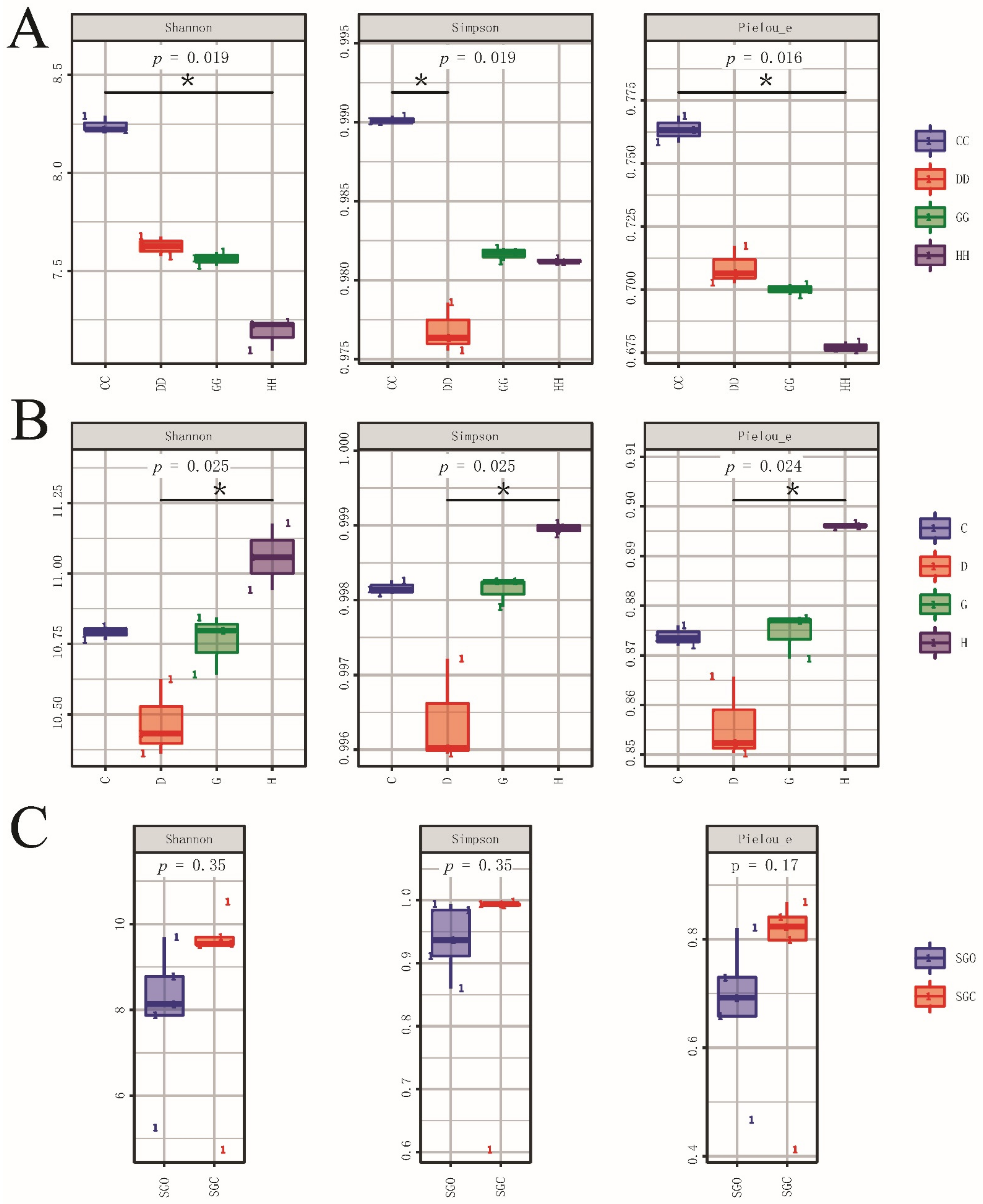
3.2. Alpha Diversity
3.3. Abundance Analysis
3.4. Taxonomic Differences in the Microbial Community and Biomarkers
3.5. β-Diversity Reveals the Effects of Different Aerators on Microbial Communities
3.6. Keystone Species Based on Network Analysis
3.7. Potential Function of the Gut Bacterial Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, C.E.; Torrans, E.L.; Tucker, C.S. Dissolved Oxygen and Aeration in Ictalurid Catfish Aquaculture. J. World Aquac. Soc. 2018, 49, 7–70. [Google Scholar] [CrossRef]
- Zhang, C.; Song, B.; Shan, J.; Ni, Q.; Wu, F.; Wang, S. Design and optimization of a new tube aeration device. Aquac. Int. 2020, 28, 985–999. [Google Scholar] [CrossRef]
- Marappan, J.; Anathaikamatchi, B.A.; Sakkarai, S.; Thiagarajan, R.; Muthusamy, D.; Kuppusamy, M.; Moturi, M.; Bera, A.; Ramasamy, P.; Shanmugam, S. Assessment of the new generation aeration systems efficiency and water current flow rate, its relation to the cost economics at varying salinities for Penaeus vannamei culture. Aquac. Res. 2020, 51, 2112–2124. [Google Scholar] [CrossRef]
- Ozaki, A.; Anongponyoskun, M.; Sirisuay, S.; Kaewjantawee, P. Verification Experiments of Aerators in Aquacultural Ponds. J. Fac. Agric. Kyushu Univ. 2013, 58, 427–432. [Google Scholar] [CrossRef]
- Kumar, A.; Moulick, S.; Mal, B.C. Selection of aerators for intensive aquacultural pond. Aquac. Eng. 2013, 56, 71–78. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, L.; Song, D.; Lin, F. Characteristics of micro-nano bubbles and potential application in groundwater bioremediation. Water Environ. Res. 2014, 86, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, E.Y.; Lee, D.; Park, B.J. Stabilization and fabrication of microbubbles: Applications for medical purposes and functional materials. Soft Matter 2015, 11, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sun, D.-W.; Zhang, Z.; Li, Y.; Cheng, L. Effects of micro-nano bubbles on the nucleation and crystal growth of sucrose and maltodextrin solutions during ultrasound-assisted freezing process. LWT-Food Sci. Technol. 2018, 92, 404–411. [Google Scholar] [CrossRef]
- Neethirajan, S.; Kobayashi, I.; Nakajima, M.; Wu, D.; Nandagopal, S.; Lin, F. Microfluidics for food, agriculture and biosystems industries. Lab Chip 2011, 11, 1574–1586. [Google Scholar] [CrossRef]
- Hu, L.; Xia, Z. Application of ozone micro-nano-bubbles to groundwater remediation. J. Hazard. Mater. 2018, 342, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Nimrat, S.; Suksawat, S.; Boonthai, T.; Vuthiphandchai, V. Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Veter. Microbiol. 2012, 159, 443–450. [Google Scholar] [CrossRef]
