Abstract
Increased knowledge suggests that disturbed gut microbiota, termed dysbiosis, might promote the development of irritable bowel syndrome (IBS) symptoms. Accordingly, gut microbiota manipulation has evolved in the last decade as a novel treatment strategy in order to improve IBS symptoms. In using different approaches, dietary management stands first in line, including dietary fiber supplements, prebiotics, and probiotics that are shown to change the composition of gut microbiota, fecal short-chain fatty acids and enteroendocrine cells densities and improve IBS symptoms. However, the exact mixture of beneficial bacteria for each individual remains to be identified. Prescribing nonabsorbable antibiotics still needs confirmation, although using rifaximin has been approved for diarrhea-predominant IBS. Fecal microbiota transplantation (FMT) has recently gained a lot of attention, and five out of seven placebo-controlled trials investigating FMT in IBS obtain promising results regarding symptom reduction and gut microbiota manipulation. However, more data, including larger cohorts and studying long-term effects, are needed before FMT can be regarded as a treatment for IBS in clinical practice.
1. Introduction
Irritable bowel syndrome (IBS) has become the most common gastrointestinal disease for referral to the gastroenterologists due to the patients’ complaints of abdominal pain, bloating and mixed bowel movements of diarrhea and/or constipation, which can range from mild to severe [1,2]. IBS can cause several extraintestinal symptoms such as headache, tiredness, fibromyalgia and poor social functioning and emotional well-being [3]. Though IBS does not cause increased mortality or cancer incidence [4], the severity of the symptoms reduces the quality of life of the patients, which leads them to skip work or school, reducing their daily productivity and causing a financial burden to society [5,6].
According to a systemic review and meta-analysis [7], the global prevalence of IBS using Rome IV criteria for the diagnosis of IBS and its subtypes [8] is 3.8% [7], but this prevalence was higher in the Western world population using the older criteria Rome III, reaching to 9.2% [7,9]. Diarrhea-predominant IBS is the most common subtype using Rome IV criteria, while mixed-type IBS was the most common subtype using Rome III criteria [7]. IBS is more common in women than in men [7]. The diagnosis of IBS is mainly a diagnosis of exclusion due to the common symptoms that can mask those of other organic diseases, most importantly, celiac disease, inflammatory bowel diseases and colorectal cancer [10].
The cause of IBS is not yet known; however, multiple factors play an important role in the pathogenesis of IBS, such as disturbed gut microbiota (dysbiosis) [11], altered enteroendocrine cells [12,13], previous infections [14,15], genetics [16] and diet [17,18]. Several mechanisms have been suggested for the pathophysiology of IBS, such as alterations in the gut–brain axis [19], abnormalities in the gut endocrine cells and the enteric nervous system [20,21], visceral hypersensitivity [19], gastrointestinal dysmotility [22], postinfectious status and low-grade inflammation [15,23], bacterial overgrowth [24], malabsorption of carbohydrates [25] and altered gut microbiota composition [19,26].
Both pharmacological and non-pharmacological treatments have been used to reduce IBS symptoms [27]. Pharmacological treatment for IBS is mainly symptomatic and of short-term effect [28,29]. Non-pharmacological treatments include dietary management, fecal microbiota transplantation (FMT), psycho- and hypnotherapy and behavioral therapy [27].
In the past decade, research has focused on dietary management and the usage of probiotics, antibiotics and FMT to treat IBS and the current review is an attempt to summarize the effect of these treatment methods on manipulating the gut microbiota.
2. Dysbiosis and IBS
The development of healthy gut microbiota essentially begins with its colonization at birth during vaginal delivery with vaginal microbes such as Lactobacillus. Delivery using cesarean section results in colonization by skin bacteria or hospital-acquired bacteria, for example, Staphylococcus and Acinetobacter [30,31], that render these babies susceptible to developing asthma and allergic rhinitis [32]. The gut microbiota matures during the first three years after birth [33] and becomes inhabited by more than 2000 different bacterial species that belong to four main phyla Bacteroides, Firmicutes, Actinobacteria and Proteobacteria [34]. A healthy composition of the gut microbiota, essential for the proper function of the gastrointestinal tract, is determined by environmental and genetic factors [35]. Some of the environmental factors include dietary habits, geographical location, surgical procedures, smoking, depression, anxiety and recurrent antibiotic treatments [36]. Dysbiosis occurs when an imbalance of the gut microbiota occurs, leading to a reduction in its diversity compared to normal (normobiosis) [37,38] and colonization of opportunistic pathogens [39]. A typical example is what occurs during pseudomembranous colitis when the colon becomes colonized with the opportunistic bacteria Clostridium difficile after using broad-spectrum antibiotics, proton pump inhibitor and immunosuppression [39]. Another example is what occurs to patients after a bout of gastroenteritis, causing post-infectious IBS with altered levels of Bacteroidetes and Clostridia [40]. Decreased richness and diversity of the gut microbiota correlates with increased IBS symptom severity [41] and increased Firmicutes to Bacteroides ratio as well as increased Clostridia and Clostridiales, which has been confirmed by a systematic review including 16 articles and involving 777 IBS patients and 461 healthy controls [42]. However, some inconsistencies regarding the microbiota profile of IBS patients exist in the literature. A systematic review of 22 study articles evaluating adults with various IBS symptoms showed that, generally, patients with IBS tend to have decreased levels of Bifidobacteria and Faecalibacterum (including Faecalibacterium prausnitzii) and increased levels of Lactobacilli and Bacteroides [43], and other studies showed increased levels of Streptococci and Ruminococcus species when compared to healthy controls [44,45,46]. Previous publications have shown that patients with diarrhea-predominant IBS have lower expression of Clostridium thermosuccinogenes phylotype [47], whereas patients with constipation-predominant IBS have increased lactate-producing bacteria that produce sulphide and hydrogen [48].
