Abstract
Leishmaniasis is an important vector-borne disease that represents a serious public health problem, including in Sicily (Italy), which is considered an endemic area. We collected canine, feline and human data from 2013 to 2021 in Sicily, while entomological surveys were conducted only in 2013 and 2021. Overall, 23,794/74,349 (34.4%) of dogs and 274/4774 (11.8%) of cats were positive in one or more diagnostic tests. A total of 467 cases of human Leishmaniasis were reported, with 71% showing cutaneous and 29% visceral involvement. The provinces with the largest number of patients were Agrigento (45.4%) and Palermo (37%). In 2013, Phlebotomus perfiliewi was the dominant sandfly species in Sicily (68.7%), followed by Phlebotomus perniciosus (17.2%) and Sergentomya minuta (14%). In 2021, Phlebotomus perfiliewi was confirmed as the most common species (61.6%), followed by Phlebotomus perniciosus (33.1%) and Sergentomya minuta (4.7%). Of particular interest was the identification of Phlebotomus papatasi (0.41%) in Agrigento. Our retrospective study can inform health authorities for the development of appropriate screening, treatment and control strategies to reduce Leishmania incidence rate. This study examined the present state of Leishmaniasis control, surveillance, and prevention in Sicily, but also highlighted deficiencies that could be addressed through the application of One-Health principles.
1. Introduction
Leishmaniasis is a vector-borne disease transmitted by obligate intracellular protozoa of the genus Leishmania [1,2,3,4,5]. Leishmania parasites cause a broad range of clinical manifestations, such as cutaneous (CL), mucocutaneous (MCL), visceral (VL), post-kala-azar dermal (PKDL), and diffuse cutaneous Leishmaniasis (DCL), depending on the Leishmania species and the immune response of the patient [4]. Over one billion people are at risk for infection and live in areas endemic for Leishmaniasis, estimating 30,000 new VL cases of and more than 1 million new CL cases each year [5,6,7]. Over 90% of annual VL incidences occur in six countries: Bangladesh, Sudan, India, Nepal, Ethiopia, Brazil and South Sudan [3,6,8,9,10,11]. East Africa is the second largest center of VL after the Indian continent and annually adds 30,000–40,000 new cases [3,6]. Leishmaniasis is a disease characterized by high epidemiological dynamism related to constantly changing transmission conditions depending on the environment, demography, human behavior, socio-economic status and the human immune system [12,13,14]. Leishmania infantum is one of the most important Leishmania spp. worldwide from both a veterinary and a public health point of view. Domestic dogs (Canis familiaris) are believed to be the principal hosts of this protozoan and the main reservoirs of zoonotic human VL, which if left untreated is potentially lethal [15,16]. Today, sero-molecular diagnostic techniques have shown that the prevalence rates of Mediterranean canine leishmaniasis are much higher than previously assumed, probably due to the high percentage of asymptomatic infections (over 50%). [17]. However, other mammals infected with L. infantum have been reported in Italy [18,19]. The wide dispersal of VL indicates new reservoirs of the disease and the likely involvement of domestic animals, such as cats in the biological cycle of Leishmania. In Italy, L. infantum transmission is related to both the abundance and geographic distribution of competent vectors, mainly represented by Phlebotomus perniciosus [20,21,22]. Phlebotomine sandflies’ activity and their geographic distribution is influenced by different environmental parameters, such as land cover, altitude, air temperature, relative humidity and vegetation [23,24]. For example, air temperature is believed to condition the distribution of vector and seasonal sandfly activity [25]. Consistently, cases of canine and human Leishmaniasis have been historically described in central and southern regions of Italy, which are typically characterized by temperate winter and hot summers and [26,27], although in recent decades, numerous cases of animal and human Leishmaniasis have been reported in Northern Italian regions, traditionally classified as nonendemic areas for Leishmaniasis [27]. Although disease notification is required, available data are based on passive case detection. In some areas, the number of people and animals exposed to infection or asymptomatically infected is greater than the number of cases detected. Therefore, a One-Health strategy incorporating entomological, environmental animal and human data has been proposed as a valid alternative to study and control the Leishmaniasis transmission [28,29]. With this study, we evaluated, through a One-Health approach, the risk of transmission of L. infantum in the region of Sicily, an island in southern Italy known to be an endemic region for Leishmania, causing human, canine and feline Leishmaniasis [30,31,32,33]. Human and veterinary medicine, environmental sciences, and wildlife conservation specialists share a common interest in limiting visceral Leishmaniasis. However, the aforementioned disciplines respond to Leishmaniasis in separate ways. This retrospective study reports the results of 9 years (from 2013 to 2021) of surveillance based on integrated clinical and epidemiological sources of human and animal Leishmaniasis in Sicily with the aim to promote inter- and intra-sectoral collaboration to plan future control strategies.
