Abstract
Considering a scenario where there is a low availability and increasing costs of fertilizers in the global agricultural market, as well as a finitude of important natural resources, such as phosphorus (P), this study tested the effect of the inoculation of rhizospheric or endophytic microorganisms isolated from Hymenaea courbaril and Butia purpurascens on the growth promotion of Glycine max (L.) Merr. The tests were conducted in a controlled greenhouse system, and the effects of biofertilization were evaluated using the following parameters: dry biomass, nutritional content, and photochemical and photosynthetic performance of plants. Seed biopriming was performed with four bacterial and four fungal isolates, and the results were compared to those of seeds treated with the commercial product Biomaphos®. Overall, microbial inoculation had a positive effect on biomass accumulation in G. max, especially in strains PA12 (Paenibacillus alvei), SC5 (Bacillus cereus), and SC15 (Penicillium sheari). The non-inoculated control plants accumulated less nutrients, both in the whole plant and aerial part, and had reduced chlorophyll index and low photosynthetic rate (A) and photochemical efficiency. Strains PA12 (P. alvei), SC5 (B. cereus), and 328EF (Codinaeopsis sp.) stood out in the optimization of nutrient concentration, transpiration rate, and stomatal conductance. Plants inoculated with the bacterial strains PA12 (P. alvei) and SC5 (B. cereus) and with the fungal strains 328EF (Codinaeopsis sp.) and SC15 (P. sheari) showed the closest pattern to that observed in plants treated with Biomaphos®, with the same trend of direction of the means associated with chlorophyll index, (A), dry mass, and concentration of important nutrients such as N, P, and Mg. We recommend the use of these isolates in field tests to validate these strains for the production of biological inoculants as part of the portfolio of bioinputs available for G. max.
1. Introduction
Soybean (Glycine max (L.) Merr.) is an important oilseed in crop rotation systems designed for high yield and efficiency [1]. Currently, this crop is affected by P deficiency that restricts plant growth. Therefore, P is often a limiting nutrient in agricultural systems and its deficiency decreases agricultural productivity. Therefore, chemical fertilizers are widely used for optimal yields; however, they are expensive, cause eutrophication of rivers, and their use in tropical acidic soils is limited by their low ion exchange capacity [2,3,4,5,6]. Although P is abundant in many soil types, most of it is not readily accessible to plants due to the high affinity of phosphate anions for Fe, Al, and CaO resulting in the formation of less soluble compounds [7]. Thus, different strategies have been developed to improve the supply of P to crops, the most promising being the use of microorganisms that participate in the transformation of soil P [8,9,10]. Currently, in Brazil, the BiomaPhos® inoculant is the only such product available in the market. It was developed with the purpose of promoting plant growth through the action of phosphate-solubilizing bacterial strains.
Studies have shown that in addition to nutrient solubilization, multifunctional microorganisms benefit plant growth and crop yield through various mechanisms, including: nitrogen fixation; ammonia production; syntheses of siderophores and growth-inducing hormones such as auxins, gibberellins, and cytokinins; control of phytopathogens by antibiosis; or synthesis of 1-aminocyclopropane-1-carboxylic acid deaminase, which increases plant growth under stress conditions, thereby improving plant resistance to heavy metal toxicity [11,12,13,14]. Rhizosphere-inhabiting microorganisms that have a beneficial effect on plant growth are known as plant growth-promoting microorganisms (PGPMs) [15]. PGPMs have been commonly used as biofertilizers in agricultural systems, and research has shown significant results, such as an increase in crop yield by 50–70%, with the use of rhizobacteria [16,17].
However, some studies show that the world fertilizer production will have to increase significantly to meet future demands, i.e., an increase of 50–100% in 2050 relative to 2005, depending on the food growth pathway [18]. Considering a scenario where there is low availability of fertilizers in the global agricultural market, with a general increase in prices, which are buoyed by oil prices, the market is threatened [19]. Moreover, given the worldwide dissemination of the environmental policy of rationalizing the use of soil resources and that the natural sources of some fertilizers, such as P, are finite [20], the selection of microbial strains that effectively promote the growth of major crops becomes essential. These strains can improve the plant’s accessibility to not only fertilization [21] but also the available nutrients (accumulated over decades of fertilizer application in crop fields). In addition to improving crop yield and nutrient supply, biofertilization integrates practices aimed at the development of a more sustainable and environmentally friendly agriculture [22].
In a previous study, Reis et al. [23] used a hydroponic system to select plant growth-promoting strains for G. max based on biometric and photosynthetic characteristics and chlorophyll a fluorescence patterns. We thus tested the hypothesis that some of these strains promote the growth of G. max cultivated in a controlled greenhouse system. Our objective was to refine the data for a coherent selection of strains that can be used in future field trials and be part of a safe portfolio of bioinputs for the cultivation of G. max.
Because the dynamics of plant–microorganism interactions depend on many factors, including physiological characters of plants and microorganisms, climatic conditions, soil type, salinity, and pH [24,25,26,27,28], we developed this preliminary study under controlled greenhouse conditions, in which it was possible to isolate factors such as competition with microorganisms already residing in the soil and abiotic stresses, including nutrient fluctuations.
We tested the effect of the inoculation of microorganisms previously isolated as rhizospheric or endophytic from the tree plants Hymenaea courbaril and Butia purpurascens. Because microorganisms play an important role in improving the nutritional [29,30,31,32,33], photochemical, and photosynthetic states of plants, we used variables associated with these parameters to evaluate the performance of microorganisms.
2. Materials and Methods
2.1. Microbial Isolates and Inoculum Preparation
Eight microbial isolates (04 fungi and 04 bacteria), rhizospheric or endophytic, were evaluated; six were previously isolated from H. courbaril (H) [34], a species widely distributed in the Cerrado biome, and two from B. purpurascens (BP) [35], an Arecaceae endemic to this biome (Table 1). These strains belong to the culture collection of the Laboratory of Agricultural Microbiology at IFGoiano, Rio Verde campus. The phosphate-solubilizing potential of these strains was previously evaluated in a hydroponic system by Reis et al. [23], by comparing it with that of the commercial product Biomaphos®, which consists of a mixture of the strains BRM034840 and BRM033112 of Bacillus megaterium and Bacillus subtilis. This study validated the effects that were previously observed in the hydroponic system. Thus, in the present study, the isolates were evaluated as growth promoters of G. max cultivated in a controlled greenhouse system. For this, the bacterial strains were reactivated in nutrient agar (NA) medium (meat extract—3 g, peptone—5 g, agar—25 g, and H2O qs 1 L) for 48 h at 30 °C in a bacterial growth chamber, while the fungal strains were reactivated in potato dextrose agar (PDA) (infusion of potato—200 g, dextrose—20 g and agar—15 g), for seven days at 30 °C.

Table 1.
Microbial isolates evaluated in the promotion of Glycine max growth in a controlled greenhouse system. In isolate code, E = endophytic; R = rhizospheric.
The bacterial inocula were obtained in nutrient broth for 24 h at 30 °C under agitation at 90 rpm. Subsequently, the cell concentration in the cultures was estimated by counting the colony forming units, and this concentration was standardized to 104 CFU mL−1, with 0.85% saline solution. The fungal mycelia were cultivated in PDA medium plates for 14 days at 30 °C. In sequence, the surface of the plates was washed with 10 mL of saline solution (0.85%) per plate, and the resulting solution was evaluated for spore concentration by counting in a Neubauer chamber, under light microscopy (magnification of 40–100×). The spore concentration of the different cultures was adjusted to 105 spores mL−1.
2.2. Soil Treatment, Seed Biopriming, and Planting
The experiment was carried out in a greenhouse belonging to the Laboratory of Plant Tissue Culture of the IFGoiano, Rio Verde campus, with the geographical coordinates 17°48′15.9″ S—50°54′19.5″ W, from April to June 2021, under a mean temperature of 31.35 °C and relative humidity of 30.19%. Before planting, the soil was sampled for chemical and physical analyses (Table 2). Subsequently, correction with limestone was performed using calcitic limestone, considering the recommendation for acidic soils, of 200 g m2, and the soil was watered for 30 days. The soil was then fertilized with the recommended doses of NPK 02-20-18 (500 g ha−1) and full-strength nutrient solution of Hoagland and Arnold [36] was applied.

Table 2.
Chemical and physical characteristics of the soil used in the experiment of growth promotion of Glycine max by microbial isolates obtained from Hymenaea courbaril and Butia purpurascens in a controlled greenhouse system.
The experiment was conducted using seeds of the Bônus 8579 RSF IPRO cultivar of G. max. The seeds were disinfected to remove epiphytic microorganisms. For this, successive rinses were performed in running water, followed by agitation in water and Tween for 5 min. Subsequently, the seeds were treated in 70% ethanol (1 min), immersion in sodium hypochlorite (2.5% active chlorine for 1 min and 30 s), and again in 70% ethanol (30 s). Finally, the seeds were rinsed three times in sterile distilled water and left to rest on sterile paper towels for 2 h.
The seed biopriming treatments were conducted separately, with each microbial culture, using 30 soybean seeds. The seeds remained immersed for 20 min, under agitation at 50 rpm, in an orbital shaker. The control treatment consisted of seeds immersed in culture medium without inoculum. The seeds were planted after being recovered from the microbial broth corresponding to each treatment.
The seeds were planted in 5 L pots filled with 4 L of the soil described above. In addition to sterilizing the seed surface, the soil was sterilized to eliminate microorganisms that could compete with the inoculum in the colonization of plant tissue. For this, the soil was autoclaved for 30 min at 121 °C. The effectiveness of sterilization was evaluated by preparing a solution of 10 g of the autoclaved soil in 90 mL of sterile distilled water and inoculating 100 µL of this solution into AN broth. The broth was incubated for 48 h at 30 °C and there was no microbial growth.
Ten soybean seeds were sown per pot and thinning was carried out at the VC (cotyledon) stage, keeping only 02 plants per pot. The plants were irrigated daily until the R1 stage, at 48 days, when the evaluations were performed.
2.3. Promotion of Growth and Nutrient Content
The promotion of plant growth by the isolates was evaluated by considering the biomass accumulation. For this, the plants were fragmented into leaves, stems and roots and the biomass was dried in an oven with forced air circulation at 65 °C until constant mass. Then, the dry mass of each plant part was determined. Total dry mass (TDM) was calculated based on the sum of the values corresponding to the biomass of each part of the plants (dry mass of aerial part − ADM + dry mass of root − RDM).
The nutritional state of the plants was evaluated by diagnosing the leaves. The samples were dried in an oven with forced air circulation at 65 °C and ground in a Willey-type mill, and the shoots (leaves and stems) and roots of the plants were collected. The laboratory analyses were performed according to the method proposed by Malavolta et al. [37] and the contents of macronutrients (N, P, K, Ca, Mg, and S) and micronutrients (Fe, B, Mn, Zn, and Cu) were determined. For this, the content of K, Ca, Mg, Fe, Mn, Zn, Cu was obtained by Atomic Absorption Spectrophotometry [38]; P, B and S by Optical Spectrophotometry [39]; and N by Kjeldahl Distiller [40].
2.4. Gas Exchange and Chlorophyll Index
The evaluation of gas exchange took place between 7 AM and 10 AM, using the third leaf, counted from the apex of the plant. The analyses were performed in an IRGA infrared gas analyzer with a fluorometer attached (model LI-6800xt, LI-COR Inc., Lincoln, NE, USA), using photosynthetically active radiation (PAR) (1000 μmol photons m−2 s−1), temperature block temperature of 27 °C, and relative humidity of approximately 70%. The following parameters were measured: net photosynthesis rate (A) (µmol of CO2 m−2 s−1), transpiration (E) (mmol of H2O m−2 s−1), internal carbon concentration (Ci) (mmol m−2 s−1), and stomatal conductance (Gsw) (mol of H2O m−2 s−1).
Pigment analysis was performed using the Dualex® sensor (Force-A, Paris, France) in the central leaflet of the third fully expanded leaf from the apex of the plant. The surface chlorophyll content (chlorophyll index) (Chl) (µg/cm2) was measured.
2.5. Chlorophyll a Fluorescence
The OJIP transient fluorescence of chlorophyll a was determined on a FluorPen FP 100 portable fluorometer (Photon Systems Instruments; Drasov, Czech Republic). The analyses were carried out on the third leaves of all sample units. These leaves were dark-adapted for 30 min for complete oxidation of the photosynthetic electron transport system. Subsequently, a pulse of 3000 µmol m−2 s−1 of blue light was offered, measuring the minimum fluorescence (F0) at 50 μs when all PSII reaction centers were open, defined as step O, followed by step J (at 2 ms), step I (at 30 ms), and maximum fluorescence (FM) when all PSII reaction centers were closed, defined as step P. The values obtained for the different steps were used for the estimation of several bioenergetic indices of PSII, according to Strasser et al. [41]: the specific light absorption flux per reaction center (ABS. RC), energy flux per reaction center at t = 0 (TRo. RC), electron transport flux per reaction center (ETo. RC), specific dissipated energy flux at the level of the chlorophyll antenna complex (Dio. RC), photosynthetic performance index (Pi_Abs), maximum quantum yield of primary photochemistry (PHI_Po), probability that a trapped exciton moves an electron into the electron transport chain after the Quinone (PSI_O), and quantum yield of electron transport (PHI_Eo), after the leaves were dark-adapted (30 min).
2.6. Experimental Design and Statistical Analyses
The experiment was conducted in a completely randomized design, considering nine treatments with microorganisms (eight isolates + the commercial product Biomaphos®) and one control treatment (without inoculation). All treatments were evaluated in 05 repetitions, with each repetition consisting of 2 plants per pot. The data obtained in the biometric and physiological analyses and in the analysis of tissue content of macro- and micronutrients were subjected to one-way ANOVA to evaluate the treatment effect. When significant, the treatment effects were evaluated using the Scott–Knott test at 5% probability.