- Foysal, M.D.J.; Gupta, S.K. A systematic meta-analysis reveals enrichment of Actinobacteria and Firmicutes in the fish gut in response to black soldier fly (Hermetica illucens) meal-based diets. Aquaculture 2022, 549, 737760. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Albores, F.; Martínez-Porchas, M.; Arvayo, M.A.; Villalpando-Canchola, E.; Gollas-Galván, T.; Porchas-Cornejo, M.A. Immunophysiological Response of Pacific White Shrimp Exposed to a Probiotic Mixture of Proteobacteria and Firmicutes in Farm Conditions. N. Am. J. Aquac. 2016, 78, 193–202. [Google Scholar] [CrossRef]
- Zheng, L.; Xie, S.; Zhuang, Z.; Liu, Y.; Tian, L.; Niu, J. Effects of yeast and yeast extract on growth performance, antioxidant ability and intestinal microbiota of juvenile Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2020, 530, 735941. [Google Scholar] [CrossRef]
- Han, S.Y.; Wang, B.J.; Liu, M.; Wang, M.Q.; Jiang, K.Y.; Qi, C.C.; Wang, L. Effect of cyclic serious/medium hypoxia stress on the survival, growth performance and resistance against Vibrio parahemolyticus of white shrimp Litopenaeus vannamei. Invertebr. Surviv. J. 2017, 14, 259–270. [Google Scholar]
- Shao, Y.; Zhong, H.; Wang, L.; Elbashier, M.M.A. Bacillus amyloliquefaciens (IAE635) and their metabolites could purify pollutants, Vibrio spp. and coliform bacteria in coastal aquaculture wastewater. Int. J. Agric. Biol. Eng. 2021, 14, 205–210. [Google Scholar] [CrossRef]
- Xue, S.; Xu, W.; Wei, J.; Sun, J. Impact of environmental bacterial communities on fish health in marine recirculating aquaculture systems. Veter. Microbiol. 2017, 203, 34–39. [Google Scholar] [CrossRef]
- Soonthornchai, W.; Rungrassamee, W.; Karoonuthaisiri, N.; Jarayabhand, P.; Klinbunga, S.; Söderhäll, K.; Jiravanichpaisal, P. Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev. Comp. Immunol. 2010, 34, 19–28. [Google Scholar] [CrossRef]
- Jiao, L.F.; Dai, T.M.; Zhong, S.Q.; Jin, M.; Sun, P.; Zhou, Q.C. Vibrio parahaemolyticus infection impaired intestinal barrier function and nutrient absorption in Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 99, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, Y.; Zhou, J.; Wu, Y. Phosphorus release from lake sediments: Effects of pH, temperature and dissolved oxygen. KSCE J. Civ. Eng. 2014, 8, 323–329. [Google Scholar] [CrossRef]
- Lou, J.; Lv, J.; Yang, D. Effects of Environmental Factors on Nitrate-DAMO Activity. Water Air Soil Pollut. 2020, 231, 263. [Google Scholar] [CrossRef]
- Kurita, Y.; Chiba, I.; Kijima, A. Physical eradication of small planktonic crustaceans from aquaculture tanks with cavitation treatment. Aquac. Int. 2017, 25, 2127–2133. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Xu, K.; Zhang, X.; Sun, H.; Fan, L.; Yan, M. White spot syndrome virus (WSSV) infection impacts intestinal microbiota composition and function in Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 84, 130–137. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef] [Green Version]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-M.; Lu, P.