3. Diet
The first-line treatment for IBS has concentrated on changing the patient’s dietary habits [49]. Dietary management has focused on reducing the consumption of certain carbohydrates that are highly fermentable but poorly absorbable, mainly the fermentable oligo-, di-, mono-saccharides and polyols (FODMAPs) that aggravate IBS symptoms, Table 1. When FODMAPs are poorly absorbed in the small intestines, they reach the colon, where they become fermented by the bacteria there, thus producing gas that causes bloating and osmotic changes leading to altered bowel motions [50]. There are at least 10 randomized controlled trials or randomized comparative trials that show that following a low FODMAPs diet improves global IBS symptoms, bloating, flatulence and diarrhea in 50–80% of the patients [51]. Previous publications have shown that changing the type of the consumed diet can change the gut microbiota in these patients with a parallel improvement in their symptoms [44,52,53,54], Table 2.

Table 1.
Types of FODMAPs and dietary fibers.

Table 2.
Randomized controlled trials investigating the effect of low FODMAP diet on gut microbiota and microbiota metabolites.
Several studies showed that using a low FODMAPs diet can reduce the abundance of several bacteria such as Bifidobacterium, Clostridium and Faecalibacterium prausnitzii in feces and increase the richness of Actinobacteria, Firmicutes and Clostridiales [44,53,54], but reported conflicting results concerning other bacterial types and the levels of microbiota metabolites, summarized in Table 2. A study by Staudacher et al. following IBS patients using a low FODMAPs diet for 12 months showed no difference in Bifidobacteria abundance in stool microbiota analysis [55]. However, there were lower concentrations of total fecal short-chain fatty acids, acetate, propionate and butyrate, before and after dietary management [55]. The study concludes that after completing all three phases of the low FODMAPs diet (restriction, reintroduction and personalization), it is safe and effective to follow a low FODMAPs diet for long-term when patients are supervised by a dietician [55]. The responsiveness to a low FODMAPs diet may be predicted by fecal microbiota profiles; for example, Phascolarctobacterium are more abundant in responders, and Firmicutes (Bacilli and Clostridia), Streptococcus, Dorea, Coprobacillus and Ruminococcus gnavus are more abundant in non-responders [56]. However, coadministration of multi-strain probiotics preparation (VSL#3) containing Streptococcus thermophilus, Bifidobacterium breve, B. longum, B. infantis, Lactobacillus acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus, to the low FODMAPs diet restores levels of Bifidobacterium species [52].
Dietary fibers play an important role as stool bulking agents as they cause water retention and may increase transit time [57]. They are divided into water-soluble and water-insoluble fibers and consist of short- and long-chain carbohydrates and lignin, Table 1. In IBS, using water-soluble fibers (psyllium) improves IBS symptoms, while water-insoluble fibers exacerbate them [20]. In addition, supplementing one’s diet with fructo- and galacto-oligosaccharides increase the abundance of Bifidobacterium due to their prebiotic activity, thus having a beneficial effect on the colon. Moreover, fermenting the dietary fibers by the colonic bacteria leads to the production of fecal short-chain fatty acids (propionate and butyrate) that reflect the activity of the gut microbiota [57,58,59,60].
In studies performed by our group, the aforementioned dietary modifications, using low FODMAPs and changing the dietary fiber intake, also showed significant changes in the densities of the enteroendocrine cells [20]. These cells are scattered throughout the whole gastrointestinal tract and are responsible for releasing the gut hormones that control the functions of the gastrointestinal tract after stimulating their microvilli with different types of nutrients [20]. In biopsies taken from different parts of the gastrointestinal tracts (stomach, small and large intestines) and dyed using special immunohistochemical staining, the densities of the different enteroendocrine cells in IBS patients at baseline were abnormal compared to healthy controls; however, they normalized toward the cells densities measured for healthy controls after dietary modifications [12,20,61,62,63,64,65,66].
4. Prebiotics, Probiotics and Antibiotics
Prebiotics, such as trans-galactooligosaccharide, are fermentable poorly-absorbable food elements that provide essential nutrients to enhance the growth of beneficial bacteria, such as Bifidobacterium and Lactobacillus, and have an anti-inflammatory effect that deters harmful pathogens in the bowels, which may contribute to the improvement of the global symptoms of IBS [67,68]. Probiotics are living microorganisms that consist of bacteria (mainly Bifidobacterium and Lactobacillus) and yeast, which are friendly to the gut and confer health benefits to the host when given in adequate amounts, usually in tablet forms or consumed in yogurt [58]. Probiotics have been used to beneficially manipulate the dysbiotic gut in IBS patients by improving the function of the gut barrier, inhibiting the overgrowth of pathogenic bacteria and producing short-chain fatty acids and several neurotransmitters [69], normalizing IL-10 and IL-12 levels and suppressing pro-inflammatory cytokine expression [70], Figure 1. Fifty-three randomized–controlled trials involving 5545 patients showed that probiotics appeared to have beneficial effects on global IBS symptoms and abdominal pain [71]. When it comes to the type of probiotics to be used, those that contain multiple bacterial strains (such as a combination of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus and Streptococcus thermophilus, known as LacClean Gold [72,73] or the combination of seven bacterial strains, namely; Lactobacillus acidophilus, L. plantarum, L. rhamnosus, Bifidobacterium breve, B. lactis, B. longum and Streptococcus thermophilus [74], are more beneficial than monostrain probiotics in alleviating IBS symptoms; however, the effects that the probiotics have on improving IBS symptoms are short-termed and do not last for a long time [58,75].
