Relationship between Bacterial Contribution and Self-Healing Effect of Cement-Based Materials
Abstract
:1. Introduction
2. Why Is Bacterial Activity Crucial for the Self-Healing Effect of Bacteria-Based Concrete?
3. Mandatory Requirements for Bacteria as Bioagents in Bacterial Concrete Technology
- Complete characterization (taxonomic identification, history of bacterial species/strain in terms of recognized pathogenicity, genetic changes, ecological properties, etc.);
- Methods for reproduction and application of the bacterium, which are in accordance with biosafety laws (e.g., antibiotic resistance, genetic mutations, etc.);
- Defining stability, activity, packaging, and storage of a biological agent in compliance with legal regulations;
- Comprehensive assessment of unpredictable environmental (and health) risks for release of (genetically modified) bacterium into the environment [19].
4. Evaluation of the Bacterial Contribution to the Self-Healing Effect
4.1. Metabolic Activities That Lead to Carbonate Ions Production
How to Find an Appropriate Bacterium for Self-Healing Concrete?
4.2. Keeping Bacterial Viability—The Key to Effective Self-Healing Effect
- Rate of bacterial activity (almost no one pays attention);
- Level of SH effect (monitoring with standardized and/or non-standardized, but well-known methodologies in concrete characterization protocols);
- Characteristics of concrete (monitoring with standardized and/or non-standardized, but well-known methodologies in concrete characterization protocols).
4.3. Physical Contribution of Individual Bacterial Cells to Self-Healing Effect
- Cell geometric compatibility (cell size, specific surface area, and volume) and motility;
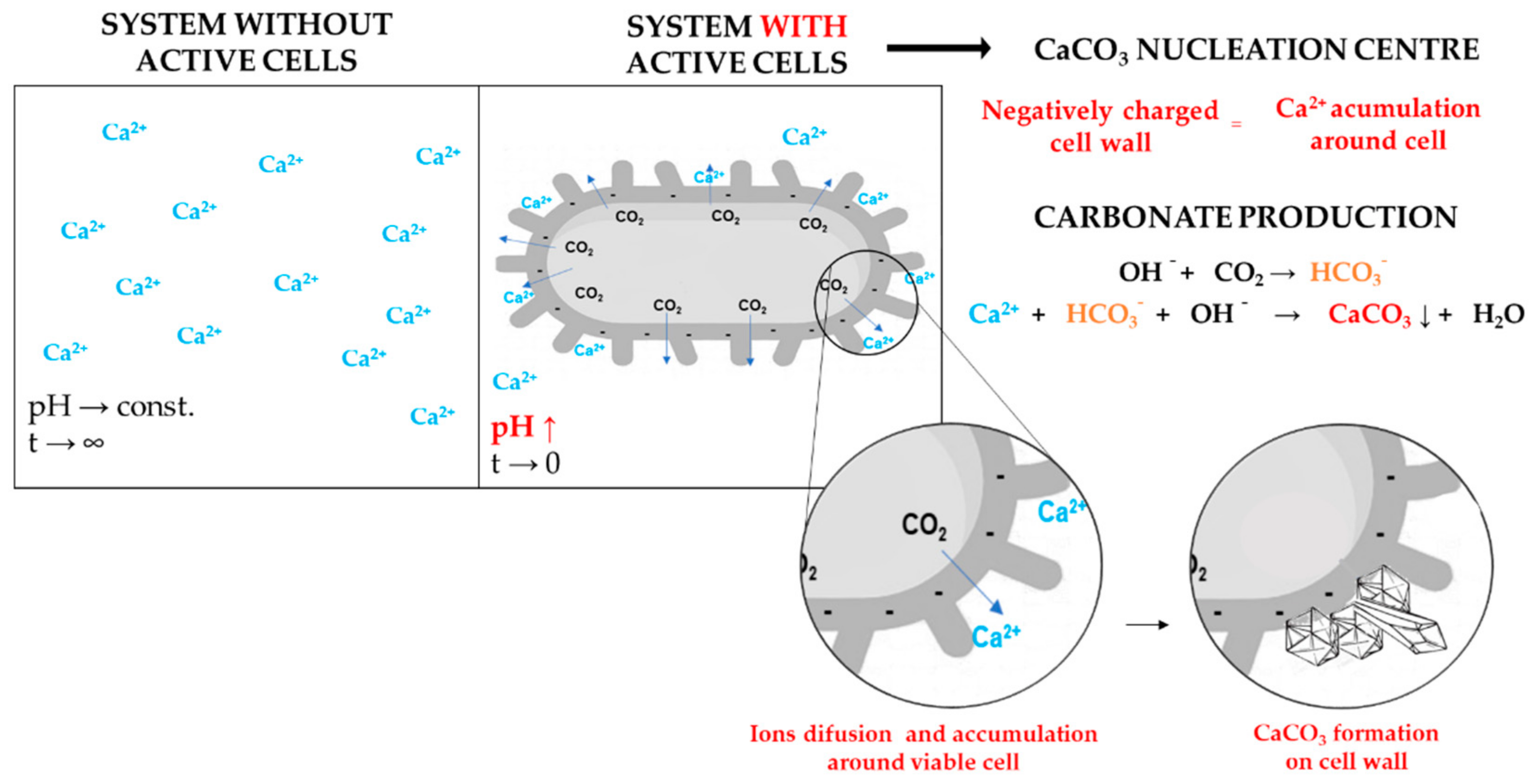
- Cell−surface electronegativity and hydrophobicity (affinity, types, and amount of chemical bonds on the cell surface);
- Cell membrane permeability (in a function of extracellular carbonate production rate);
- Biofilm production (possibility and rate of adhesion on inorganic surfaces) [88];
- The cell size can be a crucial parameter for bacterial activity in view of variable concrete porosity during dry/wet cycles in material [7]. Figure 3 also showed a correlation between average cells and pore sizes during hydration and volumetric changes in concrete. Besides water availability, pore size changes can also be a limiting parameter for spore activation and cell proliferation. Based on cell size, cell−specific area and volume of active bacteria that are involved in BICP are between 2.6 and 8.55 µm2 and between 0.3 and 1.64 µm3, respectively [88]. Considering that bacteria require availability in space, pore volume can influence bacterial activity, and as a result, CaCO3 precipitation.