-Z.; Huang, M.-S.; Dai, L.-P. Seasonal variations and aeration effects on water quality improvements and physiological responses of Nymphaea tetragona Georgi. Int. J. Phytoremediation 2013, 15, 522–535. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.; Xu, J.; Wang, H.; Zhu, H.; Lv, H. Numerical simulation and application of micro-nano bubble releaser for irrigation. Mater. Express 2021, 11, 1007–1015. [Google Scholar] [CrossRef]
- Yao, G.-J.; Ren, J.-Q.; Zhou, F.; Liu, Y.-D.; Li, W. Micro-nano aeration is a promising alternative for achieving high-rate partial nitrification. Sci. Total Environ. 2021, 795, 148899. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Liu, L.; Hou, Y.; Li, L. Nanotechnology in agriculture, livestock, and aquaculture in China: A review. Agron. Sustain. Dev. 2015, 35, 369–400. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, T.; Matsuura, Y.; Inada, M.; Takano, T.; Nakayasu, C.; Sakai, T.; Terashima, S.; Yasuike, M.; Fujiwara, A.; Nakamura, Y.; et al. An Epidemiological Study of Akoya Oyster Disease Using Polymerase Chain Reaction Targeting Spirochaetes Genes. Fish Pathol. 2018, 53, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Gu, Y.; Zhou, H.; Xu, L.; Cao, H.; Gai, C. Acinetobacter venetianus, a potential pathogen of red leg disease in freshwater-cultured whiteleg shrimp Penaeus vannamei. Aquac. Rep. 2020, 18, 100543. [Google Scholar] [CrossRef]
- Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Probiotic effects of a novel strain, Acinetobacter KU011TH, on the growth performance, immune responses, and resistance against Aeromonas hydrophila of bighead catfish (Clarias macrocephalus Günther, 1864). Microorganisms 2019, 7, e613. [Google Scholar] [CrossRef] [Green Version]
- Manan, H.; Rosland, N.A.; Deris, Z.M.; Hashim, N.F.C.; Kasan, N.A.; Ikhwanuddin, M.; Suloma, A.; Fauzan, F. 16S rRNA sequences of Exiguobacterium spp. bacteria dominant in a biofloc pond cultured with whiteleg shrimp, Penaeus vannamei. Aquac. Res. 2022, 53, 2029–2041. [Google Scholar] [CrossRef]
- Ekasari, J.; Nugroho, U.A.; Fatimah, N.; Angela, D.; Hastuti, Y.P.; Pande, G.S.J.; Natrah, F.M.I. Improvement of biofloc quality and growth of Macrobrachium rosenbergii in biofloc systems by Chlorella addition. Aquac. Int. 2021, 29, 2305–2317. [Google Scholar] [CrossRef]
- Godinho, O.; Calisto, R.; Øvreås, L.; Quinteira, S.; Lage, O.M. Antibiotic susceptibility of marine Planctomycetes. Antonie Leeuwenhoek 2019, 112, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, T.; Gahlawat, S.K.; Sihag, R.C. Elimination of pathogenic bacterium (Micrococcus sp.) by using probiotics. Turk. J. Fish. Aquat. Sci. 2012, 12, 179–181. [Google Scholar] [CrossRef]