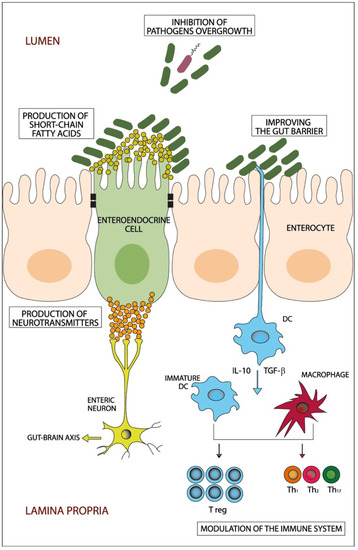
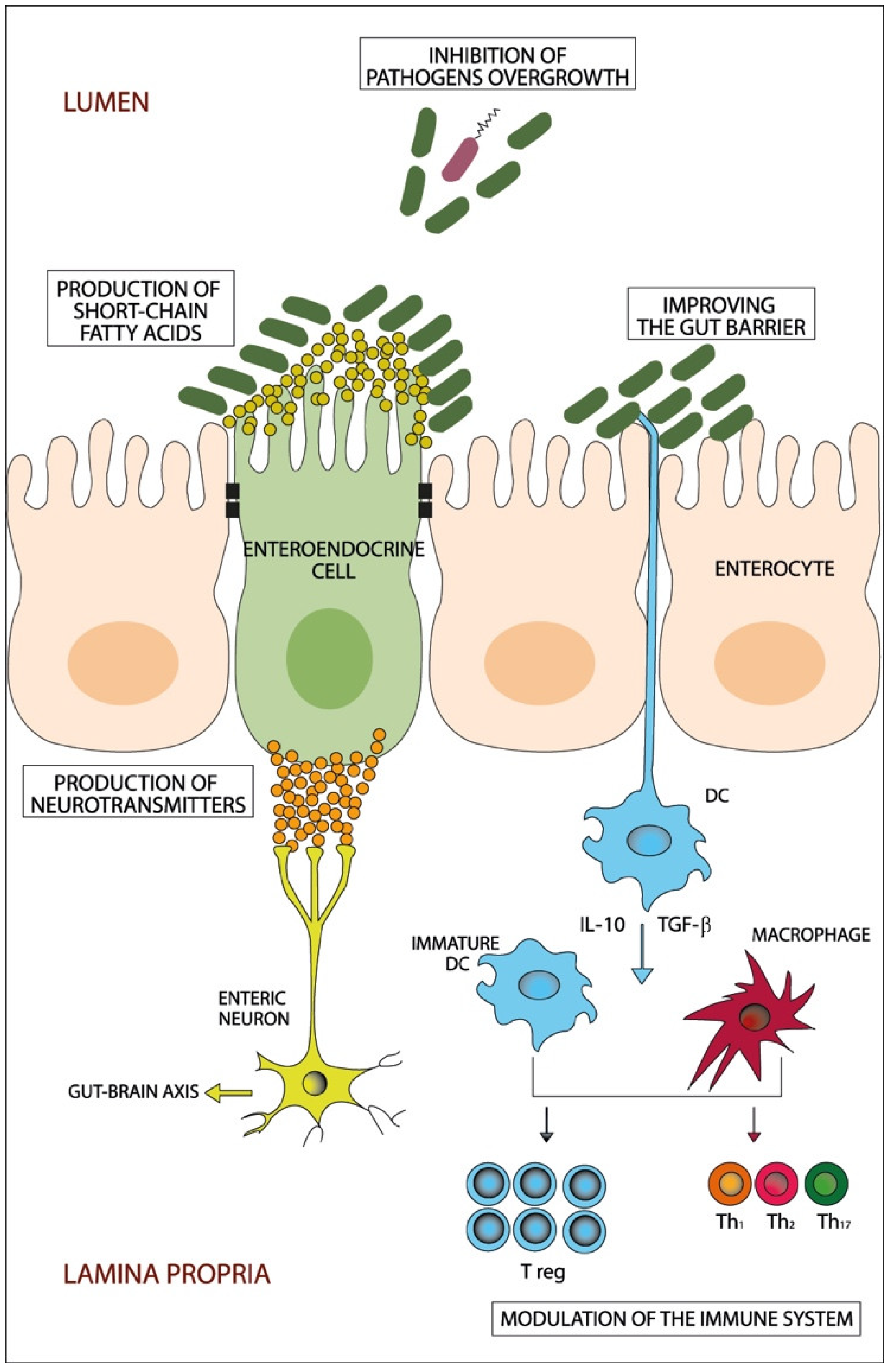
Figure 1.
A schematic diagram showing the potential beneficiary effects of probiotics on the gut. Probiotics beneficially manipulate the dysbiotic gut through different potential mechanisms that include inhibition of pathogens’ overgrowth, improving the gut barrier, production of short-chain fatty acids and neurotransmitters and modulation of the immune system. DC: dendritic cells; IL: interleukin; Th: T helper cell; T reg: T regulatory cell; TGF-β: Transforming growth factor-β.
On the other hand, the use of nonabsorbable antibiotics, such as rifixamin, has been investigated in several double-blinded and placebo-controlled randomized controlled trials [71,76] and has been approved by the US Food and Drug Administration for treating patients with diarrhea-predominant IBS [77]. It is suggested that the beneficial effect of using rifixamin in repeated courses occurs by reducing the total load of the gut microbiota and modulating intestinal permeability, thus improving bloating and diarrhea in these patients [78]. The combination of rifixamin and neomycin has improved constipation and bloating in patients with constipation-predominant IBS [79].