Subsequently, all variables that showed significant differences were jointly evaluated in a correlation matrix and associated in a principal component analysis (PCA). Because these variables had different units of measurement, correlation PCA was performed using the standardized data to have a mean equal to 0 and standard deviation equal to 1. The definition of the number of principal components occurred according to the eigenvalues (>1.0) and the explained variance (>70%). The variables were also evaluated using Pearson’s correlation coefficient, and the strength of the correlation was analyzed by the R values and the significance of the interaction (5% probability). All statistical evaluations were performed using the R software version 4.0.4 (R Core Team) [42].
3. Results
3.1. Promotion of Plant Growth and Nutrient Contents
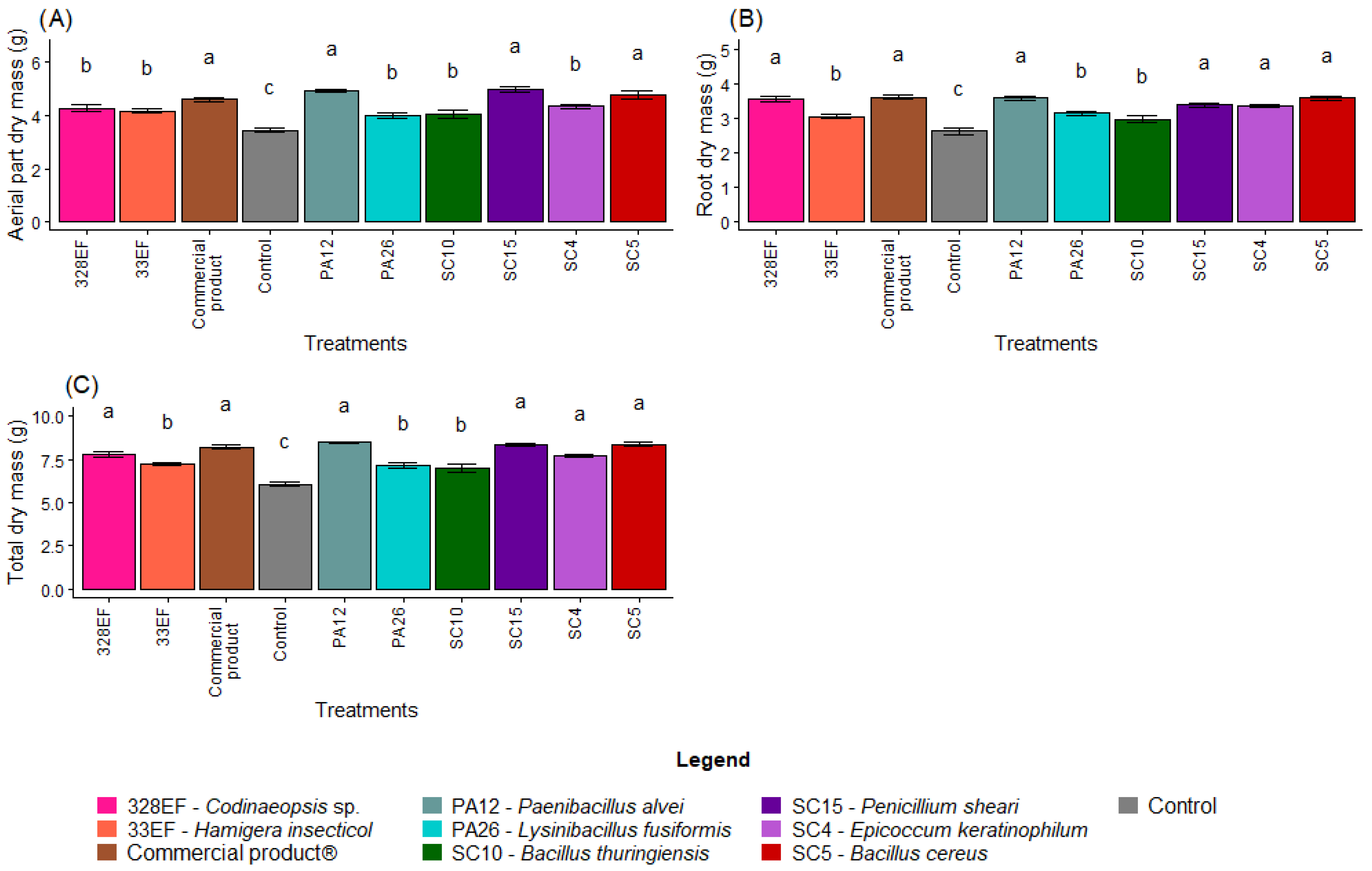
Overall, biopriming of G. max seeds with the microbial strains was more effective in promoting plant growth than the control treatment. The highest means of AMD were obtained in the plants inoculated with the strains SC15 (P. sheari), PA12 (P. alvei), SC5 (B. cereus), and with the commercial product Biomaphos® (Figure 1A). Similar results were obtained for RDM, with the highest means obtained in the plants bioprimed with the strains PA12 (P. alvei), SC5 (B. cereus), 328EF (Codinaeopsis sp.), SC15 (P. sheari), and SC4 (E. keratinophilum), and with the commercial product Biomaphos® (Figure 1B). The behavior of TDM followed that of RDM, i.e., the highest mean values were obtained in the plants inoculated with PA12 (P. alvei), SC15 (P. sheari), SC5 (B. cereus), Biomaphos®, 328EF (Codinaeopsis sp.) and SC4 (E. keratinophilum) (Figure 1C).
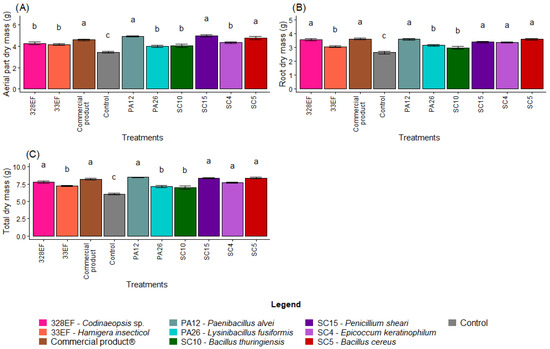
Figure 1.
Dry mass of aerial part (A), root dry mass (B) and total dry mass (C) in soybean (Glycine max) plants inoculated with fungal and bacterial strains isolated from Hymenaea courbaril and Butia purpurascens and grown in a controlled greenhouse system. Means followed by the same letter were not significantly different using the Scott–Knott test at 0.05% probability.
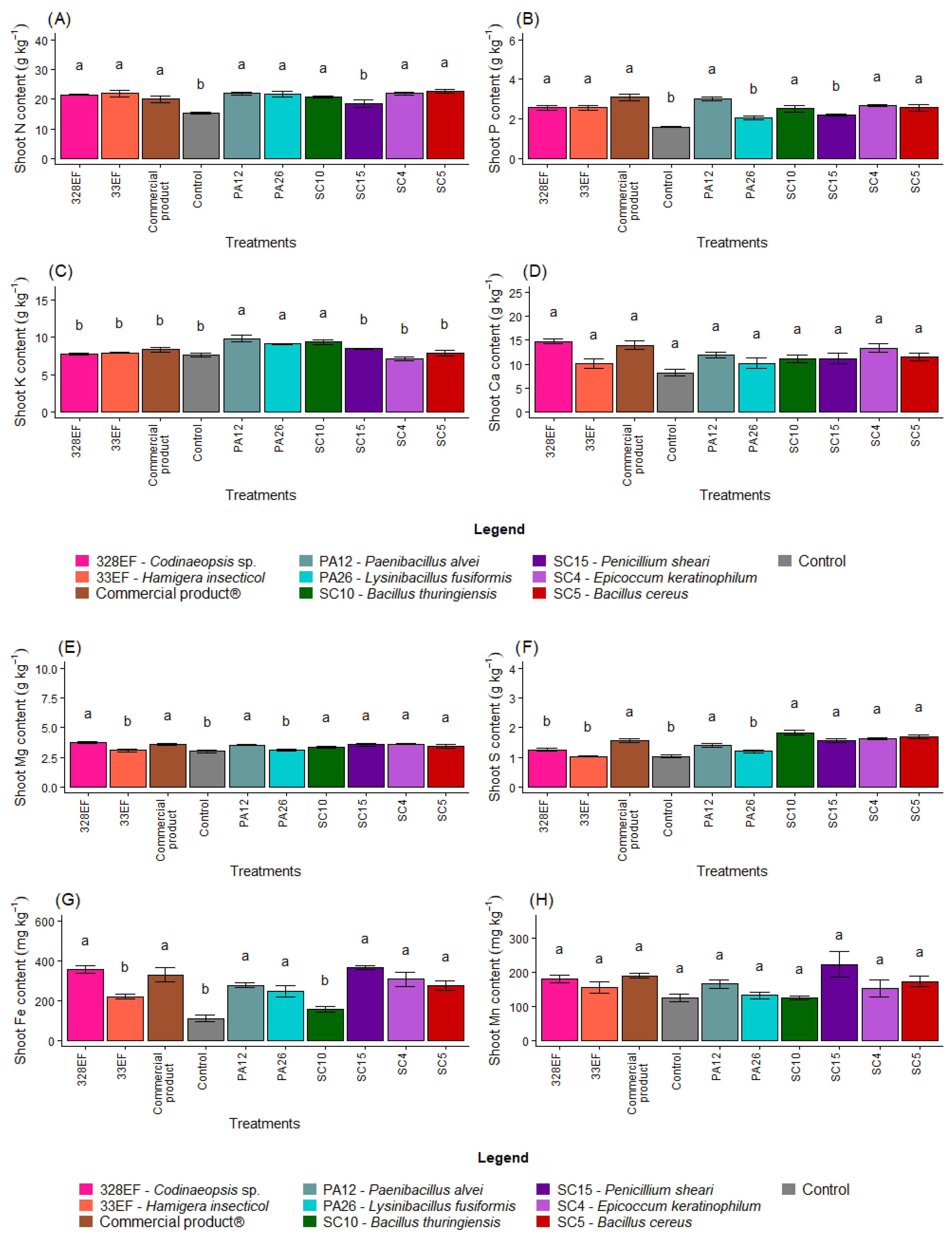
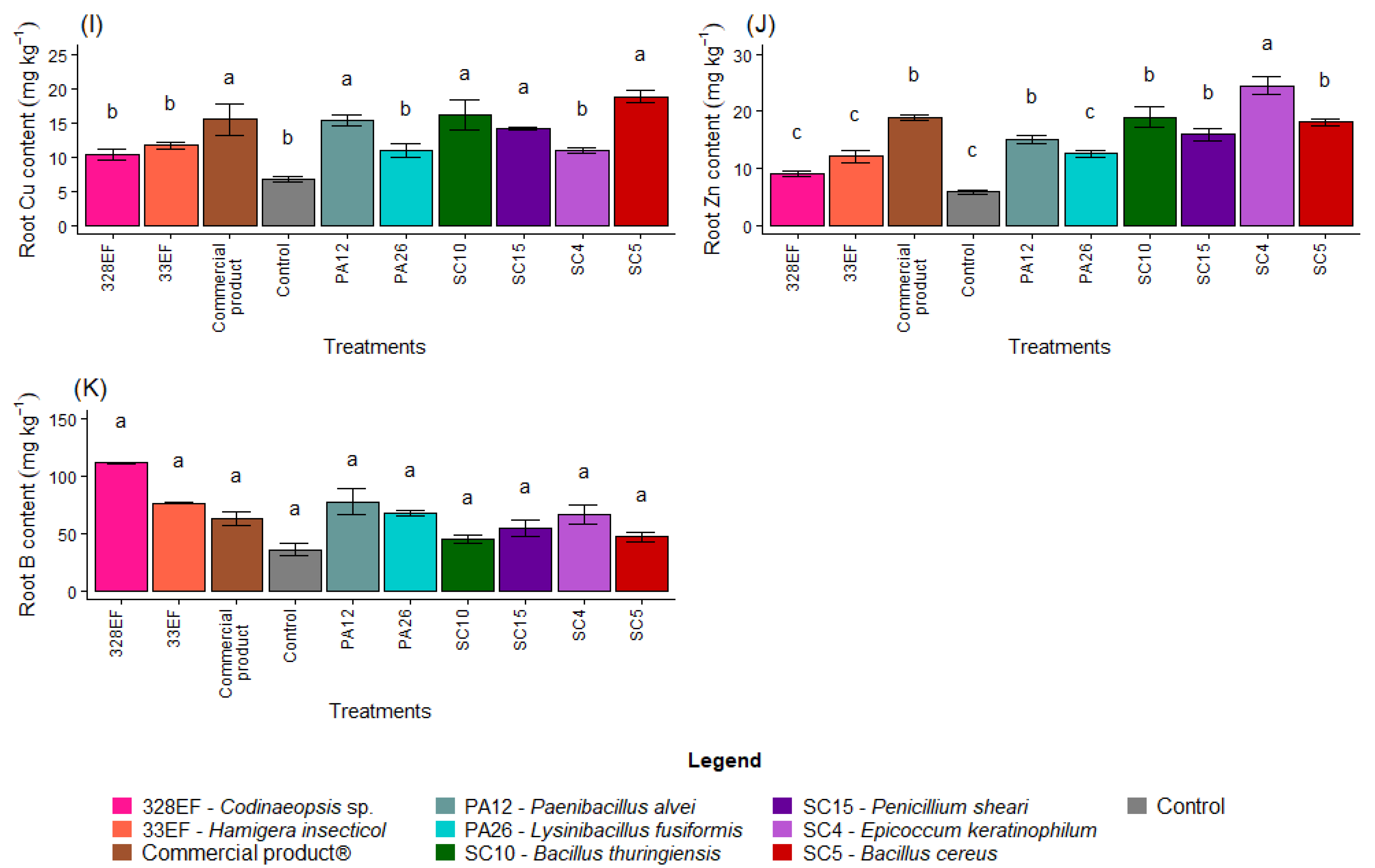
The inoculation of seeds with microorganisms increased the levels of N in the aerial part of the plants; low levels of this nutrient were observed only in control plants and in plants inoculated with SC15 (P. sheari) (Figure 2A). These treatments, as well as treatments with PA26 (L. fusiformis), also negatively affected P content in the aerial part (Figure 2B). Strains PA12 (P. alvei), PA26 (L. fusiformis), and SC10 (B. thuringiensis) promoted the uptake of K (Figure 2C). However, biopriming did not affect the contents of Ca and Mn in the aerial part of G. max (Figure 2D,H).