5. Influence of Concrete Environment on Bacteria-Based Self-Healing Effect
5.1. pH Value
5.2. Presence, Concentration, and Availability of Calcium Ions
5.3. Presence of Other Elements, Cofactors, and Inhibitors of Bacterial Activity in a Concrete Matrix
5.4. Presence and Availability of Essential Nutrients for Metabolic Activity
5.5. Other Influence
6. Influence of Concrete Characteristics on Bacteria-Based Self-Healing Effect
- Permeability (due to the water availability);
- Porosity (due to space limitation);
- Crack size (due to contact with the environment and collection of nutrients, water, and oxygen);
- Aging rate.
6.1. Porosity, Permeability, and Water Availability
6.2. Crack Appearance and Size
6.3. Age Rate
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Huseien, G.F.; Nehdi, M.L.; Faridmehr, I.; Ghoshal, S.K.; Hamzah, H.K.; Benjeddou, O.; Alrshoudi, F. Smart Bio-Agents-Activated Sustainable Self-Healing Cementitious Materials: An All-Inclusive Overview on Progress, Benefits and Challenges. Sustainability 2022, 14, 1980. [Google Scholar] [CrossRef]
- Meyer, C. Concrete Materials and Sustainable Development in the USA. Struct. Eng. Int. 2004, 14, 203–207. [Google Scholar] [CrossRef]
- Maslyk, M.; Gäb, T.; Matveeva, G.; Opitz, P.; Mondeshki, M.; Krysiak, Y.; Kolb, U.; Tremel, W. Multistep Crystallization Pathways in the Ambient-Temperature Synthesis of a New Alkali-Activated Binder. Adv. Funct. Mater. 2022, 32, 2108126. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, M.; Ding, X.; Ren, Z.; Zhao, S.; Zhao, M.; Dang, J. High-Durability Concrete with Supplementary Cementitious Admixtures Used in Corrosive Environments. Crystals 2021, 11, 196. [Google Scholar] [CrossRef]
- Wang, J.Y. Self-Healing Concrete by Means of Immobilized Carbonate Precipitating Bacteria. Ph.D. Thesis, University of Ghent, Ghent, Belgium, 2013. [Google Scholar]
- Mostavi, E.; Asadi, S.; Hassan, M.M.; Alansari, M. Evaluation of Self-Healing Mechanisms in Concrete with Double-Walled Sodium Silicate Microcapsules. J. Mater. Civ. Eng. 2015, 27, 04015035. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Qian, C.; Chen, H.; Ren, L.; Luo, M. Self-healing of early age cracks in cement-based materials by mineralization of carbonic anhydrase microorganism. Front. Microbiol. 2015, 6, 1225. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ersan, Y.C.; Boon, N. Application of microorganisms in concrete: A promising sustainable strategy to improve concrete durability. Appl. Microbiol. Biotechnol. 2016, 100, 2993–3007. [Google Scholar] [CrossRef]
- Hermawan, H.; Minne, P.; Serna, P.; Gruyaert, E. Understanding the Impacts of Healing Agents on the Properties of Fresh and Hardened Self-Healing Concrete: A Review. Processes 2021, 9, 2206. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Wei, Y.Q.; Cai, H.; Wang, Z.W.; Yang, T.; Wang, Q.H.; Wu, S.F. Microbial-Induced Carbonate Precipitation for Strengthening Soft Clay. Adv. Mater. Sci. Eng. 2020, 2020, 8140724. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zheng, M.; Li, C.; Gui, M.; Chen, Q.; Ni, J. Special role of corn flour as an ideal carbon source for aerobic denitrification with minimized nitrous oxide emission. Bioresour. Technol. 2015, 186, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Seifan, M.; Berenjian, A. Microbially induced calcium carbonate precipitation: A widespread phenomenon in the biological world. Appl. Microbiol. Biotechnol. 2019, 103, 4693–4708. [Google Scholar] [CrossRef] [PubMed]
- Tourney, J.; Ngwenya, B.T. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Siddique, R.; Chahal, N.K. Effect of ureolytic bacteria on concrete properties. Constr. Build. Mater. 2011, 25, 3791–3801. [Google Scholar] [CrossRef]
- Hoffmann, D.T.; Reeksting, B.J.; Gebhard, S. Bacteria-induced mineral precipitation: A mechanistic review. Microbiol. Res. 2021, 167, 001049. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.H.; Shin, Y.J.; So, J.S. Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 2016, 5, 250–276. [Google Scholar] [CrossRef] [Green Version]
- Vidaković, A.; Šovljanski, O.; Vučurović, D.; Racić, G.; Đilas, M.; Ćurčić, N.; Markov, S. Novel denitrifying bacteria Pseudomonas stutzeri strain D1—From isolation to the biomass production. CICEQ 2019, 25, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, V.; Stabnikov, V.; Stabnikova, O.; Kawasaki, S. Environmental safety and biosafety in construction biotechnology. World J. Microbiol. Biotechnol. 2019, 35, 26. [Google Scholar] [CrossRef]
- Erşan, Y.Ç.; de Belie, N.; Boon, N. Microbially induced CaCO3 precipitation through denitrification: An optimization study in minimal nutrient environment. Biochem. Eng. J. 2015, 101, 108–118. [Google Scholar] [CrossRef]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially Induced Calcium Carbonate Precipitation on Surface or in the Bulk of Soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Sarayu, K.; Iyer, N.R.; Murthy, A.R. Exploration on the Biotechnological Aspect of the Ureolytic Bacteria for the Production of the Cementitious Materials—A Review. Appl. Biochem. Biotechnol. 2014, 172, 2308–2323. [Google Scholar] [CrossRef] [PubMed]