5. Fecal Microbiota Transplantation
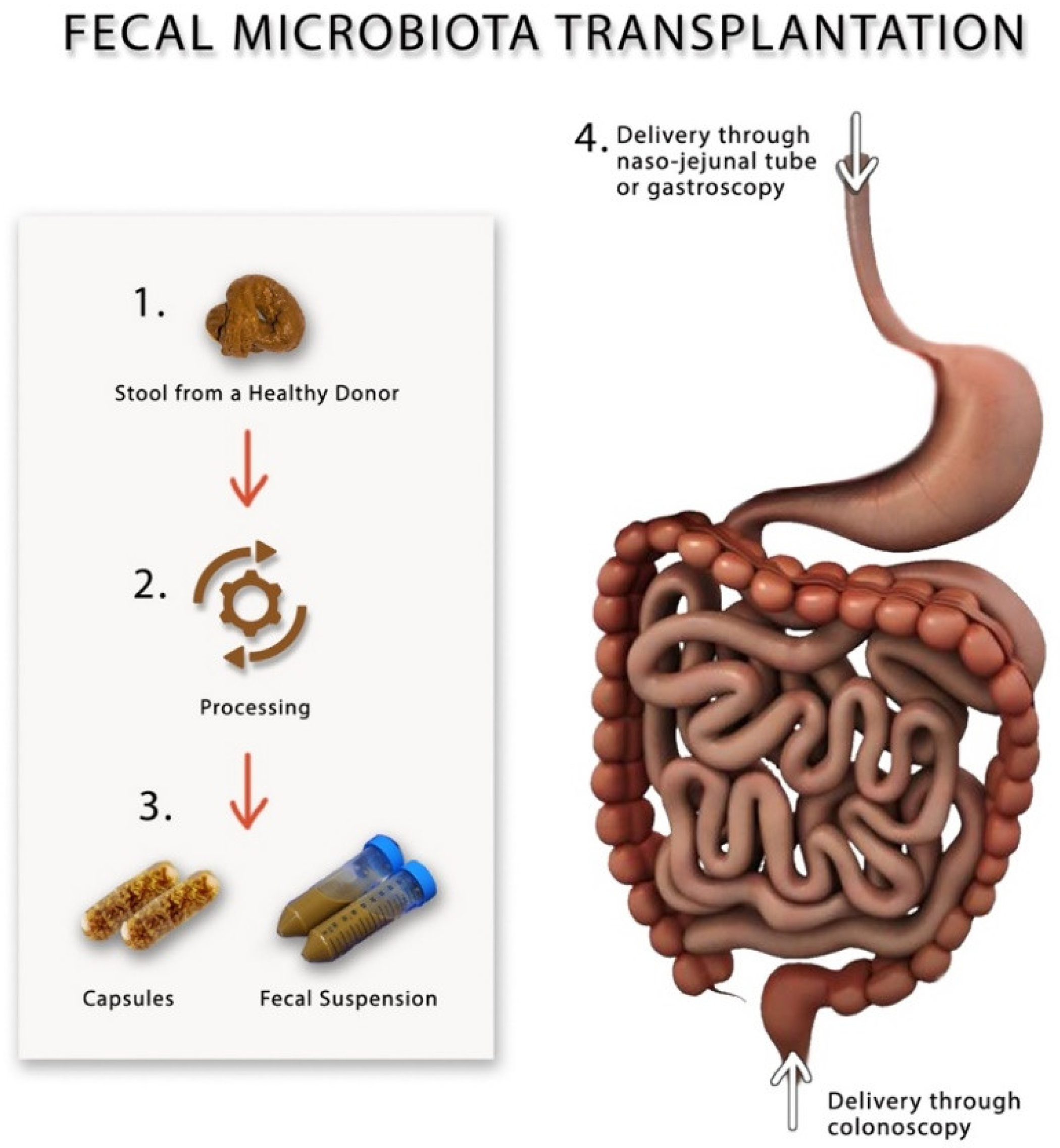
Fecal microbiota transplantation (FMT) has recently become popular as a novel method for modulating gut microbiota in gastrointestinal disorders such as inflammatory bowel syndrome, IBS and recurrent C. difficile infection [80,81,82,83], and non-gastrointestinal diseases such as chronic fatigue syndrome, obesity and even some neuropsychiatric disorders [83,84]. During FMT, a suspension made from fecal material, which is collected from healthy individuals, is infused into the gut of the patient via naso-jejunal tube, gastroscope or colonoscope [83], Figure 2. FMT is currently only used in clinical research trials and is considered a safe procedure once one adheres to the current guidelines [85]. Before performing FMT, screening of the donors should include performing thorough physical and laboratory investigations by blood and stool analysis and culture to rule out organic disorders, infectious agents and contagious diseases, most importantly, HIV, viral hepatitis, syphilis, malaria, tuberculosis and trypanosomiasis, to avoid transmitting them to the recipient [86]. It is advisable that the donors of the feces have not recently used antibiotics, travelled to tropical areas, had high-risk sexual behavior or had a bout of gastroenteritis or diarrhea within 4 weeks of donation [85]. It is not yet clear what is the correct dose or the frequency of FMT that should be performed on patients with IBS; however, according to the consensus guidelines, at least 30 g of donor feces should be added to the saline solution in order to prepare the fecal suspension that should be either stored at −80 °C or infusion directly on the same day of preparation [85]. After performing FMT, the stool will be collected from the recipients and stored in a special freezer at −80 °C for further microbial analysis [80]. Table 3 describes the different randomized controlled FMT trials, the dosages and the frequency of fecal transplants.
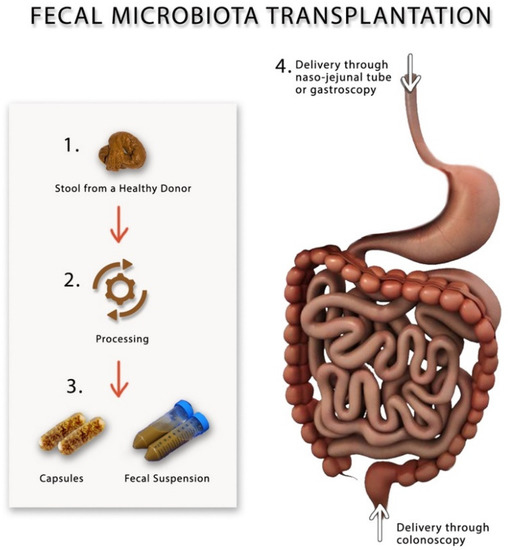
Figure 2.