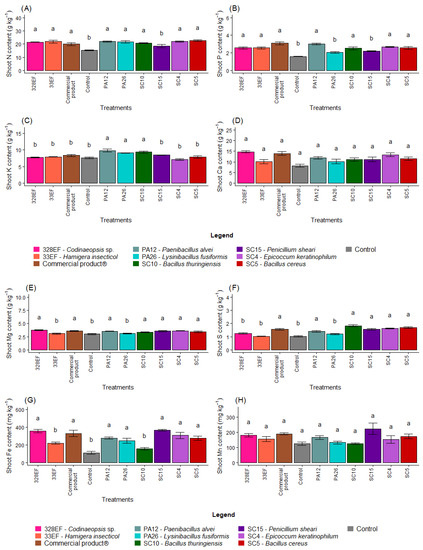
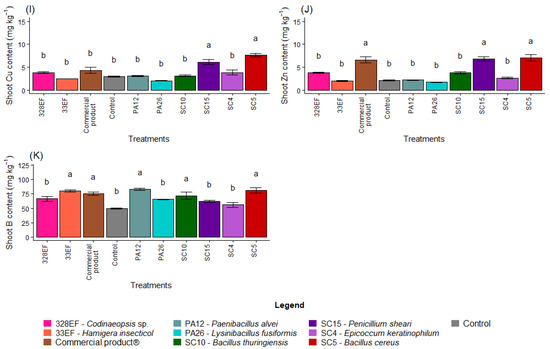
Figure 2.
Content of the macronutrients N (A), P (B), K, (C), Ca (D), Mg (E), and S (F) and of the micronutrients Fe (G), Mn (H), Cu, (I), Zn (J), and B (K) in the aerial part of soybean (Glycine max) plants inoculated with fungal and bacterial strains isolated from Hymenaea courbaril and Butia purpurascens and grown in a controlled greenhouse system. Means followed by the same letter were not significantly different using the Scott–Knott test at 0.05% probability.
The control plants and those inoculated with strains PA26 (L. fusiformis) and 33EF (H. insecticol) had low Mg content in the aerial part (Figure 2E). These three treatments and treatment 328EF (Codinaeopsis sp.) reduced the S content (Figure 2F), while Fe content was reduced in the control treatment and in the SC10 (B. thuringiensis) and 33EF (H. insecticol) treatments (Figure 2G).
The bacterium SC5 (B. cereus) and the fungus SC15 (P. sheari) stimulated the accumulation of Cu in the tissues of the aerial part, with mean values of 7.63 mg kg−1 and 6.16 mg kg−1, respectively (Figure 2I). The accumulation of Zn was also stimulated by these treatments and by the commercial product (Figure 2J). The B content was increased by treatment with PA12 (P. alvei), SC5 (B. cereus), 33EF (H. insecticol), Biomaphos®, and SC10 (B. thuringiensis) (Figure 2K).
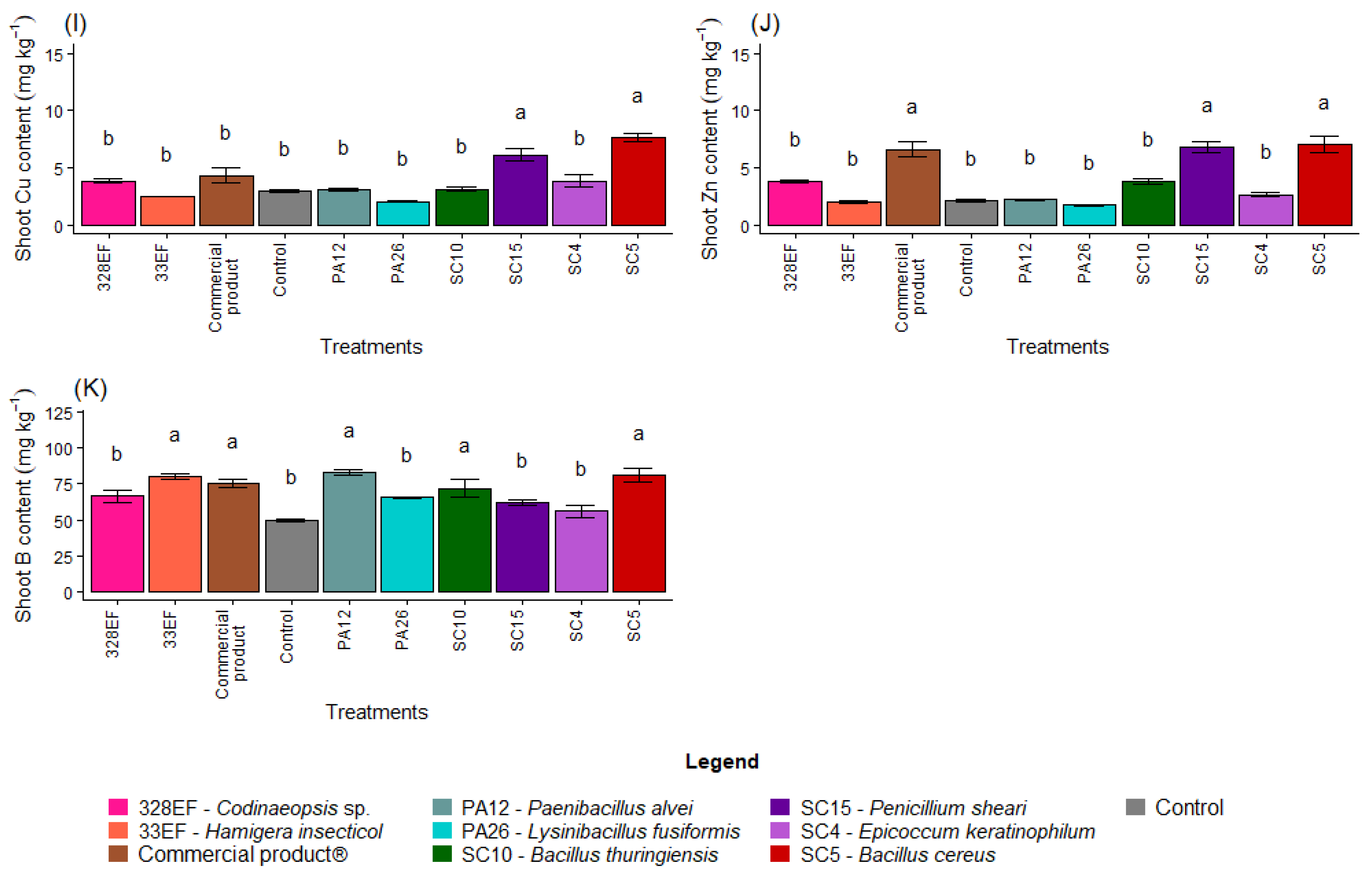
The N content in the roots was positively affected by inoculation with SC4 (E. keratinophilum), SC5 (B. cereus), and 328EF (Codinaeopsis sp.) (Figure 3A). However, P, Ca, Mg, Mn, and B contents were not differentially affected by the biopriming treatments (Figure 3B,D,E,H,K)). Furthermore, the fungus 328EF (Codinaeopsis sp.) stimulated the uptake of K by the roots of G. max plants (Figure 3C). This fungus, as well as the strains SC5 (B. cereus), SC15 (P. sheari), SC10 (B. thuringiensis), PA26 (L. fusiformis), and PA12 (P. alvei) increased the content of S in root tissues (Figure 3F). However, the control plants and those inoculated with the fungi 328EF (Codinaeopsis sp.) and SC4 (E. keratinophilum) and with Biomaphos® exhibited the lowest contents of Fe (Figure 3G).
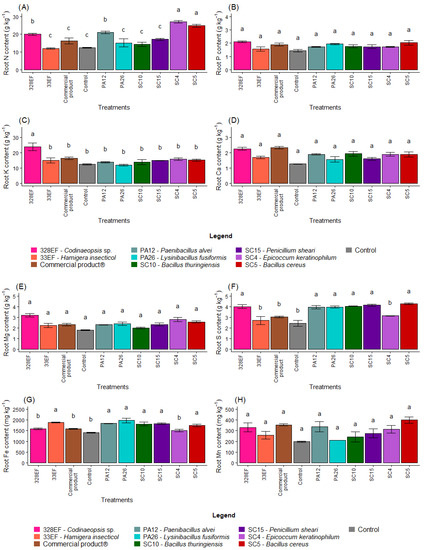
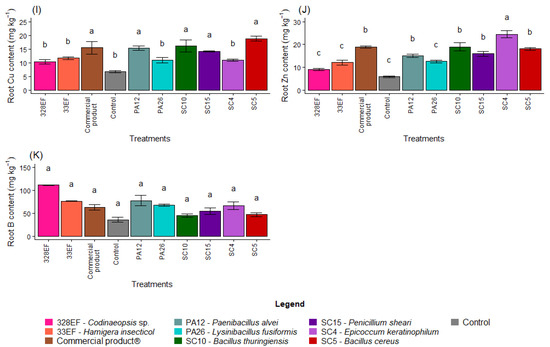
Figure 3.
Content of the macronutrients N (A), P (B), K, (C), Ca (D), Mg (E), and S (F) and of the micronutrients Fe (G), Mn (H), Cu, (I), Zn (J), and B (K) in the roots of soybean (Glycine max) plants inoculated with fungal and bacterial strains isolated from Hymenaea courbaril and Butia purpurascens and grown in a controlled greenhouse system. Means followed by the same letter were not significantly different using the Scott–Knott test at 0.05% probability.
The averages observed for the Cu content increased in the roots of plants inoculated with SC5 (B. cereus), SC10 (B. thuringiensis), Biomaphos®, PA12 (P. alvei), and SC15 (P. sheari) (Figure 3I). Furthermore, Zn content was notably higher in the plants treated with SC4 (E. keratinophilum) (Figure 3J).