- Ambus, P.; Zechmeister-Boltenstern, S. Denitrification and N-Cycling in Forest Ecosystems. In Biology of the Nitrogen Cycle; Elsevier: Amsterdam, The Netherlands, 2007; pp. 343–358. [Google Scholar]
- Ma, L.; Pang, A.P.; Luo, Y.; Lu, X.; Lin, F. Beneficial factors for biomineralization by ureolytic bacterium Sporosarcina pasteurii. Microb. Cell Factories 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X. Influence of calcium sources on microbially induced calcium carbonate precipitation by Bacillus sp. CR2. Appl. Biochem. Biotechnol. 2014, 173, 307–317. [Google Scholar] [CrossRef]
- Schwantes-Cezario, N.; Medeiros, L.P.; De Oliveira, A.G.; Nakazato, G.; Katsuko Takayama Kobayashi, R.; Toralles, B.M. Bioprecipitation of calcium carbonate induced by Bacillus subtilis isolated in Brazil. Int. Biodeterior. Biodegrad. 2017, 123, 200–205. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Williams, B.C.; Crawford, R.L. Urease Activity of Ureolytic Bacteria Isolated from Six Soils in which Calcite was Precipitated by Indigenous Bacteria. Geomicrobiol. J. 2012, 29, 389–395. [Google Scholar] [CrossRef]
- Vahabi, A.; Ramezanianpour, A.A.; Akbari Noghabi, K. A preliminary insight into the revolutionary new line in improving concrete properties using an indigenous bacterial strain Bacillus licheniformis AK01, as a healing agent. Eur. J. Environ. Civ. Eng. 2014, 19, 614–627. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, W. Current challenges and future directions for bacterial self-healing concrete. Appl. Microbiol. Biotechnol. 2019, 102, 3059–3070. [Google Scholar] [CrossRef]
- Vashisht, R.; Attri, S.; Sharma, D.; Shukla, A.; Goel, G. Monitoring biocalcification potential of Lysinibacillus sp. isolated from alluvial soils for improved compressive strength of concrete. Microbiol. Res. 2018, 207, 226–231. [Google Scholar] [CrossRef]
- Arias, D.; Cisternas, L.A.; Miranda, C.; Rivas, M. Bioprospecting of Ureolytic Bacteria From Laguna Salada for Biomineralization Applications. Front. Bioeng. Biotechnol. 2019, 6, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifan, M.; Samani, A.K.; Berenjian, A. Induced calcium carbonate precipitation using Bacillus species. Appl. Microbiol. Biotechnol. 2016, 100, 9895–9906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Teijeiro, R.; Lightfoot, D.A.; Hernandez, J.D. Effect of a Chemical Modified Urea Fertilizer on Soil Quality: Soil Microbial Populations Around Corn Roots. Commun. Soil Sci. Plant Anal. 2009, 40, 2152–2168. [Google Scholar] [CrossRef]
- Al-Thawadi, S.; Cord-Ruwisch, R. Calcium Carbonate Crystals Formation by Ureolytic Bacteria Isolated from Australian Soil and Sludge. J. Adv. Sci. Eng. Res. 2012, 2, 12–26. [Google Scholar]
- Hu, Z.-X.; Hu, X.-M.; Cheng, W.-M.; Zhao, Y.-Y.; Wu, M.-Y. Performance optimization of one-component polyurethane healing agent for self-healing concrete. Constr. Build. Mat. 2018, 179, 151–159. [Google Scholar] [CrossRef]
- Bibi, S.; Oualha, M.; Ashfaq, M.Y.; Suleiman, M.T.; Zouari, N. Isolation, differentiation and biodiversity of ureolytic bacteria of Qatari soil and their potential in microbially induced calcite precipitation (MICP) for soil stabilization. RSC Adv. 2018, 8, 5854–5863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergdale, T.E.; Pinkelman, R.J.; Hughes, S.R.; Zambelli, B.; Ciurli, S.; Bang, S.S. Engineered biosealant strains producing inorganic and organic biopolymers. J. Biotechnol. 2012, 161, 181–189. [Google Scholar] [CrossRef]
- Omoregie, A.; Gaza, K.; Ong, D.; Nissom, P. Microbial-induced carbonate precipitation using a sustainable treatment technique. Int. J. Serv. Man. Sustain. 2017, 2, 17–31. [Google Scholar]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; González-Munoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Rusznyák, A.; Akob, D.M.; Nietzsche, S.; Eusterhues, K.; Totsche, K.U.; Neu, T.R.; Küsel, K. Calcite Biomineralization by Bacterial Isolates from the Recently Discovered Pristine Karstic Herrenberg Cave. Appl. Environ. Microbiol. 2011, 78, 1157–1167. [Google Scholar] [CrossRef] [Green Version]
- Silva-Castro, G.A.; Uad, I.; Rivadeneyra, A.; Vilchez, J.I.; Martin-Ramos, D.; González-López, J.; Rivadeneyra, M.A. Carbonate Precipitation of Bacterial Strains Isolated from Sediments and Seawater: Formation Mechanisms. Geomicrobiol. J. 2013, 30, 840–850. [Google Scholar] [CrossRef]
- Kang, C.H.; Kwon, Y.J.; So, J.S. Soil Bioconsolidation Through Microbially Induced Calcite Precipitation by Lysinibacillus sphaericus WJ-8. Geomicrobiol. J. 2015, 33, 473–478. [Google Scholar] [CrossRef]