A schematic drawing showing different methods for preparing and performing fecal microbiota transplantation.
A recent meta-analysis, including seven placebo-controlled randomized controlled studies, has investigated the effect of this novel method involving 470 patients with IBS [87]. Five out of these trials used fresh/frozen fecal material [88,89,90,91,92], while the other two trials used frozen oral FMT capsules compared to placebo capsules [93,94]. These studies showed conflicting results. The meta-analysis [87] suggested that the form of transplantation used in each study had a significant effect on the study outcome, indicating that the fresh/frozen fecal material might be superior to frozen oral capsules in improving IBS global symptoms and having lasting alteration of gut microbiota, Table 3.

Table 3.
Randomized controlled trials investigating the effect of fecal microbiota transplantation on gut microbiota and microbiota metabolites.
Table 3.
Randomized controlled trials investigating the effect of fecal microbiota transplantation on gut microbiota and microbiota metabolites.
| Authors, Years | Diagnostic Criteria, Study Duration | Sample Size, IBS Subtypes | Allocation | Donors | Bowel Cleansing | FMT Route and Location (Upper/Lower GI Tract), Frequency | Dosage of FMT Group | Dosage of Control Group | Microbial Analysis | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Aroniadis et al., 2019 [93] | Rome III, 3 months | n = 48: 100% IBS-D | 1:1 | Four donors, not mixed | No | Oral capsule ( upper), multiple lasted 3 days | 25 frozen capsules (0.38 g FMT) per day | 25 placebo capsules per day | 16S rRNA | Bacterial composition of FMT recipients shifted closer to that of the donors. |
| El-salhy et al., 2019 [91] | Rome IV, 3 months | n = 165: 37.8% IBS-C; 38.4% IBS-D; 23.8% IBS-M | 1:1:1 | One donor, not mixed | No | Gastroscopy (upper), single FMT | Frozen 30 g FMT and 60 g FMT | Frozen 30 g autologous feces | 16S rRNA | Higher abundance of Eubacterium biforme, Lactobacillus spp. and Alistipes spp., lower abundance of Bacteroides spp. Inverse correlation between IBS symptoms and the concentrations of Lactobacillus spp. and Alistipes spp. Negative correlation between the Fatigue Assessment Scale score and the concentration of Alistipes spp. |
| Halkjær et al., 2018 [94] | Rome III, 6 months | n = 52: 33.3% IBS-C; 29.4% IBS-D; 37.3% IBS-M | 1:1 | Four donors, mixed FMT | Yes | Oral capsule (upper), multiple administrations lasted 12 days | 25 frozen capsules (50 g FMT) | 25 placebo capsules per day | 16S rRNA | Fecal donors had higher biodiversity than IBS patients. Microbiota of FMT recipients are more similar to the donors’ microbiota than to that of the placebo recipient. Microbiota of placebo recipient did not become more similar to the donors’ microbiota than patients with IBS before randomization. Bacteroides genus and Ruminococcaceae family correlate positively with IBS symptoms score. Blautia genus and Clostridiales correlate negatively with IBS symptoms score. |
| Holster et al., 2019 [90] | Rome III, 6 months | n = 17: 25% IBS-C; 56.3% IBS-D; 18.8% IBS-M | 1:1 | Two donors, not mixed | Yes | Colonoscopy (lower), single FMT | Frozen 30 g FMT | Frozen 30 g autologous feces | Human Intestinal Tract Chip (fecal and mucosa) | The abundance of butyrate-producing bacteria in patients’ fecal samples was not lower than the donors at baseline. Microbial composition of patients had changed to resemble that of the donor after FMT. No effect on microbial diversity was observed after FMT in fecal or mucosal microbiota. |
| Holvoet et al., 2020 [89] | Rome III, 3 months | n = 62: 100% IBS-D/IBS-M. | 2:1 | Two donors; not mixed | No | Naso-jejunal tube (upper), single FMT | Donor fresh feces | Autologous feces | 16S rRNA | Donors’ fecal samples had higher diversity than the patients. Responders to FMT had a higher microbial diversity at baseline compared to non-responders. There was a significant difference in overall bacterial composition between responder and non-responders before treatment. Bacterial composition of FMT recipients shifted closer to that of the donors. |
| Johnsen et al., 2018 [92] | Rome III, 12 months | n = 90: 53% IBS-D; 47% IBS-M | 2:1 | Two donors, mixed | Yes | Colonoscopy (lower), single FMT | Frozen or fresh 50–80 g FMT | Frozen or fresh 50– 80 g autologous feces | Not reported | Not reported |
| Lahtinen et al., 2020 [88] | Rome III, 3 months | n = 55: 51% IBS-D; 14.3% IBS-M; 28.6% IBS unsubtyped; 6.1% other | 1:1 | One donor, not mixed | Yes | Colonoscopy (lower), single FMT | Frozen 30 g FMT | Fresh 30 g autologous feces | 16S rRNA | Changes in gut microbiota profile was observed. |
IBS: irritable bowel syndrome; IBS-C: constipation-predominant IBS; IBS-D: diarrhea-predominant IBS; IBS-M: mixed-IBS; FMT: fecal microbiota transplantation; GI: gastrointestinal.