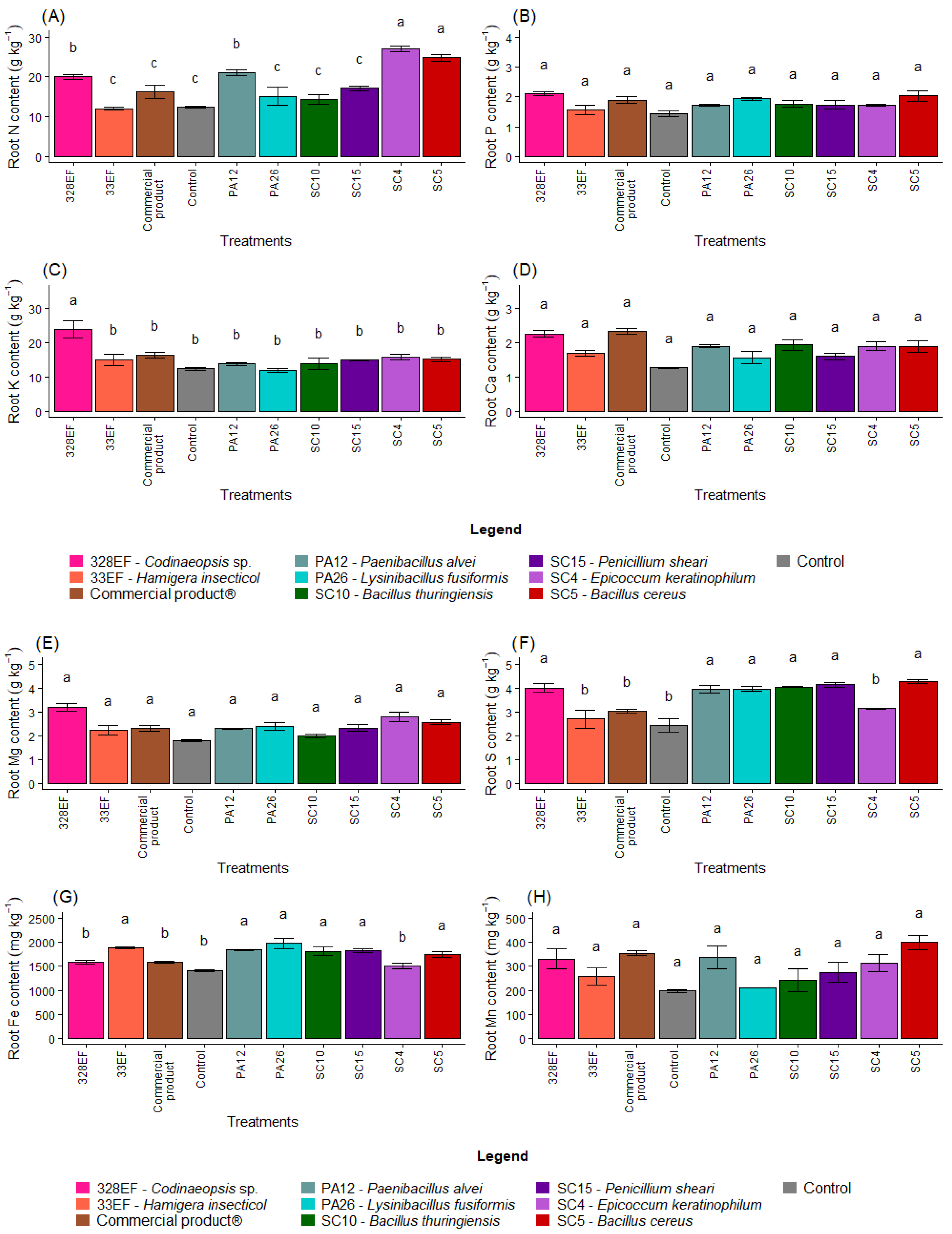
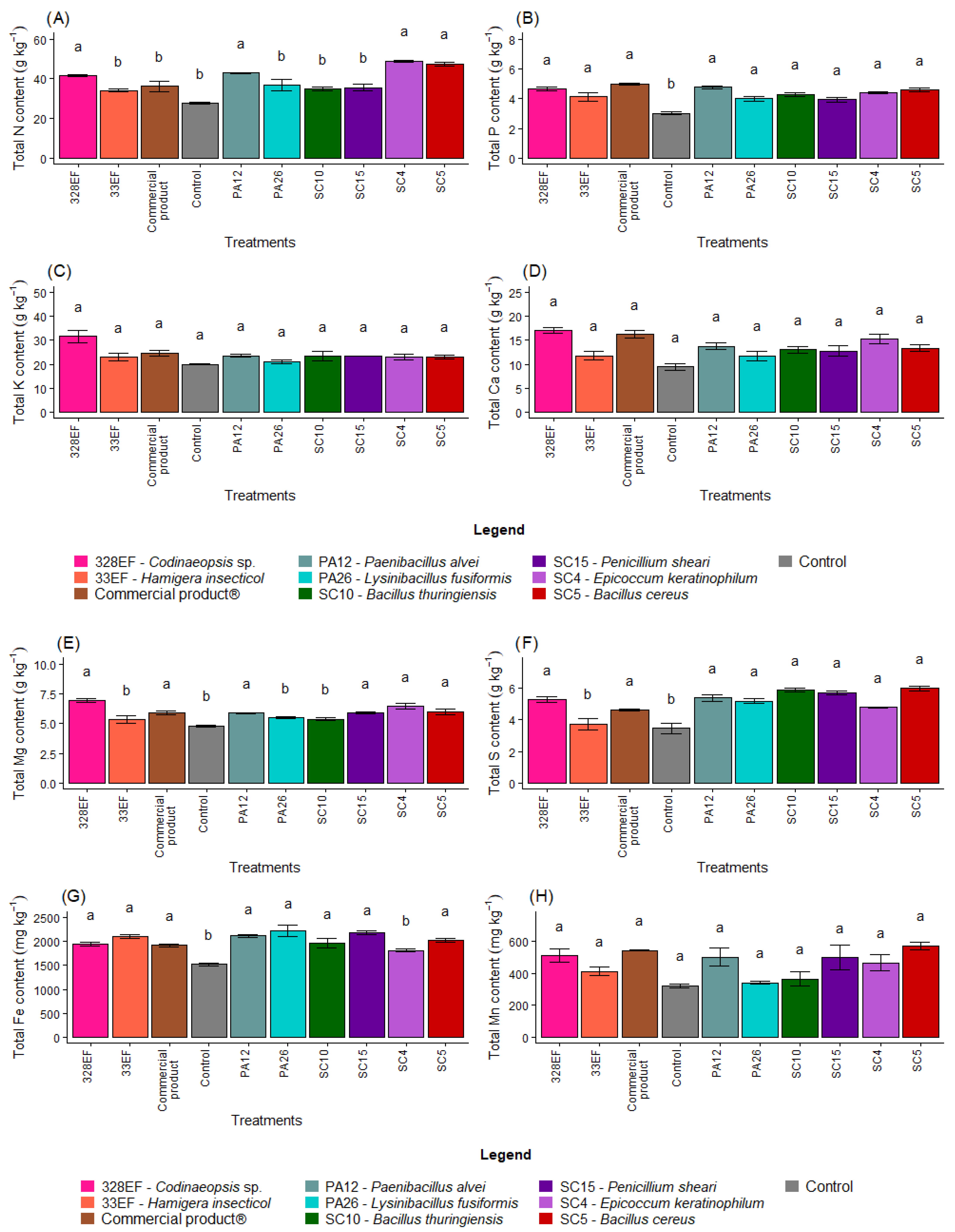
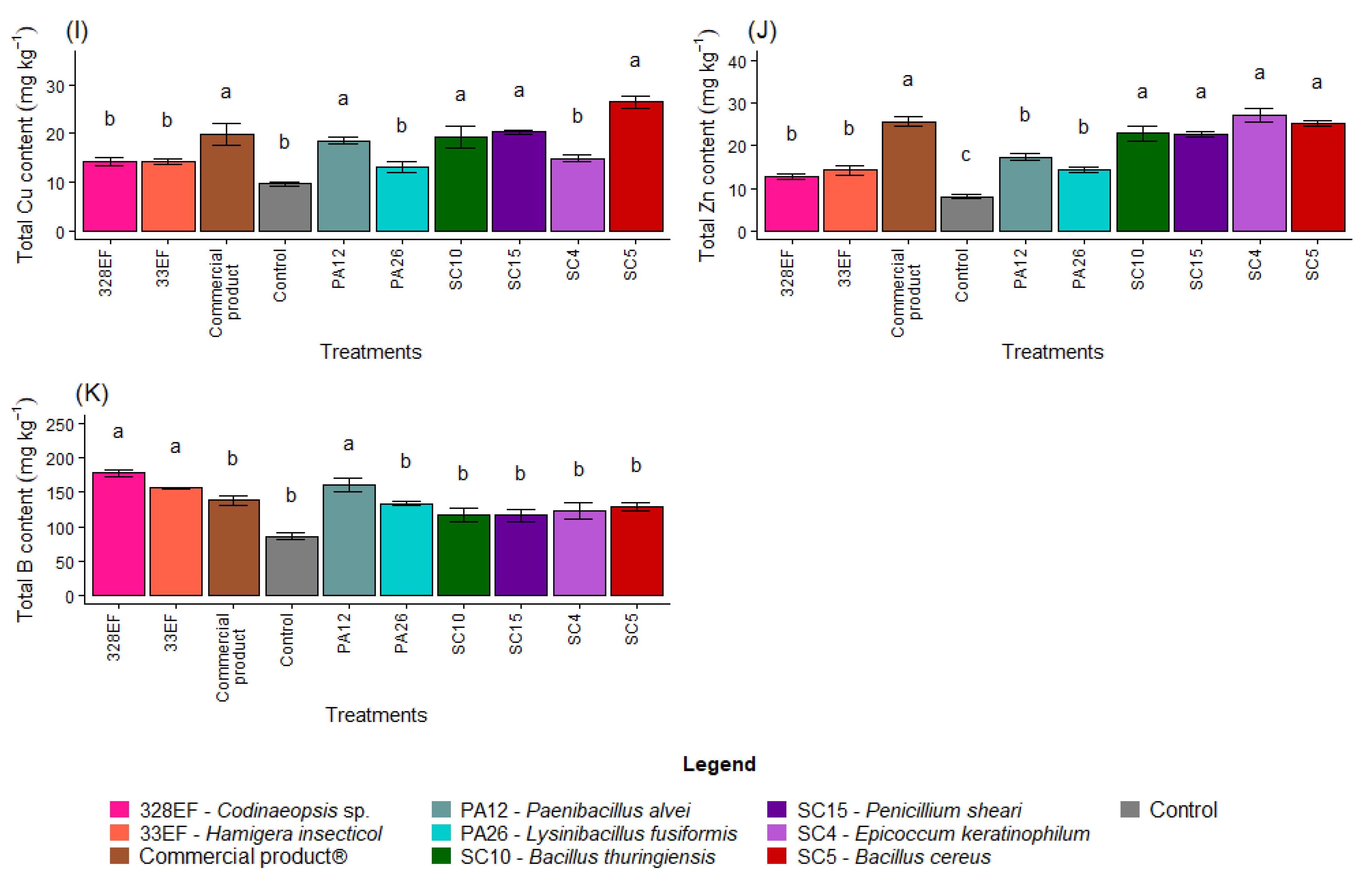
The analysis of total nutrient content showed that the plants submitted to biopriming with the strains SC4 (E. keratinophilum), SC5 (B. cereus), PA12 (P. alvei), and 328EF (Codinaeopsis sp.) had the highest mean accumulation of N (Figure 4A). The content of P, however, was negatively affected by the absence of microbial inoculation and the lowest values were obtained in the control treatment (Figure 4B). The treatments did not differentially affect the content of K, Ca, and Mn in G. max plants (Figure 4C,D,H).
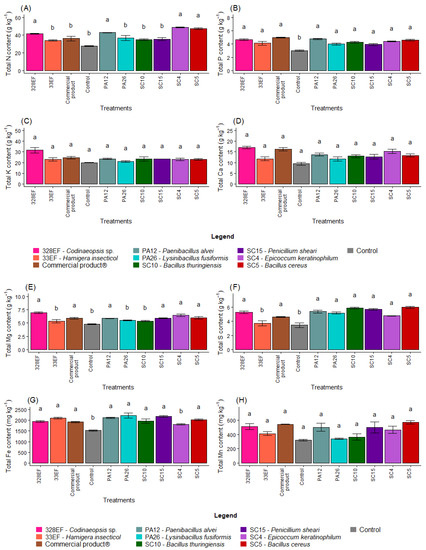
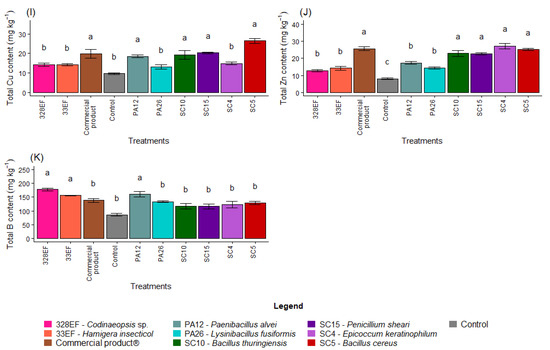
Figure 4.
Total content of the macronutrients N (A), P (B), K, (C), Ca (D), Mg (E), and S (F) and of the micronutrients Fe (G), Mn (H), Cu, (I), Zn (J), and B (K) in soybean (Glycine max) plants inoculated with fungal and bacterial strains isolated from Hymenaea courbaril and Butia purpurascens and grown in a controlled greenhouse system. Means followed by the same letter were not significantly different using the Scott–Knott test at 0.05% probability.
The mean content of Mg was higher in the plants inoculated with 328EF (Codinaeopsis sp.), SC4 (E. keratinophilum), SC5 (B. cereus), Biomaphos®, SC15 (P. sheari), and PA12 (P. alvei) (Figure 4E). Total S content was reduced in the control treatment and in the plants treated with the fungus 33EF (H. insecticol) (Figure 4F). Total Fe was also reduced in the plants of the control treatment as well as in those inoculated with SC4 (E. keratinophilum) (Figure 4G).
Total Cu content in the roots was also affected by the inoculation treatments, with the highest means obtained in plants inoculated with SC5 (B. cereus), SC15 (P. sheari), Biomaphos®, SC10 (B. thuringiensis), and PA12 (P. alvei) (Figure 4I). Zn content was also affected by SC5 (B. cereus), SC15 (P. sheari), Biomaphos®, and SC10 (B. thuringiensis) and by the fungus SC4 (E. keratinophilum) (27.20 mg kg−1) (Figure 4J). B content was positively affected by the PA12 bacterium (P. alvei) and by the fungi 328EF (Codinaeopsis sp.) and 33EF (H. insecticol), respectively (Figure 4K).
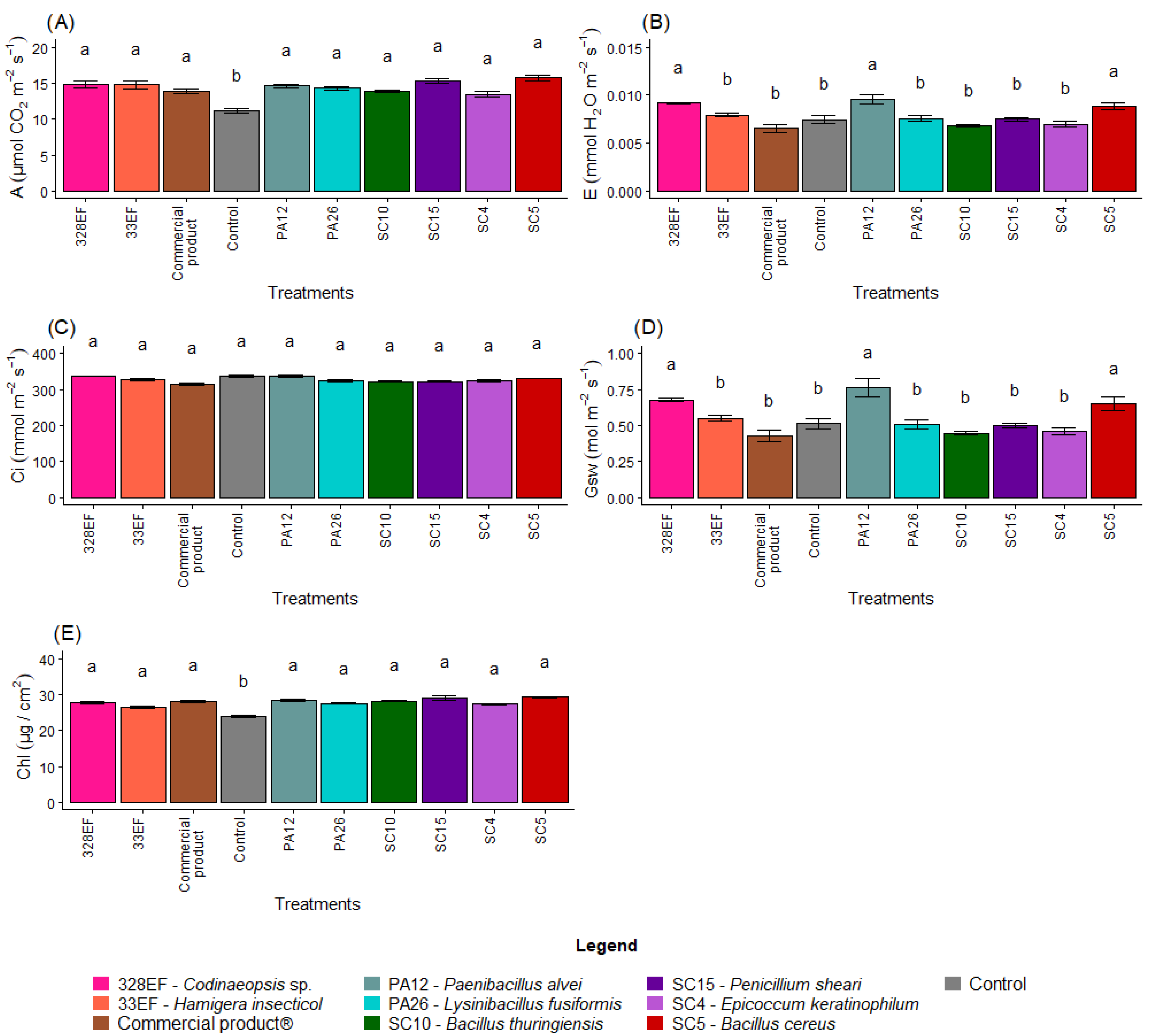
3.2. Gas Exchange and Chlorophyll Index
Overall, microbial inoculation had a positive effect on the photosynthetic rate. Thus, plants of the control treatment exhibited the lowest rates (11.18) (Figure 5A). However, the transpiration rate was low in control plants (0.007); the highest transpiration rates were obtained in the plants treated with PA12 (P. alvei), 328EF (Codinaeopsis sp.), and SC5 (B. cereus), respectively (Figure 5B). The treatments did not affect Ci (Figure 5C); however, Gsw followed the same behavior of E, with the highest rates obtained in the plants treated with PA12 (P. alvei), 328EF (Codinaeopsis sp.), and SC5 (B. cereus) (Figure 5D).