- López-Moreno, A.; Sepúlveda-Sánchez, J.D.; Mercedes Alonso Guzmán, E.M.; Le Borgne, S. Calcium carbonate precipitation by heterotrophic bacteria isolated from biofilms formed on deteriorated ignimbrite stones: Influence of calcium on EPS production and biofilm formation by these isolates. Biofouling 2014, 30, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Omoregie, A.I.; Ong, D.E.L.; Nissom, P.M. Assessing ureolytic bacteria with calcifying abilities isolated from limestone caves for biocalcification. Lett. Appl. Microbiol. 2018, 68, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeksting, B.J.; Hoffmann, T.D.; Tan, L.; Paine, K.; Gebhard, S. In-depth profiling of calcite precipitation by environmental bacteria reveals fundamental mechanistic differences with relevance to application. Appl. Environ. Microbiol. 2020, 86, e02739-19. [Google Scholar] [CrossRef] [Green Version]
- Hammes, F.; Boon, N.; de Villiers, J.; Verstraete, W.; Siciliano, S.D. Strain-specific ureolytic microbial calcium carbonate precipitation. App. Environ. Microbiol. 2003, 69, 4901–4909. [Google Scholar] [CrossRef] [Green Version]
- Heirman, G.; De Graef, B.; De Windt, W.; Herremans, T.; Vangheel, T.; Van Gemert, D.; De Belie, N.; Verstraete, W. Biological repair of damaged concrete and mortar surfaces: Biomineralisation. In Proceedings of the 6th International Conference on Materials Science and Restoration (MSR VI), Karlsruhe, Germany, 16–18 September 2003. [Google Scholar]
- Dick, J.; De Wind, W.; De Graef, B.; Saveyn, H.; Van der Meeren, P.; De Belie, N.; Verstraete, W. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species. Biodegradation 2006, 17, 357–367. [Google Scholar] [CrossRef]
- Baskar, R.; Baskar, S.; Mauclaire, L.; McKenzie, J.A. Microbially induced calcite precipitation in culture experiments: Possible origin for stalactites in Sahastradhara caves, Dehradun, India. Curr. Sci. 2006, 90, 58–64. [Google Scholar]
- Ettenauer, J.; Piñar, G.; Sterflinger, K.; Gonzalez-Muñoz, M.T.; Jroundi, F. Molecular monitoring of the microbial dynamics occurring on historical limestone buildings during and after the in-situ application of different bio-consolidation treatments. Sci. Total Environ. 2011, 409, 5337–5352. [Google Scholar] [CrossRef] [Green Version]
- Heidari Nonakaran, S.; Pazhouhandeh, M.; Keyvani, A.; Abdollahipour, F.Z.; Shirzad, A. Isolation and identification of Pseudomonas azotoformans for induced calcite precipitation. World J. Microbiol. Biotechnol. 2015, 31, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Helmi, F.M.; Elmitwalli, H.R.; Elnagdy, S.M.; El-Hagrassy, A.F. Calcium carbonate precipitation induced by ureolytic bacteria Bacillus licheniformis. Ecol. Eng. 2016, 90, 367–371. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Youn, H. Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Appl. Sci. 2016, 8, 1277. [Google Scholar] [CrossRef] [Green Version]
- Jonkers, H.; Schlangen, E. Development of a bacteria-based self-healing concrete. In Tailor Made Concrete Structures; Walraven, S., Ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Chen, H.J.; Huang, Y.H.; Chen, C.C.; Maity, J.P.; Chen, C.Y. Microbial Induced Calcium Carbonate Precipitation (MICP) Using Pig Urine as an Alternative to Industrial Urea. Waste Biomass Valorization 2018, 10, 2887–2895. [Google Scholar] [CrossRef]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzym. Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Sarda, D.; Choonia, H.S.; Sarode, D.D.; Lele, S.S. Biocalcification by Bacillus pasteurii urease: A novel application. J. Ind. Microbiol. Biotechnol. 2009, 36, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, C.M.; Han, S.H.; Kim, S.G.; Park, J.Y.; Kang, C.H.; Jeong, J.H.; So, J.S. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Wang, J.; Jonkers, H.; de Belie, N. Bacillus sphaericus LMG 22257 is physiologically suitable for self-healing concrete. Appl. Microbiol. Biotechnol. 2017, 101, 5101–5114. [Google Scholar] [CrossRef] [PubMed]
- Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Elert, K.; Martín-Sánchez, I.; González-Muñoz, M.T.; Rodriguez-Navarro, C. Protection and consolidation of stone heritage by self-inoculation with indigenous carbonatogenic bacterial communities. Nat. Commun. 2017, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Qian, C. Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase. Process Biochem. 2019, 91, 271–281. [Google Scholar] [CrossRef]
- Martin, D.; Dodds, K.; Ngwenya, B.T.; Butler, I.B.; Elphick, S.C. Inhibition of Sporosarcina pasteurii under anoxic conditions: Implications for subsurface carbonate precipitation and remediation via ureolysis. Environ. Sci. Technol. 2012, 46, 8351–8355. [Google Scholar] [CrossRef]
- Heinrich, K.; Leslie, D.J.; Jonas, K. Modulation of Bacterial Proliferation as a Survival Strategy. Adv. Appl. Microbiol. 2015, 92, 127–171. [Google Scholar] [PubMed]
- Logan, N.A.; Vos, P. Endospore-forming Soil Bacteria. In Soil Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Leggett, M.J.; McDonnell, G.; Denyer, S.P.; Setlow, P.; Maillard, J.Y. Bacterial spore structures and their protective role in biocide resistance. J. App. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Mandic-Mulec, I.; Prosser, J.I. Diversity of Endospore-forming Bacteria in Soil: Characterization and Driving Mechanisms. In Endospore-Forming Soil Bacteria; Springer: Berlin/Heidelberg, Germany, 2011; pp. 31–59. [Google Scholar]