- Liao, Z.; Gong, Y.; Zhao, W.; He, X.; Wei, D.; Niu, J. Comparison effect of Rhodobacter sphaeroides protein replace fishmeal on growth performance, intestinal morphology, hepatic antioxidant capacity and immune gene expression of Litopenaeus vannamei under low salt stress. Aquaculture 2021, 547, 737488. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Ma, J.; Sun, L.; Liu, Y.; Ren, H.; Shen, Y.; Bi, F.; Zhang, T.; Wang, X. Alter between gut bacteria and blood metabolites and the anti-tumor effects of Faecalibacterium prausnitzii in breast cancer. BMC Microbiol. 2020, 20, 1–19. [Google Scholar] [CrossRef]
- Davidovich, N.; Pretto, T.; Blum, S.E.; Baider, Z.; Grossman, R.; Kaidar-Shwartz, H.; Dveyrin, Z.; Rorman, E. Mycobacterium gordonae infecting redclaw crayfish Cherax quadricarinatus. Dis. Aquat. Org. 2019, 135, 169–174. [Google Scholar] [CrossRef]
- Jhunkeaw, C.; Khongcharoen, N.; Rungrueng, N.; Sangpo, P.; Panphut, W.; Thapinta, A.; Senapin, S.; St-Hilaire, S.; Dong, H.T. Ozone nanobubble treatment in freshwater effectively reduced pathogenic fish bacteria and is safe for Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 534, 736286. [Google Scholar] [CrossRef]
- Luu, T.Q.; Truong, P.N.H.; Zitzmann, K.; Nguyen, K.T. Effects of Ultrafine Bubbles on Gram-Negative Bacteria: Inhibition or Selection. Langmuir 2019, 35, 13761–13768. [Google Scholar] [CrossRef]





| Water | Sediment | Shrimp Intestine | ||||
|---|---|---|---|---|---|---|
| Nano-Aerator | Control | Nano-Aerator | Control | Nano-Aerator | Control | |
| Total sequences | 98,576 ± 6462 | 96,102 ± 2841 | 116,117 ± 2919 | 111,931 ± 7987 | 170,603 ± 18,535 | 148,246 ± 22,084 |
| Sequences after denoising | 89,907 ± 5948 | 89,555 ± 2709 | 100,837 ± 2642 | 96,398 ± 7344 | 156,481 ± 17409 | 135,701 ± 21,330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, L.; Lou, S.; Tian, J.; Sun, S.; Li, X.; Li, Y. Effects of Nano-Aerators on Microbial Communities and Functions in the Water, Sediment, and Shrimp Intestine in Litopenaeus vannamei Aquaculture Ponds. Microorganisms 2022, 10, 1302. https://doi.org/10.3390/microorganisms10071302
Xu Y, Li L, Lou S, Tian J, Sun S, Li X, Li Y. Effects of Nano-Aerators on Microbial Communities and Functions in the Water, Sediment, and Shrimp Intestine in Litopenaeus vannamei Aquaculture Ponds. Microorganisms. 2022; 10(7):1302. https://doi.org/10.3390/microorganisms10071302
Chicago/Turabian StyleXu, Yingkai, Lisong Li, Suo Lou, Jiashen Tian, Shuhao Sun, Xiaodong Li, and Yingdong Li. 2022. "Effects of Nano-Aerators on Microbial Communities and Functions in the Water, Sediment, and Shrimp Intestine in Litopenaeus vannamei Aquaculture Ponds" Microorganisms 10, no. 7: 1302. https://doi.org/10.3390/microorganisms10071302
APA StyleXu, Y., Li, L., Lou, S., Tian, J., Sun, S., Li, X., & Li, Y. (2022). Effects of Nano-Aerators on Microbial Communities and Functions in the Water, Sediment, and Shrimp Intestine in Litopenaeus vannamei Aquaculture Ponds. Microorganisms, 10(7), 1302. https://doi.org/10.3390/microorganisms10071302