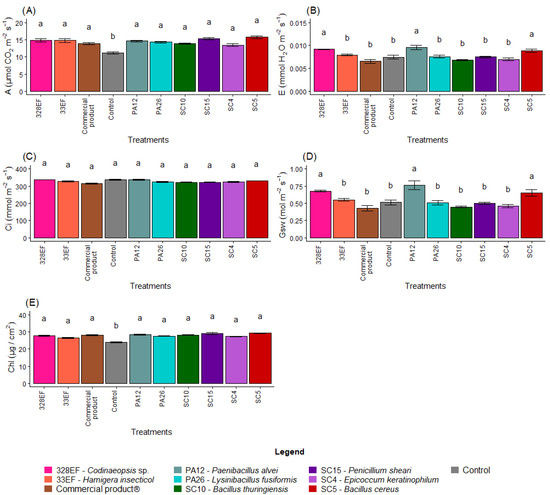
Figure 5.
Photosynthetic rate, A (A); transpiration rate, E (B); internal concentration of CO2, Ci (C); stomatal conductance, Gsw (D); and chlorophyll index (E) in soybean plants (Glycine max) inoculated with fungal and bacterial strains isolated from Hymenaea courbaril and Butia purpurascens and cultivated in a controlled greenhouse system. Means followed by the same letter were not significantly different using the Scott–Knott test at 0.05% probability.
The chlorophyll index was also affected by the microbial biopriming treatments and the lowest indices were obtained in the control plants (23.84 µg cm2) (Figure 5E).
3.3. Chlorophyll a Fluorescence
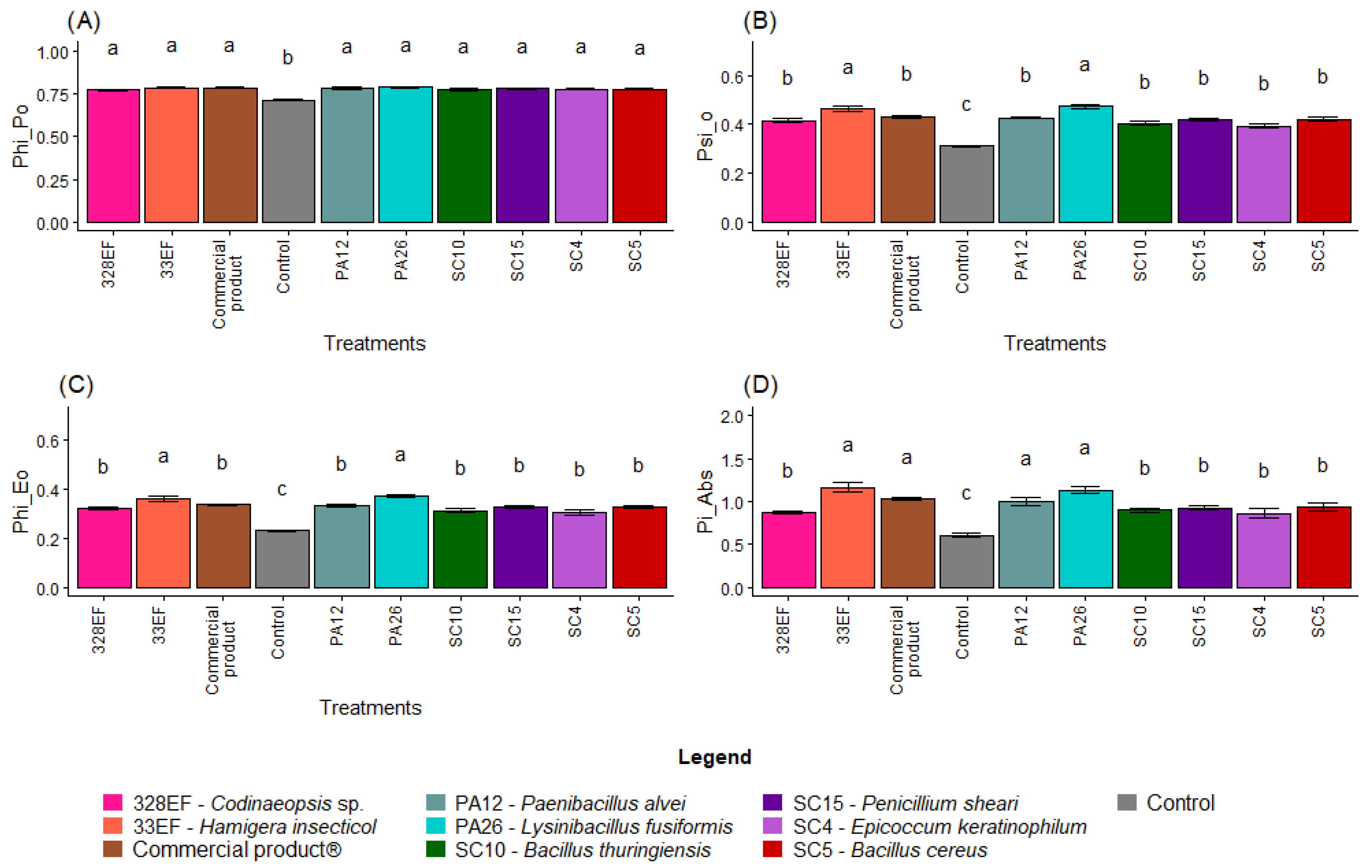
Overall, non-inoculated plants showed lower photochemical performance, with a lower mean value of PHI_Po (0.71), than the inoculated plants (Figure 6A). PSI_O was also lower in non-inoculated plants (0.31), while the highest means were obtained in plants inoculated with PA26 (L. fusiformis) and 33EF (H. insecticol) (Figure 6B). A similar behavior was observed for PHI_Eo, with these same plants exhibiting the highest values (Figure 6C). Pi_Abs, however, was positively affected not only by PA26 (L. fusiformis) and 33EF (H. insecticol) but also by PA12 (P. alvei) (1.00) and the commercial product (1.03) (Figure 6D).
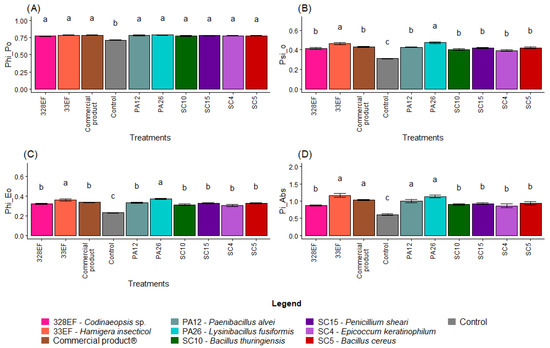
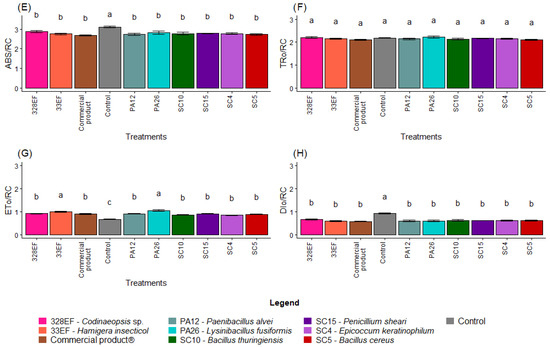
Figure 6.
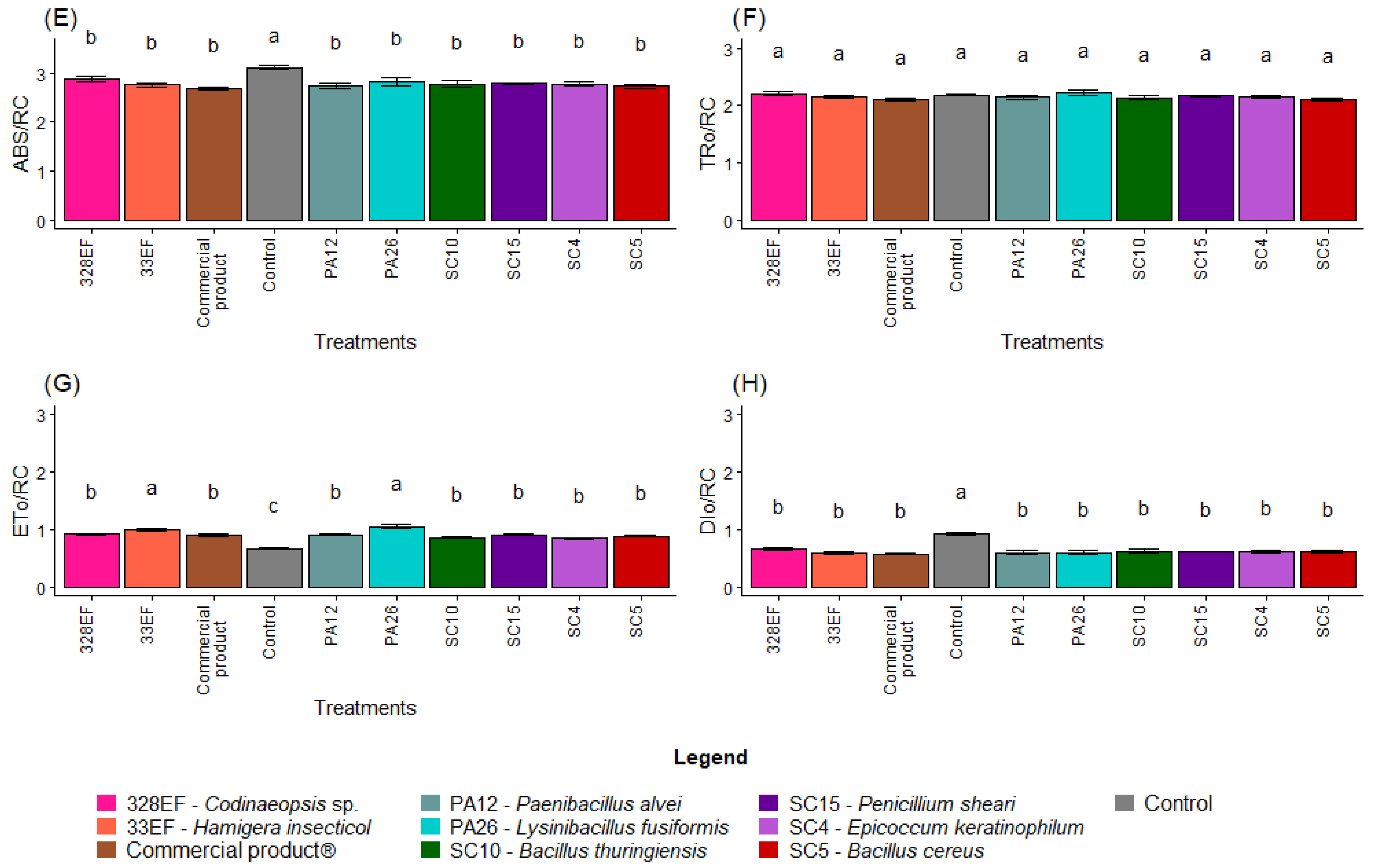
Maximum quantum yield of primary photochemistry (PHI_Po) (A), probability that a trapped exciton moves an electron into the electron transport chain beyond Quinone (Qa) (PSI_O) (B), quantum yield of electron transport (PHI_Eo) (C), photosynthetic performance index (Pi_Abs) (D), absorption flux per RC (ABS. RC) (E), energy flux per RC at t = 0 (TR0. RC) (F), electron transport flux per RC at t = 0 (ET0/RC) (G), and specific dissipated energy flux (DI0. RC) (H) in soybean (Glycine max) plants inoculated with fungal and bacterial strains isolated from Hymenaea courbaril and Butia purpurascens and grown in a controlled greenhouse system. Means followed by the same letter were not significantly different using the Scott–Knott test at 0.05% probability.
As expected, ABS. RC and DI0. RC, two indicators of photochemical stress, were higher in the control plants than in inoculated plants (Figure 6E,H). However, there was no difference in TR0. RC between the treatments (Figure 6F). PA26 (L. fusiformis) and 33EF (H. insecticol) had a positive effect on the ET0/RC means (Figure 6G).