- Jorquera, G.; Thiel, M.; Portflitt-Toro, M.; Dewitte, B. Marine protected areas invaded by floating anthropogenic litter: An example from the South Pacific. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 245–259. [Google Scholar] [CrossRef] [Green Version]
- Espitia Nery, M.E.; Corredor Pulido, D.E.; Castaño Oliveros, P.A.; Rodriguez Medina, J.A.; Ordoñez Bello, Q.Y.; Perez Fuentes, M.S. Mechanisms of encapsulation of bacteria in self-healing concrete: Review. Dyna 2019, 86, 17–22. [Google Scholar] [CrossRef] [Green Version]
- De Koster, S.A.L.; Morsb, R.M.; Nugteren, H.W.; Jonkers, H.M.; Meesters, G.M.H.; Van Ommen, J.R. Geopolymer coating of bacteria-containing granules for use in self-healing concrete. Procedia Eng. 2015, 102, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; De Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. 2011, 39, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, H. Bacteria-based self-healing concrete. HERON 2011, 56, 1–12. [Google Scholar]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Hosseini-Balam, N.; Mostofinejad, D.; Eftekhar, M. Effects of bacterial remediation on compressive strength, water absorption, and chloride permeability of lightweight aggregate concrete. Constr. Build. Mater. 2017, 145, 107–116. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Feng, T.; Zhou, M.; Zhao, L.; Zhou, A.; Li, Z. Immobilizing bacteria in expanded perlite for the crack self-healing in concrete. Constr. Build. Mater. 2017, 148, 610–617. [Google Scholar] [CrossRef]
- Chen, H.; Qian, C.; Huang, H. Self-healing cementitious materials based on bacteria and nutrients immobilized respectively. Constr. Build. Mater. 2016, 126, 297–303. [Google Scholar] [CrossRef]
- Wang, J.; Van Tittelboom, W.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Wang, J.; Snoeck, D.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Vijay, K.; Murmu, M.; Deo, S.V. Bacteria based self-healing concrete—A review. Constr. Build. Mat. 2017, 152, 1008–1014. [Google Scholar] [CrossRef]
- Luo, M.; Qian, C.; Li, R. Factors affecting crack repairing capacity of bacteria-based self-healing concrete. Constr. Build. Mater. 2015, 87, 1–7. [Google Scholar] [CrossRef]
- Zheng, T.; Su, Y.; Zhang, X.; Zhou, H.; Qian, C. Effect and Mechanism of Encapsulation-Based Spores on Self-Healing Concrete at Different Curing Ages. ACS Appl. Mater. Interfaces 2020, 12, 52415–52432. [Google Scholar] [CrossRef]
- Meera, L.; Chenyan, E. Durability and Self-Healing Behaviour of Bacterial Impregnated Concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 14887. [Google Scholar]
- Mirshahmohammad, M.; Rahmani, H.; Maleki-Kakelar, M.; Bahari, A. A Comparative Study on Self-Healing Methods of Concretes by Sporosarcina pasteurii Bacteria. Res. Sq. 2021, 1–24. [Google Scholar] [CrossRef]
- Hussein, Z.M.; Abedali, A.H.; Ahmead, A.S. Improvement Properties of Self—Healing Concrete by Using Bacteria. IOP Conf. Ser. Mater. Sci. Eng. 2019, 584, 012034. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, C.; Zhou, A.; Yang, C.; Zhao, L.; Li, Z. Aragonite formation induced by open cultures of microbial consortia to heal cracks in concrete: Insights into healing mechanisms and crystal polymorphs. Constr. Build. Mater. 2019, 224, 815–822. [Google Scholar] [CrossRef]
- Hassan, M.; Milla, J.; Rupnow, T.; Soysal, A. Self-Healing Concrete using Encapsulated Bacterial Spores in a Simulated Hot Subtropical Climate. Digit. Comment 2019, 34, 1–44. [Google Scholar]
- Šovljanski, O.; Pezo, L.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Contribution of bacterial cells as nucleation centers in microbiologically induced CaCO3 precipitation—A mathematical modeling approach. J. Basic Microbiol. 2021, 61, 835–848. [Google Scholar] [CrossRef]
- Kłodzińska, E.; Szumski, M.; Dziubakiewicz, E.; Hrynkiewicz, K.; Skwarek, E.; Janusz, W.; Buszewski, B. Effect of zeta potential value on bacterial behavior during electrophoretic separation. Electrophoresis 2010, 31, 1590–1596. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Factors Affecting Improvement in Engineering Properties of Residual Soil through Microbial-Induced Calcite Precipitation. J. Geotech. Geoenvironmental Eng. 2014, 140, 04014006. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors Affecting Efficiency of Microbially Induced Calcite Precipitation. J. Geotech. Geoenvironmental Eng. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Behnood, A.; Van Tittelboom, K.; De Belie, N. Methods for measuring pH in concrete: A review. Constr. Build. Mater. 2016, 105, 176–188. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Sathyanarayanan, K.S.; Darshan, B.S.; Balaji Raja, R.; India, T. Studies on the characterisation of Biosealant properties of Bacillus sphaericus. Int. J. Eng. Sci. Technol. 2010, 2, 270–277. [Google Scholar]