In several studies, butyrate-producing bacteria in fecal samples of the recipient IBS patients were not lower than that of the donors, for example, Eubacterium halli, Eubacterium rectale, Megasphera elsdenii, Faecalibacterium prausnitzii [90], Alistipes spp. [90,91], Eubacterium biforme and Lactobacillus spp. [91] were increased, while Bacteroides spp. was decreased in responders following FMT [91]. Moreover, Lactobacillus spp. was negatively correlated with the clinical outcome of IBS-symptom severity score [91]. In addition, our group and other publications also reported that IBS patients with low fecal Alistipes spp. were most likely to not respond to FMT [41,95] and that FMT also increased the total fecal short-chain fatty acids levels, namely; butyric acid [41,96], which was inversely correlated with IBS symptoms [96]. Donor selection seems to be important, but it remains to be investigated whether single or mixed donors is the preferred choice and at which time intervals should FMT be performed. Several trials showed contradictory results when using either single [91,93,97] or mixed donors [92,94], but most of them showed that the bacterial composition of FMT recipients shifted closer to that of the donors, Table 3. A study showed that increasing the dose of fecal transplant to 60 g and/or using repetitive FMT may increase the response rate in IBS patients to FMT [98].
In several open-labeled studies performed by our group, we investigated the effect of FMT from the healthy relatives of the patients on IBS symptoms, gut microbiota, short-chain fatty acids, stem cells and enteroendocrine cells in diarrhea-predominant IBS patients [41,66,80,99,100]. According to our studies, the IBS symptom severity score improved significantly following FMT [41,80]. The bacterial strains signals for Ruminococcus gnavus, Actinobacteria and Bifidobacteria and the fecal short-chain fatty acids were significantly different between IBS patients and their donors, which became insignificantly different starting at 3 weeks after FMT and lasting up to 6 months following FMT [41]. However, the beneficial effect of FMT on IBS symptoms tends to fade over time, as observed in other trials [41,80,89,91]. The gut microbiota profile for IBS patients became more or less similar to that of their donors following FMT [41,80], which is consistent with the findings of several randomized control trials mentioned in Table 3. Furthermore, our studies also showed that altering the gut microbiota following FMT was paralleled by changes in the densities of the duodenal stem cells progenitors and the densities of the enteroendocrine cells in the duodenum and colon toward the densities measured in healthy controls [66,99,100]. This suggests that manipulating the gut microbiota by FMT changes the so-called “gut microenvironment” in the gastrointestinal tract of IBS patients, which may be responsible for improving the global symptoms of IBS.
6. Conclusions
There is strong evidence that dysbiosis plays an important role in the pathophysiology of IBS. There are different non-pharmacological methods that can improve the symptoms of IBS, which affect the gut microenvironment. Manipulating the gut microenvironment not only changes the composition of gut microbiota but also affects the other components of the gut microenvironment, namely short-chain fatty acids that represent the gut microbiota function and the enteroendocrine cells densities with a total impact on the symptoms of IBS. More studies with larger cohorts and for longer terms are required to investigate this issue.
Funding
This research received no external funding.
Acknowledgments
The author would like to thank the graphic designer Marcelle Mazzawi for preparing the figures.
Conflicts of Interest
The author declares no conflict of interest.
References
- Schuster, M.M. Defining and diagnosing irritable bowel syndrome. Am. J. Manag. Care 2001, 7 (Suppl. 8), S246–S251. [Google Scholar] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Irritable bowel syndrome: Recent developments in diagnosis, pathophysiology, and treatment. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-Reported Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated With More Severe Symptoms and Reduced Quality of Life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Ye, Z.; Wang, J.; Liao, X.; Liv, M.; Svn, Z. Risk of Colorectal Cancer in Patients With Irritable Bowel Syndrome: A Meta-Analysis of Population-Based Observational Studies. Front. Med. 2022, 9, 819122. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.A.; Yan, S.; Strassels, S. Impact of Irritable Bowel Syndrome on Quality of Life and Resource Use in the United States and United Kingdom. Digestion 1999, 60, 77–81. [Google Scholar] [CrossRef]
- Patrick, D.L.; Drossman, D.A.; Frederick, I.O.; Dicesare, J.; Puder, K.L. Quality of Life in Persons with Irritable Bowel Syndrome (Development and Validation of a New Measure). Am. J. Dig. Dis. 1998, 43, 400–411. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Hungin, A.P.S.; Whorwell, P.J.; Tack, J.; Mearin, F. The prevalence, patterns and impact of irritable bowel syndrome: An international survey of 40 000 subjects. Aliment. Pharmacol. Ther. 2003, 17, 643–650. [Google Scholar] [CrossRef]
- Rayman, R.B. Irritable bowel syndrome: Aeromedical considerations. Aviat. Space Environ. Med. 2011, 82, 1061–1063. [Google Scholar] [CrossRef]
- Hong, S.N.; Rhee, P.L. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J. Gastroenterol. 2014, 20, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; El-Salhy, M. Changes in duodenal enteroendocrine cells in patients with irritable bowel syndrome following dietary guidance. Exp. Biol. Med. 2017, 242, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Mazzawi, T.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Changes in the symptom pattern and the densities of large-intestinal endocrine cells following Campylobacter infection in irritable bowel syndrome: A case report. BMC Res. Notes 2013, 6, 391. [Google Scholar] [CrossRef]