3.4. Correlation Matrix between Variables and PCA
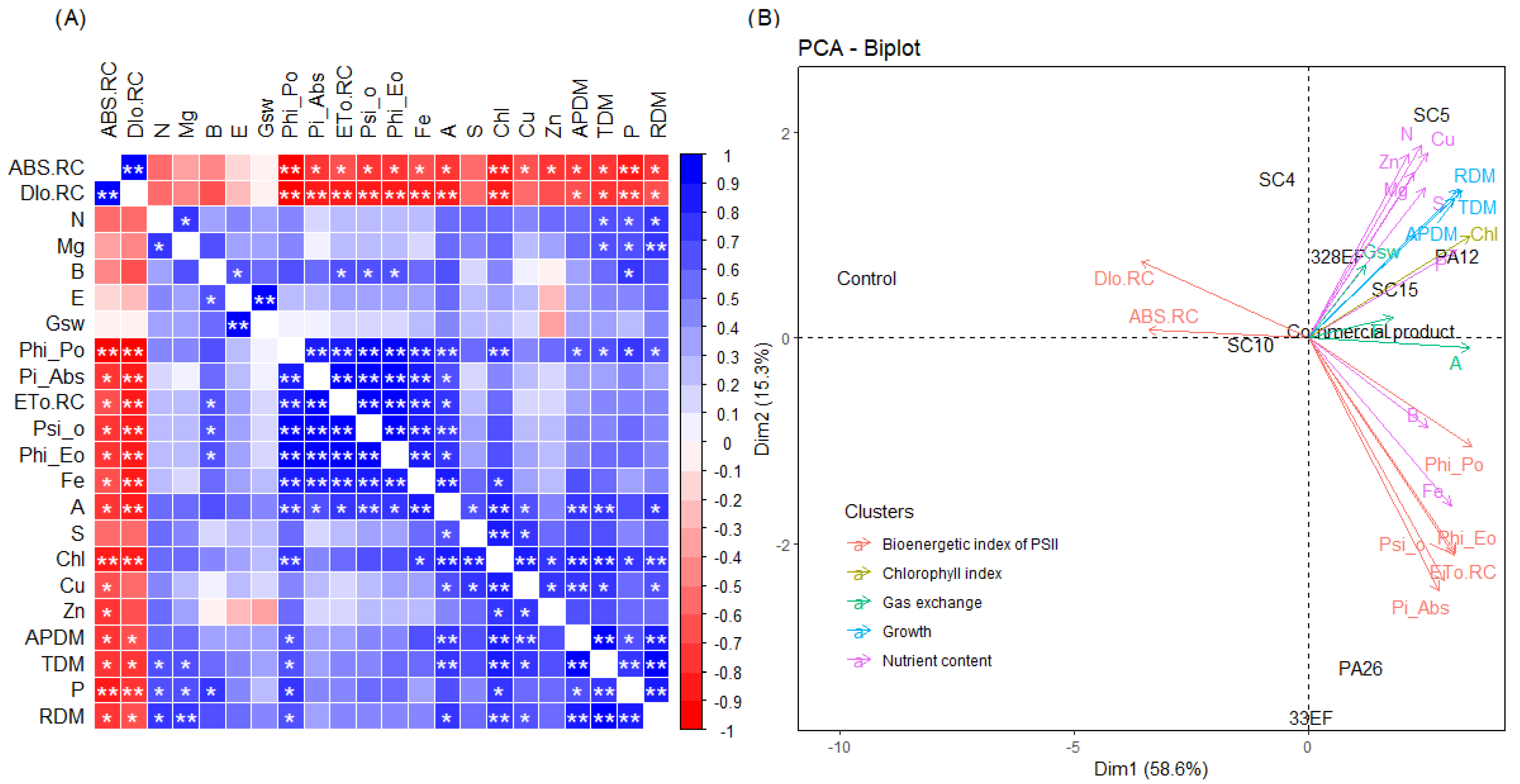
A negative and significant correlation was observed between ABS. RC and Dio. RC and the concentrations of most nutrients, A, chlorophyll index, ADM, and RDM. As expected, Phi_Po, Pi_Abs, ETo. RC, Psi_o, and Phi_Eo correlated positively and significantly with photosynthetic rate and the latter correlated positively with stomatal conductance. Moreover, the concentrations of N and P correlated positively with dry biomass (Figure 7A).
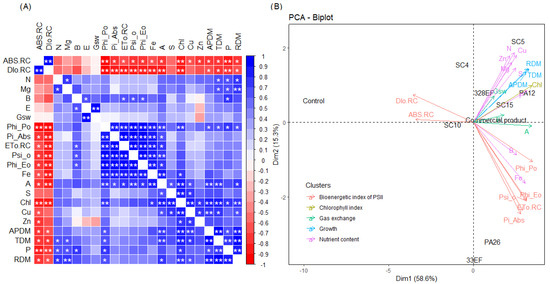
Figure 7.
Correlations between the means of dry biomass, nutrient content, total chlorophyll, gas exchange, and chlorophyll fluorescence a parameters (A) and principal component analysis of these variables (B) in soybean plants (Glycine max) inoculated with fungal and bacterial strains isolated from Hymenaea courbaril and Butia purpurascens and grown in a controlled greenhouse system. SC5 = Bacillus cereus, SC10 = Bacillus thuringiensis, PA12 = Paenibacillus alvei, PA26 = Lysinibacillus fusiformis, SC15 = Penicillium sheari, SC4 = Epicoccum keratinophilum, 33EF = Hamigera insecticola, 328EF = Codinaeopsis sp., Biomaphos® = Bacillus megaterium and Bacillus subtilis, Control = without microorganisms. * significant at 0.5 and ** significant at 0.1 probability.
PCA highlighted the opposite behavior of the indicators of photochemical stress, ABS. RC and Dio. RC, and the chlorophyll index, photosynthetic rate, nutrient concentration, and dry biomass. Thus, the control (non-inoculated) plants tended to have higher ABS. RC and Dio. RC, while the inoculated plants had higher dry biomass, nutrient content, and chlorophyll content, in addition to better photochemical and photosynthetic performance. The results of treatment with the bacterial strains PA12 (P. alvei) and SC5 B. cereus and the fungal strains 328EF (Codinaeopsis sp.) and SC15 (P. sheari) were the closest to the pattern observed for the commercial product Biomaphos®, with the same trend of direction of the means associated with chlorophyll index, A, dry mass, and concentration of important nutrients such as N, P, and Mg (Figure 7B).
4. Discussion
4.1. Microbial Inoculation Had a Positive Effect on the Growth of G. max, Especially with Strains PA12 (P. alvei), SC5 (B. cereus), and SC15 (P. sheari)
Microbial inoculation positively affected the accumulation of biomass in G. max plants, and strains PA12 (P. alvei), SC5 (B. cereus), and SC15 (P. sheari) acted as plant growth promoters, inducing the growth of the aerial part, roots, and whole plant. The strains PA12 (P. alvei) and SC5 (B. cereus) were previously identified as phosphate solubilizers in the study by Reis et al. [23]. Paenibacillus is one of the genera of Firmicutes predominantly found in association with plants [43] and P. alvei has been described in the literature as a disease biocontroller and inducer of crop growth and productivity [44,45,46]. Similarly, B. cereus has been related to phosphate solubilization and promotion of G. max growth by other authors [47]. A commercial product developed from strain 905 was used on approximately 3 million acres planted with wheat after being registered as a biopesticide [48]. Ku et al. [49] demonstrated that this species colonizes the roots of soybean, wheat, and Chinese cabbage, thereby promoting the growth of the three crops. Furthermore, Baliyan et al. [50] suggested that the plant growth-promoting (PGP) effect of B. cereus is related to its ability to synthesize gibberellins. Zeng et al. [51] performed comparative genomic and functional analyses in four different strains of this bacterium and showed that genes related to traits that promote plant growth are highly conserved.
With regard to P. sheari, although it was identified as a rhizospheric species [34], it has not yet been described to have a PGP effect. However, Dwivedi and Sangeeta [52] reported it as an antagonist of Sclerotinia sclerotiorum. Our study highlights the potential of this understudied species.
4.2. Non-Inoculated Plants Had Lower Nutrient Contents in the Aerial Part and Whole Plant, Reduced Chlorophyll Index, and Low Photosynthetic Rate and Photochemical Efficiency Compared to the Inoculated Plants
Biopriming of the seeds with the inocula significantly affected nutrient contents in G. max tissues. The total contents of N, P, Mg, S, Fe, Cu, Zn, and B in the tissues of the non-inoculated plants were lower than that in the treated plants. This finding accentuates the importance of microbial inoculation (biofertilization) for the bioavailability of nutrients in crops [21,53,54]. This availability is directly associated with organic acid production (solubilization of complexed nutrients) and nitrogen fixation [55,56]; however, microorganisms in general participate in (1) mineralization of soil organic matter and thus nutrient cycling [57], (2) improvement of soil structure [58], (3) interaction with other rhizosphere microorganisms [59], (4) production of bioactive compounds such as plant hormones and enzymes [59,60], and (5) control of phytopathogens [59,60,61,62,63,64].
Non-inoculated plants accumulated low concentrations of Mg. Studies show that microbial cells demand Mg for vital metabolic activities [65,66]. In plants, this nutrient is present in high concentrations and has a number of important functions: it acts as an enzymatic activator or cofactor in metabolic reactions involving ATP, stabilizes DNA, RNA, and cell membranes, and is a component of chlorophyll [67,68,69].
An increase in total S was observed in all treatments except in plants inoculated with the fungus 33EF (H. insecticola) and in the control treatment. This result was expected because most of the S (>90%) is unavailable to plants because it is bound in organic form. Thus, plants depend on microorganisms that make SO42− available in the rhizosphere by mineralization to the soil solution [70,71,72]. S is thus transported from the roots to the aerial part through the xylem mainly in the inorganic form of SO42− [73,74]. Like N, S is essential for vital functions and processes, including the formation of compounds such as amino acids, proteins, coenzymes, lipids, sulfolipids, flavonoids, polysaccharides, and nucleotides [73,75,76,77].
Although Fe is a poorly mobile nutrient, it is an essential micronutrient for plants because it has a role in essential metabolic processes such as chlorophyll biosynthesis, cell division, photosynthesis, and respiration [78,79]. The results obtained for Fe content show that this nutrient accumulated mostly in the roots, an effect commonly associated with poor soils like the one in this study, in which microorganisms promote the increase of lateral roots and the elongation of root hairs for greater nutrient uptake [80,81,82]. In addition, microorganisms produce siderophores and phosphatases that respectively chelate Fe and release available forms of P [11,83,84,85] and other nutrients such as Cu, Mg, Zn, and Fe to plants. Vitorino et al. [86] confirmed the ability of strains used in this work, such as SC10 (B. thuringiensis) and SC5 (B. cereus), to solubilize different sources of phosphates. On the other hand, in the work developed by Reis et al. [23], PA12 (P. alvei) and PA26 (L. fusiformes) increased phosphorus acquisition by G. max. This may explain the results observed here.
The increase in Zn uptake found in the inoculated plants may also be associated with the production of siderophores. Studies show that siderophores, due to their chelation capacity, can mobilize heavy metals such as Zn, in addition to Fe [87,88,89]. In plants, Zn is taken up by the roots in the form of the Zn2+ ion and is essential for a number of metabolic functions such as oxidative reactions [90], enzyme composition [91], structural and catalytic activities [92], ribosome stability [93], DNA replication [94], translation and energy transfer reactions [95], photosynthesis (as it catalyzes the synthesis of fructose-6-phosphate, an important metabolite in glycolysis, and is an essential constituent in carbon anhydrase activity affecting chlorophyll synthesis) [96,97], protein synthesis, and increasing the level of antioxidant enzymes [98].
With regard to chlorophyll index, the highest mean values were also obtained in the inoculated plants. This is due to the increased uptake of N, Fe, and Mg by these plants. These nutrients are essential for chlorophyll synthesis [99]. Some studies show that photosynthesis, in addition to being affected by N, Fe, and Mg deficiency, is impaired by P, S, B, Cu, and Zn deficiency in leaves [100,101,102,103]. Therefore, increased uptake of these nutrients resulted in improved photosynthesis of the inoculated soybean plants compared with the control treatment plants.
The results of the chlorophyll a fluorescence confirm this statement. The control treatment plants had the lowest values for yield (Phi_Po and Phi_Eo), flux ratios (Psi_O), and photochemical performance index (Pi_Abs), the highest values for ABS. RC and Dio. RC, and the lowest value for ETo/RC. The increase in ABS. RC in the control plants indicates that energy uptake was not effective. Thus, as expected, the Dio. RC of these plants was above average. Excess energy affects the electron transport chain, causing limitations in electron transport to PSII and the cytb6f complex, resulting in an excessive reduction of Qa [99,104]. This increased dissipation, accompanied by a lower electron transport flux per reaction center (ETo. RC), is responsible for a decrease in PHI_Eo, PSI_O, and Pi_Abs.
All microorganism-inoculated plants had higher values of PHI_Po and Pi_Abs than the control plants, whose photosynthetic apparatus were less developed due to photoinhibition and photodamage [105]. These results suggest that the control treatment plants had marked damage to their antenna pigments and impairment of electron fluxes between PSII and PSI through the thylakoid membrane due to nutrient deficiency [100,106,107]. This nutritional deficiency leads to increased production of reactive oxygen species that inhibit PSII repair and induce oxidative damage and degradation of the thylakoid membrane, which may correlate with decreased chlorophyll content [108,109,110].
4.3. The Strains PA12 (P. alvei), SC5 (B. cereus), and 328EF (Codinaeopsis sp.) Stood Out in Optimizing Nutrient Concentration, Transpiration Rate, and Stomatal Conductance in G. max
Although no differences were observed between the inocula in improving the uptake of P by the plants, the entire absorption of P was effectively due to microbial activity because there was no P remaining in the soil (see Table 2). The bacteria PA12 (P. alvei) and SC5 (B. cereus) and the fungi 328EF (Codinaeopsis sp.) and SC4 (E. keratinophilum) increased the total content of N. The effect observed for the bacteria is directly associated with the solubilizing capacity of the strains. Increases in the content of plant-available P, through solubilization, directly affect the content of N in the samples. Studies show that biological fixation of N is affected by the amount of P available to the plant. In addition to being essential for soybean energy metabolism, as a constituent of the ATP molecule, P contributes to nodulation and atmospheric nitrogen fixation [111,112].