- Gat, D.; Tsesarsky, M.; Shamir, D.; Ronen, Z. Accelerated microbial-induced CaCO3 precipitation in a defined coculture of ureolytic and non-ureolytic bacteria. Biogeosciences 2014, 11, 2561–2569. [Google Scholar] [CrossRef] [Green Version]
- Rampelotto, P.H. Resistance of Microorganisms to Extreme Environmental Conditions and Its Contribution to Astrobiology. Sustainability 2010, 2, 1602–1623. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Che, Y.; Leng, F. Calcium leaching behavior of cementitious materials in hydrochloric acid solution. Sci. Rep. 2018, 8, 8806. [Google Scholar] [CrossRef]
- Chatterjee, S.; Fördös, Z.; Thaulow, N. Alkali-Silica Reaction—Danish Experience; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Sinnelä, M.; Park, Y.; Lee, J.; Jeong, K.; Kim, Y.W.; Hwang, H.J.; Mah, J.-H. Effects of Calcium and Manganese on Sporulation of Bacillus Species Involved in Food Poisoning and Spoilage. Foods 2019, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Ksara, M.; Newkirk, R.; Langroodi, S.K.; Althoey, F.; Sales, C.M.; Schauer, C.L.; Farnam, Y. Microbial damage mitigation strategy in cementitious materials exposed to calcium chloride. Constr. Build. Mater. 2019, 195, 1–9. [Google Scholar] [CrossRef]
- Riad, I.M.; Elshami, A.A.; Elshikh, M.M.Y. Influence of concentration and proportion prepared bacteria on properties of self-healing concrete in sulfate environment. Innov. Infrastruct. Solut. 2022, 7, 71. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Van Tittelboom, K.; Palin, D.; Wang, J.; Sierra-Beltrán, M.G.; Erşan, Y.Ç.; Mors, R.; Wiktor, V.; Jonkers, H.M.; Schlangen, E.; et al. Bio-Based Self-Healing Concrete: From Research to Field Application. Adv. Polym. Sci. 2016, 273, 345–385. [Google Scholar]
- Dunuweera, S.P.; Rajapakse, R.M.G. Cement Types, Composition, Uses and Advantages of Nanocement, Environmental Impact on Cement Production, and Possible Solutions. Adv. Mater. Sci. Eng. 2018, 2018, 4158682. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.T.; King, A.D.; Goodman, N. Isolation and characterization of urease from Aspergillus niger. J. Gen. Microb. 1993, 139, 957–962. [Google Scholar] [CrossRef]
- Markov, S.; Vidaković, A. Testing methods for antimicrobial activity of TiO2 photocatalyst. Acta Period. Technol. 2014, 45, 141–152. [Google Scholar] [CrossRef]
- Bachmeier, K.L.; Williams, A.E.; Warmington, J.R.; Bang, S.S. Urease activity in microbiologically-induced calcite precipitation. J. Biotechnol. 2002, 93, 171–181. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, K.E.; Cha, I.T.; Park, W. Optimization of bacterial sporulation using economic nutrient for self-healing concrete. J. Microb. 2020, 58, 288–296. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2009, 36, 197–210. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Stanaszek-Tomal, E. Bacterial Concrete as a Sustainable Building Material? Sustainability 2020, 12, 696. [Google Scholar] [CrossRef] [Green Version]
- Achal, V.; Mukherjee, A.; Reddy, M.S. Biocalcification by Sporosarcina pasteurii using corn steep liquor as the nutrient source. Ind. Biotechnol. 2010, 6, 170–174. [Google Scholar] [CrossRef]
- Hong, M.; Jang, I.; Son, Y.; Yi, C.; Park, W. Agricultural by-products and oyster shell as alternative nutrient sources for microbial sealing of early age cracks in mortar. AMB Express 2021, 11, 11. [Google Scholar] [CrossRef]
- Vermeer, C.M.; Rossi, E.; Tamis, J.; Jonkers, H.M.; Kleerebezem, R. From waste to self-healing concrete: A proof-of-concept of a new application for polyhydroxyalkanoate. Resour. Conserv. Recycl. 2021, 164, 105206. [Google Scholar] [CrossRef]
- Khan, M.B.E.; Shen, L.; Dias-da-Costa, D. Self-healing behaviour of bio-concrete in submerged and tidal marine environments. Constr. Build. Mater. 2021, 277, 122332. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Stanojev, J.; Bajac, B.; Kovač, S.; Tóth, E.; Ristić, I.; Tomić, A.; Ranitović, A.; Cvetković, D.; et al. Comprehensive profiling of microbiologically induced CaCO3 precipitation by ureolytic Bacillus isolates from alkaline soils. Microorganisms 2021, 9, 1691. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Grahovac, J.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Best-performing Bacillus strains for microbiologically induced CaCO3 precipitation: Screening of relative influence of operational and environmental factors. J. Biotechnol. 2022, 350, 31–41. [Google Scholar] [CrossRef]
- Tan, L.; Reeksting, B.; Ferrandiz-Mas, V.; Heath, A.; Gebhard, S.; Paine, K. Effect of carbonation on bacteria-based self-healing of cementitious composites. Constr. Build. Mater. 2020, 257, 119501. [Google Scholar] [CrossRef]
- Vučetic, S.; Miljevic, B.; Šovljanski, O.; van der Bergh, J.M.; Markov, S.; Hirsenberger, H.; Tzoutzouli Malesevic, M.; Ranogajec, J. Functional mortars for conservation of cultural heritage structures. Mater. Sci. Eng. 2020, 949, 012091. [Google Scholar] [CrossRef]
- Koehler, E.P. Aggregates in Self-Consolidating Concrete. Ph.D. Thesis, University of Texas, Austin, TX, USA, 2007. Available online: http://hdl.handle.net/2152/3298 (accessed on 14 May 2022).