- Grover, M.; Camilleri, M.; Smith, K.; Linden, D.R.; Farrugia, G. On the fiftieth anniversary Postinfectious irritable bowel syndrome: Mechanisms related to pathogens. Neurogastroenterol. Motil. 2014, 26, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Tada, Y.; Fukuba, N.; Oka, A.; Kusunoki, R.; Mishima, Y.; Oshima, N.; Moriyama, I.; Yuki, T.; Kawashima, K.; et al. Pathogenesis of Irritable Bowel Syndrome—Review Regarding Associated Infection and Immune Activation. Digestion 2013, 87, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.A. The Role of Genetics in IBS. Gastroenterol. Clin. N. Am. 2011, 40, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-Related Gastrointestinal Symptoms in the Irritable Bowel Syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome-etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, K.S. Irritable bowel syndrome: Emerging paradigm in pathophysiology. World J. Gastroenterol. 2014, 20, 2456–2469. [Google Scholar] [CrossRef]
- Mazzawi, T.; El-Salhy, M. Effect of diet and individual dietary guidance on gastrointestinal endocrine cells in patients with irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 943–952. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. Is irritable bowel syndrome an organic disorder? World J. Gastroenterol. 2014, 20, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Chen, C.Y.; Chang, F.Y.; Lee, S.D. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: An Oriental study. Clin. Sci. 1998, 95, 165–169. [Google Scholar] [CrossRef]
- Spiller, R. Inflammation as a basis for functional GI disorders. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Srivastava, D. Irritable bowel syndrome and small intestinal bacterial overgrowth: Meaningful association or unnecessary hype. World J. Gastroenterol. 2014, 20, 2482–2491. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.; Braverman, D.; Stankiewicz, H. Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. Isr. Med Assoc. J. IMAJ 2000, 2, 583–587. [Google Scholar]
- Camilleri, M. Physiological underpinnings of irritable bowel syndrome: Neurohormonal mechanisms. J. Physiol. 2014, 592 Pt 14, 2967–2980. [Google Scholar] [CrossRef]
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent advances in the treatment of irritable bowel syndrome. Pol. Arch. Intern. Med. 2021, 131, 709–715. [Google Scholar] [CrossRef]
- Tack, J.; Vanuytsel, T.; Corsetti, M. Modern Management of Irritable Bowel Syndrome: More Than Motility. Dig. Dis. 2016, 34, 566–573. [Google Scholar] [CrossRef]
- Rao, S.; Weber, H.C. New treatment targets for the management of irritable bowel syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 9–14. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Isolauri, E.; Salminen, S. Gut microbiota: A source of novel tools to reduce the risk of human disease? Pediatr. Res. 2015, 77, 182–188. [Google Scholar] [CrossRef]
- Huurre, A.; Kalliomäki, M.; Rautava, S.; Rinne, M.; Salminen, S.; Isolauri, E. Mode of delivery-effects on gut microbiota and humoral immunity. Neonatology 2008, 93, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Rushing, J. Cesarean Versus Vaginal Delivery: Long-term Infant Outcomes and the Hygiene Hypothesis. Clin. Perinatol. 2011, 38, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Wilson, B.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients 2019, 11, 1824. [Google Scholar] [CrossRef]
- Bennet, S.M.; Ohman, L.; Simren, M. Gut Microbiota as Potential Orchestrators of Irritable Bowel Syndrome. Gut Liver 2015, 9, 318–331. [Google Scholar] [CrossRef]
- Chang, C.; Lin, H. Dysbiosis in gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 3–15. [Google Scholar] [CrossRef]
- Bartlett, J.G. Narrative Review: The New Epidemic of Clostridium difficile–Associated Enteric Disease. Ann. Intern. Med. 2006, 145, 758–764. [Google Scholar] [CrossRef]
- Jalanka, J.; Salojärvi, J.; Salonen, A.; Immonen, O.; Garsed, K.; Kelly, F.M.; Zaitoun, A.; Palva, A.; Spiller, R.; De Vos, W.M. Faecal microbiota composition and host–microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014, 63, 1737–1745. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; Hov, J.R.; Valeur, J.; Sangnes, D.A.; El-Salhy, M.; Gilja, O.H.; Hatlebakk, J.G.; Lied, G.A. Clinical response to fecal microbiota transplantation in patients with diarrhea-predominant irritable bowel syndrome is associated with normalization of fecal microbiota composition and short-chain fatty acid levels. Scand. J. Gastroenterol. 2019, 54, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of Gut Microbiota in Patients With Irritable Bowel Syndrome Based on 16S rRNA-Targeted Sequencing: A Systematic Review. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e8. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-S.; Chang, P.-F.; Liao, C.-H.; Lee, T.-H.; Chen, Y.; Lee, Y.-C.; Wu, M.-S.; Wang, H.-P.; Ni, Y.-H. Differences of microbiota in small bowel and faeces between irritable bowel syndrome patients and healthy subjects. Scand. J. Gastroenterol. 2015, 51, 410–419. [Google Scholar] [CrossRef]
- Lyra, A.; Rinttilä, T.; Nikkilä, J.; Krogius-Kurikka, L.; Kajander, K.; Malinen, E.; Mättö, J.; Mäkelä, L.; Palva, A. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J. Gastroenterol. 2009, 15, 5936–5945. [Google Scholar] [CrossRef]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’Homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Vasant, D.H.; A Paine, P.; Black, C.J.; A Houghton, L.; A Everitt, H.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.; Irving, P.M.; et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef] [PubMed]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 2017, 67, 872–881. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef]
- Dale, H.F.; Lied, G.A. Gut microbiota and therapeutic approaches for dysbiosis in irritable bowel syndrome: Recent developments and future perspectives. Turk. J. Med Sci. 2020, 50, 1632–1641. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Flourié, B.; Riottot, M.; Bisetti, N.; Gailing, M.; Guibert, A.; Bornet, F.; Rambaud, J. Effects of fructo-oligosaccharides ingestion on fecal bifidobacteria and selected metabolic indexes of colon carcinogenesis in healthy humans. Nutr. Cancer 1996, 26, 21–29. [Google Scholar] [CrossRef]