PA12 (P. alvei), SC5 (B. cereus), and SC15 (P. sheari) also increased the Cu content in soybean plants. This effect may be associated with the production of phenolic compounds, a process observed in dicotyledons under Fe deficiency. These compounds affect the rhizosphere microbial community, leading to increased synthesis of siderophores and metal chelators that facilitate root Cu uptake [113,114,115,116].
Moreover, inoculation with PA12 (P. alvei) and 328EF (Codinaeopsis sp.) increased B uptake. B is commonly affected by microbial activity [117,118]. It is preferentially taken up via soil due to its very limited mobility in the phloem, being mainly translocated through the xylem [119,120]. B has a role in cell wall formation and stability, lignification, and root nodulation, and its deficiency affects biological N2 fixation and, consequently, N uptake [121,122].
Strains PA12 (P. alvei), SC5 (B. cereus), and 328EF (Codinaeopsis sp.) were the most effective in improving the transpiration rate and stomatal conductance in G. max plants. In rice plants, microbial inoculation also increased E and Gsw [123]. This is because mucilaginous exudates and polysaccharides released by plant roots (mucilage) and root-associated microorganisms (mucigel) have an impact on the stability of soil aggregates, generating macropores and influencing hydraulic processes in the rhizosphere [124]. The mucilage network reinforces the soil matrix potential around the roots, helping keep the rhizosphere moist and preventing sudden drops in water flow, especially around the root tips [125,126]. Improved access to water allows plants to maintain higher rates of transpiration and stomatal conductance. Moreover, fungal hyphae can access places in the soil that roots do not reach to obtain water and nutrients. Studies have shown that hyphae increase root hydraulic conductivity and plant water uptake [127]. Gharizadeh et al. [128] showed that Codinaeopsis gonytrichoides effectively interacts with wood. Thus, a symbiotic association with a species of this genus that improves water uptake by soybean plants and increases E and Gsw was established in this study.
Bacterial inoculants stand out in the agricultural market, mainly because they are easy to obtain. Preparations with endospore-forming Bacillus strains are more required because their long-term viability facilitates the development of commercial products. Currently, the potential for inoculant production from Paenibacillus, originally included in the genus Bacillus, has also been widely evaluated. Bacteria of the genera Bacillus and Paenibacillus exploit a wide variety of organic and inorganic substrates as nutrient sources [129] and Paenibacillus has already been described as an N2-fixing bacterium [32,130]. Moreover, the production of antimicrobial substances and sporulation capacity of Bacillus and Paenibacillus strains provide them with a double advantage in terms of competition for resources and survival in different habitats [51].
We validated the potential of the phosphate-solubilizing strains described by Reis et al. [23], PA12 (P. alvei) and SC5 (B. cereus), in promoting G. max growth in a controlled greenhouse system and as candidates for the formulation of inoculant products in the future. However, similar to other studies [47], we indicate that the use of B. cereus strains to be marketed as biofertilizers is valid provided that the strains are non-pathogenic.
PCA showed that the results of the plants inoculated with the bacterial strains PA12 (P. alvei) and SC5 (B. cereus) and with the fungal strains 328EF (Codinaeopsis sp.) and SC15 (P. sheari) were the closest to the pattern observed for the commercial product Biomaphos®, with the same trend of direction of the means associated with the chlorophyll index, A, dry mass and concentration of important nutrients such as N, P, and Mg.
5. Conclusions
Here we demonstrate the ability of new strains PA12 (P. alvei), SC5 (B. cereus), 328EF (Codinaeopsis sp.) and SC15 (P. sheari) to perform better in terms of chlorophyll index, (A), dry mass, and concentration of important nutrients such as N, P, and Mg, in plants of G. max. The activity of these strains was compatible with the growth promotion pattern presented by a commercial product available on the market. Thus, we recommend the use of these isolates in field tests to validate these strains for the production of biological inoculants that will comprise the portfolio of bioinputs available for sustainable agricultural practices.
Author Contributions
Conceptualization, L.A.B. and L.C.V.; methodology, M.N.O.R. and L.L.L.; formal analysis, M.N.O.R. and L.L.L.; investigation, M.N.O.R. and L.C.V.; resources, M.N.O.R. and L.C.V.; writing—original draft preparation, M.N.O.R. and L.C.V.; writing—review and editing, L.A.B.; visualization, M.N.O.R. and L.C.V.; supervision, L.A.B.; project administration, L.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting the Master’s scholarship to Mateus Neri Oliveira Reis; the Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG) and IFGoiano, Rio Verde Campus for the infrastructure; and the students for helping in the study. We also thank Simple Agro Agribusiness Systems for their support and inputs.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gawęda, D.; Nowak, A.; Haliniarz, M.; Woźniak, A. Yield and economic effectiveness of soybean grown under different cropping systems. Int. J. Plant Prod. 2020, 14, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S.; Yang, L.T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.B.; Perkins-Veazie, P.; McCall, I.; Whipker, B.E. Restricted phosphorus fertilization increases the betacyanin concentration and red foliage coloration of alternanthera. J. Am. Soc. Hortic. Sci. 2019, 144, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.R.; Cunha, D.G.F.; Kurian, M. A water-energy-food nexus perspective on the challenge of eutrophication. Water 2018, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.L.; Maguire, R.O.; Alley, M.M.; Thomason, W.E.; Whitehurst, G.B. Plant-available phosphorus after application of synthetic chelating agents. Commun. Soil Sci. Plant Anal. 2016, 47, 433–446. [Google Scholar] [CrossRef]
- Chen, R.; Song, S.; Li, X.; Liu, H.; Huang, D. Phosphorus deficiency restricts plant growth but induces pigment formation in the flower stalk of Chinese kale. Hortic. Environ. Biotechnol. 2013, 54, 243–248. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef] [Green Version]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A.; Rchiad, Z. Benefits of phosphate solubilizing bacteria on belowground crop performance for improved crop acquisition of phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef]
- Estrada-Bonilla, G.A.; Durrer, A.; Cardoso, E.J. Use of compost and phosphate-solubilizing bacteria affect sugarcane mineral nutrition, phosphorus availability, and the soil bacterial community. Appl. Soil Ecol. 2021, 157, 103760. [Google Scholar] [CrossRef]
- Lucero, C.T.; Lorda, G.S.; Anzuay, M.S.; Ludueña, L.M.; Taurian, T. Peanut endophytic phosphate solubilizing bacteria increase growth and P content of soybean and maize plants. Curr. Microbiol. 2021, 78, 1961–1972. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Riaz, U.; Murtaza, G.; Anum, W.; Samreen, T.; Sarfraz, M.; Nazir, M.Z. Plant Growth-Promoting Rhizobacteria (PGPR) as biofertilizers and biopesticides. In Microbiota and Biofertilizers; Springer: Cham, Switzerland, 2021; pp. 181–196. [Google Scholar] [CrossRef]
- Nor, M.N.M. Isolation and Characterization of Effective Microorganism from Oil Palm Rhizhopheric Soil and Evaluation of Their Potential as Biofertiliser. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 515, p. 012040. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant growth-promoting microorganisms for environmental sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Jiang, X.; Wang, Q.; Guan, D.; Li, L.; Ongena, M.; Li, J. Isolation and identification of PGPR strain and its effect on soybean growth and soil bacterial community composition. Int. J. Agric. Biol. 2018, 20, 1289–1297. [Google Scholar] [CrossRef]
- Blanco, M. Supply of and Access to Key Nutrients NPK for Fertilizers for Feeding the World in 2050; UPM: Madrit, Spain, 2011. [Google Scholar]
- Kazakova, I. Characteristic features of the formation of the global and domestic markets of mineral fertilizers. Econ. Forecast 2015, 2, 104–118. [Google Scholar] [CrossRef] [Green Version]
- Cordell, D.; White, S. Peak phosphorus: Clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef] [Green Version]
- Miransari, M. Soil microbes and plant fertilization. Appl. Microbiol. Biotechnol. 2011, 92, 875–885. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 16. [Google Scholar] [CrossRef]
- Reis, M.N.O.; Bessa, L.A.; de Jesus, A.P.; Silva, F.G.; Moreira, M.A.; Vitorino, L.C. Efficiency of the Hydroponic System as an Approach to Confirm the Solubilization of CaHPO4 by Microbial Strains Using Glycine max as a Model. Front. Plant Sci. 2021, 12, 759463. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haney, C.H.; Samuel, B.S.; Bush, J.; Ausubel, F.M. Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat. Plants 2015, 1, 15051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [Green Version]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y.; Wang, S.; Shi, X.; Li, L.; Liang, J. Effects of phosphate solubilizing bacteria on the growth, photosynthesis, and nutrient uptake of Camellia oleifera abel. Forests 2019, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Rafi, M.M.; Krishnaveni, M.S.; Charyulu, P.B.B.N. Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. In Recent Developments in Applied Microbiology and Biochemistry; Academic Press: Dordrecht, The Netherlands, 2019; pp. 223–233. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Li, Y.; Guan, G.; Chen, S. Paenibacillus strains with nitrogen fixation and multiple beneficial properties for promoting plant growth. PeerJ. 2019, 7, e7445. [Google Scholar] [CrossRef] [Green Version]
- Dash, N.; Pahari, A.; Dangar, T.K. Functionalities of phosphate-solubilizing bacteria of rice rhizosphere: Techniques and perspectives. In Recent Advances in Applied Microbiology; Springer: Singapore, 2017; pp. 151–163. [Google Scholar] [CrossRef]
- de Souza Rocha, A.F.; Vitorino, L.C.; Bessa, L.A.; Costa, R.R.G.F.; da Silva Brasil, M.; Souchie, E.L. Soil parameters affect the functional diversity of the symbiotic microbiota of Hymenaea courbaril L., a Neotropical fruit tree. Rhizosphere 2020, 16, 100237. [Google Scholar] [CrossRef]
- da Silva, C.F.; Vitorino, L.C.; Soares, M.A.; Souchie, E.L. Multifunctional potential of endophytic and rhizospheric microbial isolates associated with Butia purpurascens roots for promoting plant growth. Antonie Van Leeuwenhoek 2018, 111, 2157–2174. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; California Agriculture Experiment Station Circular 347-2; University of California College of Agriculture: Berkeley, CA, USA, 1950. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; p. 319. [Google Scholar]
- de Oliveira, S.R.; Raposo, J.L., Jr.; Neto, J.A.G. Fast sequential multi-element determination of Ca, Mg, K, Cu, Fe, Mn and Zn for foliar diagnosis using high-resolution continuum source flame atomic absorption spectrometry: Feasibility of secondary lines, side pixel registration and least-squares background correction. Spectrochim. Acta B At. Spectrosc. 2009, 64, 593–596. [Google Scholar] [CrossRef]
- Oliveira, T.C. Otimização Multivariada e Validação de Métodos Para A Determinação de Boro, Enxofre, Fósforo e Molibdênio em Fertilizante Mineral por ICP OES. 2016. Available online: https://www.lume.ufrgs.br/handle/10183/142151 (accessed on 27 July 2021).
- Guebel, D.V.; Nudel, B.C.; Giulietti, A.M. A simple and rapid micro-Kjeldahl method for total nitrogen analysis. Biotechnol. Tech. 1991, 5, 427–430. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; CRC Press: Boca Raton, FL, USA, 2000; pp. 445–483. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 27 March 2022).