- Dommergues, Y.R.; Belser, L.W.; Schmidt, E.L. Limiting Factors for Microbial Growth and Activity in Soil. In Advances in Microbial Ecology; Alexander, M., Ed.; Springer: Dordrecht, The Netherlands, 1978; p. 2. [Google Scholar]
- Dong, H.; Gao, P.; Ye, G. Characterization and comparison of capillary pore structures of digital cement pastes. Mater. Struct. 2017, 50, 154. [Google Scholar] [CrossRef] [Green Version]
- Boyd, A.J.; Hooton, R.D. Long-term scaling performance of concretes containing supplementary cementing materials. J. Mater. Civ. Eng. 2007, 19, 820–825. [Google Scholar] [CrossRef]
- Xiong, Q.; Baychev, T.G.; Jivkov, A.P. Review of pore network modelling of porous media: Experimental characterisations, network constructions and applications to reactive transport. J. Contam. Hydrol. 2016, 192, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chidiac, S. Self-healing concrete: A critical review. In Proceedings of the 2019 CSCE Annual Conference, Laval, QC, Canada, 12–15 June 2019; p. MAT152-1-MAT152-10. [Google Scholar]
- Roig-Flores, M.; Serna, P. Concrete Early-Age Crack Closing by Autogenous Healing. Sustainability 2020, 12, 4476. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; Gruyaert, E.; Rahier, H.; De Belie, N. Influence of mix composition on the extent of autogenous crack healing by continued hydration or calcium carbonate formation. Constr. Build. Mater. 2012, 37, 349–359. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Koh, H.J. Application of biochar from food and wood waste as green admixture for cement mortar. Sci. Total Environ. 2018, 619–620, 419–435. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, G.; Wang, Y.; Li, Z. Optimization of sporulation and germination conditions of functional bacteria for concrete crack-healing and evaluation of their repair capacity. ACS Appl. Mater. Interfaces 2020, 12, 10938–10948. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Reddy, M.S. Microbial Concrete: Way to Enhance the Durability of Building Structures. J. Mater. Civ. Eng. 2011, 23, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Ghorbel, E.; Fares, H.; Cousture, A. Bacterial self-healing of concrete and durability assessment. Cem. Concr. Compos. 2019, 104, 103340. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Huang, Y.; Zhang, J.; Fang, C.; Yu, K.; Chen, Q.; Li, T.; Han, R.; Yang, Z.; et al. Laboratory and field study on the performance of microcapsule-based self-healing concrete in tunnel engineering. Constr. Built. Mater. 2019, 220, 90–101. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Van der Bergh, J.M.; Miljević, B.; Vučetić, S.; Šovljanski, O.; Markov, S.; Riley, M.; Ranogajec, J.; Bras, A. Comparison of Microbially Induced Healing Solutions for Crack Repairs of Cement-Based Infrastructure. Sustainability 2021, 13, 4287. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Koren, K.; Löbmann, K.; Hinge, M.; Scoma, A.; Kjeldsen, K.U.; Røy, H. Constraints on CaCO3 precipitation in superabsorbent polymer by aerobic bacteria. Appl. Microbiol. Biotechnol. 2019, 104, 365–375. [Google Scholar] [CrossRef]
- Tan, L.; Ke, X.; Li, Q.; Gebhard, S.; Ferrandiz-Mas, V.; Paine, K.; Chen, W. The effects of biomineralization on the localised phase and microstructure evolutions of bacteria-based self-healing cementitious composites. Cem. Concr. Compos. 2022, 128, 104421. [Google Scholar] [CrossRef]
- Wang, A.; Zhan, Q.; Fu, C.; Wang, Y.; Zhou, J. Study on improving the self-repairing effect of cement-based materials by microbial mineralization coupled with inorganic minerals. Case Stud. Constr. Mater. 2022, 17, e01279. [Google Scholar] [CrossRef]
- Safiuddin, M.; Kaish, A.; Woon, C.-O.; Raman, S. Early-Age Cracking in Concrete: Causes, Consequences, Remedial Measures, and Recommendations. Appl. Sci. 2018, 8, 1730. [Google Scholar] [CrossRef] [Green Version]
- Schlangen, E.; ter Heide, N.; van Breugel, K. Crack healing of early age cracks in concrete. In Measuring, Monitoring and Modeling Concrete Properties; Springer: Dordrecht, The Netherlands, 2006; pp. 273–284. [Google Scholar]
- Jaroenratanapirom, D.; Sahamitmongkol, R. Self-crack closing ability of mortar with different additives. J. Met. Mater. Miner. 2011, 21, 9–17. [Google Scholar]
- Gagné, R.; Argouges, M. A study of the natural self-healing of mortars using air-flow measurements. Mater. Struct. Constr. 2012, 45, 1625–1638. [Google Scholar] [CrossRef]
- Justo-Reinoso, I.; Reeksting, B.J.; Heath, A.; Gebhard, S.; Paine, K. Evaluation of Cyclic Healing Potential of Bacteria-Based Self-Healing Cementitious Composites. Sustainability 2022, 14, 6845. [Google Scholar] [CrossRef]





| Isolation Site | ||
|---|---|---|
| Sediments | ||
| Bacteria | Ref. | |
| Sporosarcina pasteurii S. luteola Bacillus lentus | [40] | |
| Brevundimonas dimitiuda | [41] | |
| Arthrobacter sp. Flavobacterium sp. Pseudomonas sp. | [42] | |
| B. pumilis P. grimonti Halomonas sp. | [43] | |