- Mazzawi, T.; El-Salhy, M. Changes in small intestinal chromogranin A-immunoreactive cell densities in patients with irritable bowel syndrome after receiving dietary guidance. Int. J. Mol. Med. 2016, 37, 1247–1253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzawi, T.; El-Salhy, M. Dietary guidance and ileal enteroendocrine cells in patients with irritable bowel syndrome. Exp. Ther. Med. 2016, 12, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Gundersen, D.; Hausken, T.; El-Salhy, M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol. Med. Rep. 2014, 10, 2322–2326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur. J. Clin. Nutr. 2016, 70, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur. J. Clin. Nutr. 2015, 69, 519–524. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; El-Salhy, M. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand. J. Gastroenterol. 2022, 1–5. [Google Scholar] [CrossRef]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Canakis, A.; Haroon, M.; Weber, H.C. Irritable bowel syndrome and gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 28–35. [Google Scholar] [CrossRef]
- Chlebicz-Wójcik, A.; Śliżewska, K. Probiotics, Prebiotics, and Synbiotics in the Irritable Bowel Syndrome Treatment: A Review. Biomolecules 2021, 11, 1154. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Sohn, W.; Lee, O.Y.; Lee, S.P.; Lee, K.N.; Jun, D.W.; Lee, H.L.; Yoon, B.C.; Choi, H.S.; Chung, W.-S.; et al. Effect of multispecies probiotics on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2014, 29, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Park, Y.S.; Lee, D.H.; Seo, J.-G.; Shin, C.M.; Kim, N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Biochem. Nutr. 2015, 57, 129–134. [Google Scholar] [CrossRef]
- Cha, B.K.; Jung, S.M.; Choi, C.H.; Song, I.D.; Lee, H.W.; Kim, H.J.; Hyuk, J.; Chang, S.K.; Kim, K.; Chung, W.S.; et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Shayto, R.H.; Mrad, R.A.; I Sharara, A. Use of rifaximin in gastrointestinal and liver diseases. World J. Gastroenterol. 2016, 22, 6638–6651. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Pimentel, M.; Rao, S.S.; Schoenfeld, P.; Cash, B.; Weinstock, L.B.; Paterson, C.; Bortey, E.; Forbes, W.P. Repeat Treatment With Rifaximin Is Safe and Effective in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2016, 151, 1113–1121. [Google Scholar] [CrossRef]
- Pimentel, M.; Chang, C.; Chua, K.S.; Mirocha, J.; DiBaise, J.; Rao, S.; Amichai, M. Antibiotic Treatment of Constipation-Predominant Irritable Bowel Syndrome. Am. J. Dig. Dis. 2014, 59, 1278–1285. [Google Scholar] [CrossRef]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef]
- Kelly, C.R.; Ihunnah, C.; Fischer, M.; Khoruts, A.; Surawicz, C.; Afzali, A.; Aroniadis, O.; Barto, A.; Borody, T.; Giovanelli, A.; et al. Fecal Microbiota Transplant for Treatment of Clostridium difficile Infection in Immunocompromised Patients. Am. J. Gastroenterol. 2014, 109, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- Aroniadis, O.C.; Brandt, L.J. Fecal microbiota transplantation: Past, present and future. Curr. Opin. Gastroenterol. 2013, 29, 79–84. [Google Scholar] [CrossRef]
- Fretheim, H.; Chung, B.K.; Didriksen, H.; Bækkevold, E.S.; Midtvedt, Ø.; Brunborg, C.; Holm, K.; Valeur, J.; Tennøe, A.H.; Garen, T.; et al. Fecal microbiota transplantation in systemic sclerosis: A double-blind, placebo-controlled randomized pilot trial. PLoS ONE 2020, 15, e0232739. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilic-Stojanovic, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, L.; Wang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2022, 12, 827395. [Google Scholar] [CrossRef]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.-J.; Tillonen, J.; Satokari, R.; et al. Randomised clinical trial: Faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef]
- Holster, S.; Lindqvist, C.M.; Repsilber, D.; Salonen, A.; de Vos, W.M.; König, J.; Brummer, R.J. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin. Transl. Gastroenterol. 2019, 10, e00034. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Kristoffersen, A.B.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2019, 69, 859–867. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J.; Oneto, C.; Feuerstadt, P.; Sherman, A.; Wolkoff, A.W.; Kassam, Z.; Sadovsky, R.G.; Elliott, R.J.; Budree, S.; et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: A double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 675–685. [Google Scholar] [CrossRef]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.; Günther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Mazzawi, T.; Hausken, T.; Hatlebakk, J.G. Irritable bowel syndrome patients who are not likely to respond to fecal microbiota transplantation. Neurogastroenterol. Motil. 2022, e14353. [Google Scholar] [CrossRef]
- El-Salhy, M.; Kristoffersen, A.B.; Valeur, J.; Casen, C.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Long-term effects of fecal microbiota transplantation (FMT) in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2021, 34, e14200. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, T.; Joossens, M.; Wang, J.; Boelens, J.; Verhasselt, B.; Laukens, D.; van Vlierberghe, H.; Hindryckx, P.; De Vos, M.; De Looze, D.; et al. Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut 2016, 66, 980–982. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hausken, T.; Hatlebakk, J.G. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS). Nutrients 2019, 11, 1415. [Google Scholar] [CrossRef]
- Mazzawi, T.; Eikrem, Ø.; Lied, G.A.; Hausken, T. Abnormal Uroguanylin Immunoreactive Cells Density in the Duodenum of Patients with Diarrhea-Predominant Irritable Bowel Syndrome Changes following Fecal Microbiota Transplantation. Gastroenterol. Res. Pract. 2020, 2020, 3520686. [Google Scholar] [CrossRef]
- Mazzawi, T.; El-Salhy, M.; Lied, G.A.; Hausken, T. The Effects of Fecal Microbiota Transplantation on the Symptoms and the Duodenal Neurogenin 3, Musashi 1, and Enteroendocrine Cells in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 403. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).