- Yadav, A.N.; Verma, P.; Singh, B.; Chauhan, V.S.; Suman, A.; Saxena, A.K. Plant growth promoting bacteria: Biodiversity and multifunctional attributes for sustainable agriculture. Adv. Biotechnol. Microbiol. 2017, 5, 555671. [Google Scholar] [CrossRef]
- Kalaiselvi, P.; Jayashree, R.; Poornima, R. Plant growth promoting Bacillus spp. and Paenibacillus alvei on the growth of Sesuvium portulacastrum for phytoremediation of salt affected soils. Int. J. Curr. Microbiol. Appl. Sci 2019, 8, 2847–2858. [Google Scholar] [CrossRef]
- Kumar, P.; Khare, S.; Dubey, R.C. Diversity of bacilli from disease suppressive soil and their role in plant growth promotion and yield enhancement. N. Y. Sci. J. 2012, 5, 90–111. [Google Scholar]
- Schoina, C.; Stringlis, I.A.; Pantelides, I.S.; Tjamos, S.E.; Paplomatas, E.J. Evaluation of application methods and biocontrol efficacy of Paenibacillus alvei strain K-165, against the cotton black root rot pathogen Thielaviopsis basicola. Biol. Control 2011, 58, 68–73. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Ding, H.; Niu, B.; Fan, H.; Li, Y.; Wang, Q. Draft genome sequence of Bacillus cereus 905, a plant growth-promoting rhizobacterium of wheat. Genome Announc. 2016, 4, e00489-16. [Google Scholar] [CrossRef] [Green Version]
- Ku, Y.; Xu, G.; Tian, X.; Xie, H.; Yang, X.; Cao, C. Root colonization and growth promotion of soybean, wheat and Chinese cabbage by Bacillus cereus YL6. PLoS ONE 2018, 13, e0200181. [Google Scholar] [CrossRef] [Green Version]
- Baliyan, N.; Dhiman, S.; Dheeman, S.; Kumar, S.; Arora, N.K.; Maheshwari, D.K. Optimization of gibberellic acid production in endophytic Bacillus cereus using response surface methodology and its use as plant growth regulator in chickpea. J. Plant Growth Regul. 2021, 1–11. [Google Scholar] [CrossRef]
- Zeng, Q.; Xie, J.; Li, Y.; Gao, T.; Xu, C.; Wang, Q. Comparative genomic and functional analyses of four sequenced Bacillus cereus genomes reveal conservation of genes relevant to plant-growth-promoting traits. Sci. Rep. 2018, 8, 17009. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.K.; Sangeeta. Role of antagonistic microbes in management of phytopathogenic fungi of some important crops. In Microbial Diversity and Biotechnology in Food Security; Springer: New Delhi, India, 2014; pp. 273–292. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Miransari, M. Soil microbes and the availability of soil nutrients. Acta Physiol. Plant. 2013, 35, 3075–3084. [Google Scholar] [CrossRef]
- Hajjam, Y.; Cherkaoui, S. The influence of phosphate solubilizing microorganisms on symbiotic nitrogen fixation: Perspectives for sustainable agriculture. J. Mater. 2017, 8, 801–808. [Google Scholar]
- Li, Y.; Li, Q.; Guan, G.; Chen, S. Phosphate solubilizing bacteria stimulate wheat rhizosphere and endosphere biological nitrogen fixation by improving phosphorus content. PeerJ 2020, 8, e9062. [Google Scholar] [CrossRef]
- Coonan, E.C.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.R.; Strong, C.L.; Richardson, A.E. Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutr. Cycl. Agroecosyst. 2020, 117, 273–298. [Google Scholar] [CrossRef]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [Green Version]
- Pathan, S.I.; Ceccherini, M.T.; Sunseri, F.; Lupini, A. Rhizosphere as hotspot for plant-soil-microbe interaction. In Carbon and Nitrogen Cycling in Soil; Springer: Singapore, 2020; pp. 17–43. [Google Scholar] [CrossRef]
- Ali, S.; Xie, L. Plant growth promoting and stress mitigating abilities of soil born microorganisms. Recent Pat. Food Nutr. Agric. 2020, 11, 96–104. [Google Scholar] [CrossRef]
- Pirog, T.P.; Iutynska, G.O.; Leonova, N.O.; Beregova, K.A.; Shevchuk, T.A. Microbial synthesis of phytohormones. Biotechnol. Acta 2018, 11, 5–24. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Garrido, C.; Collado, I.G. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phytochem. Rev. 2020, 19, 721–740. [Google Scholar] [CrossRef]
- Pigoleva, S.V.; Zakharchenko, N.S.; Furs, O.V.; Tarlachkov, S.V.; Funtikova, T.V.; Filonov, A.E.; Aripovskii, A.V.; Dyachenko, O.V.; Buryanov, Y.I.; Shevchuk, T.V. Effects of Associative Microorganisms on Plant Growth and Resistance to Xenobiotics and Phytopathogens. Appl. Biochem. Microbiol. 2020, 56, 473–482. [Google Scholar] [CrossRef]
- Sidorova, T.M.; Asaturova, A.M.; Homyak, A.I. Biologically active metabolites of Bacillus subtilis and their role in the control of phytopathogenic microorganisms. Agric. Biol. 2018, 53, 29–37. [Google Scholar] [CrossRef]
- Santos Júnior, V.D.; Nizoli, É.; Galvan, D.; Gomes, R.J.; Biz, G.; Ressutte, J.B.; Rocha, T.D.S.; Spinosa, W.A. Micronutrient requirements and effects on cellular growth of acetic acid bacteria involved in vinegar production. Food Sci. Technol. 2022, 2, e05121. [Google Scholar] [CrossRef]
- Subramani, S.; Perdreau-Dahl, H.; Morth, J.P. The magnesium transporter A is activated by cardiolipin and is highly sensitive to free magnesium in vitro. Elife 2016, 5, e11407. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Tanoi, K.; Kobayashi, N. Leaf Senescence by Magnesium Deficiency. Plants 2015, 4, 756–772. [Google Scholar] [CrossRef] [Green Version]
- Arigony, A.L.V.; de Oliveira, I.M.; Machado, M.; Bordin, D.L.; Bergter, L.; Prá, D.; Pegas Henriques, J.A. The influence of micronutrients in cell culture: A reflection on viability and genomic stability. BioMed Res. Int. 2013, 2013, 597282. [Google Scholar] [CrossRef] [Green Version]
- Suran, P.; Kulhánek, M.; Balík, J.; Černý, J.; Sedlář, O. Evaluation of Soil S Pools under 23 Years of Maize Monoculture. Agronomy 2021, 11, 2376. [Google Scholar] [CrossRef]
- Ma, Q.; Wen, Y.; Pan, W.; Macdonald, A.; Hill, P.W.; Chadwick, D.R.; Wu, L.; Jones, D.L. Soil carbon, nitrogen, and sulphur status affects the metabolism of organic S but not its uptake by microorganisms. Soil Biol. Biochem. 2020, 149, 107943. [Google Scholar] [CrossRef]
- Fox, A.; Kwapinski, W.; Griffiths, B.S.; Schmalenberger, A. The role of sulfur-and phosphorus-mobilizing bacteria in biochar-induced growth promotion of Lolium perenne. FEMS Microbiol. Ecol. 2014, 90, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2022, 2030082. [Google Scholar] [CrossRef] [PubMed]
- Udayana, S.K.; Singh, P.; Jaison, M.; Roy, A. Sulphur: A boon in agriculture. Pharma Innov. J. 2021, 10, 912–921. [Google Scholar]
- Jeon, J.S.; Etalo, D.W.; Carreno-Quintero, N.; de Vos, R.C.; Raaijmakers, J.M. Effects of Sulfur Assimilation in Pseudomonas fluorescens SS101 on Growth, Defense, and Metabolome of Different Brassicaceae. Biomolecules 2021, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Kulczycki, G. The effect of elemental sulfur fertilization on plant yields and soil properties. Adv. Agron. 2021, 167, 105–181. [Google Scholar] [CrossRef]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef]
- Lurthy, T.; Pivato, B.; Lemanceau, P.; Mazurier, S. Importance of the Rhizosphere Microbiota in Iron Biofortification of Plants. Front. Plant Sci. 2021, 12, 744445. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free Radic. Biol. Med. 2019, 133, 11–20. [Google Scholar] [CrossRef]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef]
- Holz, M.; Zarebanadkouki, M.; Carminati, A.; Becker, J.N.; Spohn, M. The effect of root hairs on rhizosphere phosphatase activity. J. Plant Nutr. Soil Sci. 2020, 183, 382–388. [Google Scholar] [CrossRef]
- Holz, M.; Zarebanadkouki, M.; Kuzyakov, Y.; Pausch, J.; Carminati, A. Root hairs increase rhizosphere extension and carbon input to soil. Ann. Bot. 2018, 121, 61–69. [Google Scholar] [CrossRef] [Green Version]
- McRose, D.L.; Seyedsayamdost, M.R.; Morel, F.M. Multiple siderophores: Bug or feature? JBIC J. Biol. Inorg. Chem. 2018, 23, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Goteti, P.K.; Emmanuel, L.D.A.; Desai, S.; Shaik, M.H.A. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 869697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: New York, NY, USA, 2010. [Google Scholar]
- Vitorino, L.C.; de Souza Rocha, A.F.; Bessa, L.A.; Lourenço, L.L.; da Costa, A.C.; Silva, F.G. Symbiotic microorganisms affect the resilience of Hymenaea courbaril L., a neotropical fruit tree, to water restriction. Plant Stress 2022, 5, 100092. [Google Scholar] [CrossRef]
- Eshaghi, E.; Nosrati, R.; Owlia, P.; Malboobi, M.A.; Ghaseminejad, P.; Ganjali, M.R. Zinc solubilization characteristics of efficient siderophore-producing soil bacteria. Iran. J. Microbiol. 2019, 11, 419. [Google Scholar] [CrossRef]
- Kumar, A.; Dewangan, S.; Lawate, P.; Bahadur, I.; Prajapati, S. Zinc-solubilizing bacteria: A boon for sustainable agriculture. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 139–155. [Google Scholar] [CrossRef]
- Hussein, K.A.; Joo, J.H. Zinc ions affect siderophore production by fungi isolated from the Panax ginseng rhizosphere. J. Microbiol. Biotechnol. 2019, 29, 105–113. [Google Scholar] [CrossRef]
- Nandal, V.; Solanki, M. Zn as a vital micronutrient in plants. J. Microbiol. Biotechnol. Food Sci. 2021, 11, 262–271. [Google Scholar] [CrossRef]
- Gondal, A.H.; Zafar, A.; Zainab, D.; Toor, M.D.; Sohail, S.; Ameen, S.; Ijaz, A.B.; Ch, B.I.; Hussain, I.; Haider, S.; et al. A detailed review study of zinc involvement in animal, plant and human nutrition. Indian J. Pure Appl. Biosci. 2021, 9, 262–271. [Google Scholar] [CrossRef]
- Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Yuvaraj, M.; Subramanian, K.S. Significance of Zinc in Plant Nutrition. Biotica Res. Today 2020, 2, 823–825. [Google Scholar]
- Gautam, A.; Dubey, R.S. Metal toxicity in plants: Induction of oxidative stress, antioxidative defense system, metabolic alterations and phytoremediation. In Molecular Physiology of Abiotic Stresses in Plant Productivity; American Scientific Publishers: Valencia, CA, USA, 2018; pp. 256–290. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 191–248. [Google Scholar]
- Bangera, M.; Gowda, K.G.; Sagurthi, S.R.; Murthy, M.R.N. Structural and functional insights into phosphomannose isomerase: The role of zinc and catalytic residues. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 475–487. [Google Scholar] [CrossRef]
- Hacisalihoglu, G. Zinc (Zn): The last nutrient in the alphabet and shedding light on Zn efficiency for the future of crop production under suboptimal Zn. Plants 2020, 9, 1471. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, R.; Kalaji, H.M.; Valizadeh, G.R.; Khalilvand Behrozyar, E.; Hemati, A.; Gharavi-Kochebagh, P.; Ghassemi, A. Effects of humic acid on photosynthetic efficiency of rapeseed plants growing under different watering conditions. Photosynthetica 2018, 56, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Morales, F.; Pavlovič, A.; Abadía, A.; Abadía, J. Photosynthesis in poor nutrient soils, in compacted soils, and under drought. In The Leaf: A Platform for Performing Photosynthesis; Springer: Cham, Switzerland, 2018; pp. 371–399. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Ohnishi, M.; Furutani, R.; Sohtome, T.; Suzuki, T.; Wada, S.; Tanaka, S.; Ifuku, K.; Ueno, D.; Miyake, C. Photosynthetic parameters show specific responses to essential mineral deficiencies. Antioxidants 2021, 10, 996. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Li, L.; Aro, E.M.; Millar, A.H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular Mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Contador, C.A.; Lo, S.K.; Chan, S.H.; Lam, H.M. Metabolic analyses of nitrogen fixation in the soybean microsymbiont Sinorhizobium fredii using constraint-based modeling. MSystems 2020, 5, e00516-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Contador, C.A.; Fan, K.; Lam, H.M. Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front. Plant Sci. 2018, 9, 1860. [Google Scholar] [CrossRef] [Green Version]
- González-Guerrero, M.; Matthiadis, A.; Sáez, Á.; Long, T.A. Fixating on metals: New insights into the role of metals in nodulation and symbiotic nitrogen fixation. Front. Plant Sci. 2014, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Sugiyama, A. The soybean rhizosphere: Metabolites, microbes, and beyond—A review. J. Adv. Res. 2019, 19, 67–73. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef] [Green Version]
- Mandal, M.; Naik, S.K.; Das, D.K. Effect of boron and sulfur interaction on some important biological indices in an inceptisol. J. Plant Nutr. 2018, 41, 197–209. [Google Scholar] [CrossRef]
- Bilen, S.; Bilen, M.; Bardhan, S. The effects of boron management on soil microbial population and enzyme activities. Afr. J. Biotechnol. 2011, 10, 5311–5319. [Google Scholar]
- Pereira, G.L.; Siqueira, J.A.; Batista-Silva, W.; Cardoso, F.B.; Nunes-Nesi, A.; Araújo, W.L. Boron: More than an essential element for land plants? Front. Plant Sci. 2021, 11, 2234. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.T.; Pan, J.F.; Hu, N.J.; Chen, H.H.; Jiang, H.X.; Lu, Y.B.; Chen, L.S. Citrus physiological and molecular response to boron stresses. Plants 2021, 11, 40. [Google Scholar] [CrossRef]
- Mehboob, N.; Hussain, M.; Minhas, W.A.; Yasir, T.A.; Naveed, M.; Farooq, S.; Alfarraj, S.; Zuan, A.T.K. Soil-applied boron combined with boron-tolerant bacteria (Bacillus sp. mn54) improve root proliferation and nodulation, yield and agronomic grain biofortification of chickpea (Cicer arietinum L.). Sustainability 2021, 13, 9811. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef] [Green Version]
- Haider, F.U.; Coulter, J.A.; Cheema, S.A.; Farooq, M.; Wu, J.; Zhang, R.; Shuaijie, G.; Liqun, C. Co-application of biochar and microorganisms improves soybean performance and remediate cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 214, 112112. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente Cantó, C.; Simonin, M.; King, E.; Moulin, L.; Bennett, M.J.; Castrillo, G.; Laplaze, L. An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. Plant J. 2020, 103, 951–964. [Google Scholar] [CrossRef] [Green Version]
- Carminati, A.; Zarebanadkouki, M.; Kroener, E.; Ahmed, M.A.; Holz, M. Biophysical rhizosphere processes affecting root water uptake. Ann. Bot. 2016, 118, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Carminati, A. Rhizosphere wettability decreases with root age: A problem or a strategy to increase water uptake of young roots? Front. Plant Sci. 2013, 4, 298. [Google Scholar] [CrossRef] [Green Version]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef]
- Gharizadeh, K.H.; Sheykholeslami, A.; Khodaparast, S.A. A study on the identification of wood inhabiting hyphomycetes in Chalus vicinity (Iran). Rostaniha 2007, 8, 93–109. [Google Scholar]
- Hamdache, A.; Azarken, R.; Lamarti, A.; Aleu, J.; Collado, I.G. Comparative genome analysis of Bacillus spp. and its relationship with bioactive nonribosomal peptide production. Phytochem. Rev. 2013, 12, 685–716. [Google Scholar] [CrossRef]
- Anand, R.; Chanway, C. N2-fixation and growth promotion in cedar colonized by an endophytic strain of Paenibacillus polymyxa. Biol. Fertil. Soils 2013, 49, 235–239. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).