| Lysinibacillus sphaericus | [44] | |
| Enterobacter calcerogenus B. subtilis B. cereus | [45] | |
| B. lentus B. fortis Sporosarcina sp. Pseudogracibacillus sp. | [46] | |
| B. licheniformis B. muralis | [47] | |
| Cement-Based Materials | ||
| Bacteria | Ref. | |
| B. sphaericus | [48] | |
| Bacillus sp. Paenibacillus sp. Arthrobacter sp. | [49] | |
| B. lentus B. sphaericus | [50] | |
| B. thuringiensis B. pumilis | [51] | |
| Bacillus sp. Brevibacillus sp. | [52] | |
| P. azotoformanis | [53] | |
| B. licheniformis | [54] | |
| Bacillus sp., Sporosarcina sp. | [55] | |
| Reference Strains | ||
| Bacteria | Collection of Microorganisms | Ref. |
| Sporosarcina pasteurii DSM 33 B. cohnii DSM 6307 B. pseudofirmus DSM 8715 | DSM 1 | [56,57] |
| S. pasteurii ATCC 11859 | ATCC 2 | [58] |
| S. pasteurii NCIM 2477 | NCIM 3 | [59] |
| S. pasteurii KCTC 3558 | KTTC 4 | [60] |
| B. sphaericus LMG 22257 | LMG 5 | [61] |
| B. lentus NCIB 8773 | NCIB 6 | [62] |
| Myxococcus xanthus CECT 422T | CECT 7 | [41] |
| B. mucilaginous L3 | CICC 8 | [63] |
| References | [81] | [56] | [82] | [83] | [84] | [85] | [20] | [86] | [87] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Materials | Ordinary Portland Cement (OPC) | ||||||||||
| Bacteria | Spore-forming alkali-resistant bacterium | B. cohnii DSM 6307 B. halodurans DSM 497 B. pseudofirmus DSM 8715 | B. mucilaginous L3 | B. subtilis jc3 | S. pasteurii DSM 33 | B. subtilis | P. aeruginosa Diaphorobacter nitroreducens | Anaerobic consortium (Pseudomonas, Azotobacter) | B. pseudofirmus D. nitroreducens | ||
| Metabolic Activity | Ammonification | Ureolysis | Denitrification | ||||||||
| Vegetative cells (V) or spores (S) | V | S | S | nd | V | V | V | V | V | ||
| Initial bacterial concentration (CFU) a | 109 | 107 | 1010 | 105 | 107 | 103–109 | 107 | 108 | 107 | ||
| Monitoring of bacterial activity | Nd b | Assessment of spore activation | nd | nd | nd | nd | Checking viability c | nd | nd | ||
| Monitoring | self-healing effect | Scanning electronic microscopy | + | − | + | − | + | + | − | + | + |
| X-ray diffraction analysis | + | − | + | − | + | − | − | + | − | ||
| Optical microscopy | − | − | + | + | − | − | + | + | + | ||
| Chloride permeability | − | − | + | − | − | − | − | − | − | ||
| Fourier−transform IR spectroscopy | − | − | − | − | + | − | − | − | − | ||
| Surface resistivity | − | − | − | − | − | − | − | − | + | ||
| Healing ratio d | + | − | − | − | − | − | − | − | − | ||
| CaCO3 precipitation potential | − | + | − | − | − | − | − | − | − | ||
| concrete performance | Compressive strength | − | + | + | − | + | + | − | − | + | |
| Tensile strength | − | + | − | − | + | − | − | − | − | ||
| Water permeability | + | − | + | + | + | − | − | + | − | ||
| Water absorption | − | − | − | − | − | + | + | − | − | ||
| Durability assessment | − | − | − | + | − | − | − | − | − | ||
| Concrete density | − | − | + | − | − | − | − | − | − | ||
| Ultrasonic pulse velocity | − | − | − | − | − | + | − | − | − | ||
| Concrete slump test | − | − | + | − | − | − | − | − | − | ||
| Setting time test | − | − | + | − | − | − | − | − | − | ||
| Static modulus of elasticity | − | − | − | − | − | − | − | − | + | ||
| Bacteria | Inoculation Level (CFU) | After the SH Effect a | Healed Crack Width (μm) | Ref. | |
|---|---|---|---|---|---|
| Permeability | Porosity | ||||
| B. mucilaginous L3 | 1010 | ↓ * | nd ** | 300–500 | [82] |
| B. sphaericus LMG 22257 | ↓ | ↓ | 200–900 | [126] | |
| Spore-forming alkali-resistant bacterium | 109 | ↓ | ↓ | 100–800 | [81] |
| B. cohnii DSM 6307 | ↓ | nd | 1240 | [127] | |
| B. sphaericus LMG 22257 | ↓ | ↓ | 970 | [5] | |
| Anaerobic consortium | 108 | ↓ | nd | 100–1200 | [86] |
| Bacillus sp. CT5 | ↓ | ↓ | 3000 | [128] | |
| B. subtilis 5265T | ↓ | ↑ *** | 400 | [129] | |
| S. pasteurii DSM 33 | 107 | ↓ | nd | 200 | [84] |
| B. cereus CS1 | ↓ | nd | 800 | [130] | |
| B. subtilis jc3 | 105 | ↓ | ↓ | 200 | [83] |
| B. sphaericus LMG 22257 | nd | ↓ | nd | 250–400 | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šovljanski, O.; Tomić, A.; Markov, S. Relationship between Bacterial Contribution and Self-Healing Effect of Cement-Based Materials. Microorganisms 2022, 10, 1399. https://doi.org/10.3390/microorganisms10071399
Šovljanski O, Tomić A, Markov S. Relationship between Bacterial Contribution and Self-Healing Effect of Cement-Based Materials. Microorganisms. 2022; 10(7):1399. https://doi.org/10.3390/microorganisms10071399
Chicago/Turabian StyleŠovljanski, Olja, Ana Tomić, and Siniša Markov. 2022. "Relationship between Bacterial Contribution and Self-Healing Effect of Cement-Based Materials" Microorganisms 10, no. 7: 1399. https://doi.org/10.3390/microorganisms10071399
APA StyleŠovljanski, O., Tomić, A., & Markov, S. (2022). Relationship between Bacterial Contribution and Self-Healing Effect of Cement-Based Materials. Microorganisms, 10(7), 1399. https://doi.org/10.3390/microorganisms10071399