2. Materials and Methods
2.1. Study Area
Sicily is an island in the south of Italy with a surface of 25.711 km2 (Longitude: 14.0153557, Latitude: 37.5999938, mean elevation: 622 m/2041 feet, Barometric Pressure: 94 Kpa). The island is predominantly mountainous, characterized by the presence of Mount Etna (10,900 feet, 3220 metres), with intense volcanic activity. The region of Sicily is divided into nine provinces: Palermo (PA), Trapani (TP) and Agrigento (AG) in the west, Caltanissetta (CL) and Enna (EN) in the center and Ragusa (RG), Siracusa (SR), Catania (CT) and Messina (ME) in the east.
2.2. Clinical Samples
Clinical samples from human patients were collected for diagnostic purposes and were examined at the dermatology and infectious diseases units of Sicilian province hospitals. VL patients presented such symptoms as: fever, hepatomegaly and/or splenomegaly; cutaneous lesions were erythematous papules, itchy, slow-growing papules that over a few weeks/months may progress into nodules and later to ulcers. Patients of VL were given treatment with liposomal amphotericin B (AmBisome®) 3 mg/kg administered intravenously (IV) once at days 1–5, 14, and 21. CL cases were treated with pentavalent antimonial (SbV) therapy, using a standard daily dose of 20 mg of SbV per kg, administered IV or via intramuscular route (IM). Overall, 341 Sera and 341 bone marrows samples were collected from humans with suspected VL; 699 tissue/biopsies were collected from humans with suspected CL, 74,349 lymph node needle aspirations and 74,349 sera were collected from dogs and 4774 lymph node needle aspirations and 4774 sera were collected from cats from veterinary medical centers (Table S1).
2.3. Diagnosis
All samples analyzed were from patients whose Leishmania infection status was screened for different reasons such as confirmation or exclusion of the disease, early diagnosis and investigation after therapy. Diagnosis of the disease was conducted by indirect immunofluorescence testing (IFAT) on serum matrices and detection of parasite DNA in bone marrow, tissue/biopsies and lymph node needle aspiration by real-time PCR (qPCR)). Serological examination included IFAT assays following the protocol according to the Office International des Epizooties (OIE) Terrestrial Manual (sensitivity 0.96, specificity 0.98) [34]. Sera were prepared by serial two-fold dilutions (1:40 to 1:5120 for canine samples; 1:80 to 1:5120 for human and feline samples) in phosphate buffered saline (PBS) at pH 7.2 and added to antigen coated wells. The IFAT slides were incubated for 30 min at 37 °C and positive and negative controls were included in each series of analyzed samples. The slides were subjected to three consecutive washes in PBS (10 min each), incubated with fluorescein labeled goat immunoglobulin (i.e., anti-cat, anti-dog and anti-human IgG, Sigma Aldrich, Saint Louis, MO, USA) and incubated at 37 °C for 30 min. The slides were washed three times (10 min each) in PBS and the sera reactivity was detected using a Leica DM 4000B fluorescence microscope (Leica, Heerbrugg, Switzerland, 40× magnification). Sera showed reactivity were subjected to analysis at dilutions 1:40 and 1:5120. The samples that showed titers of ≥1:160 were considered positive [34]. DNA was extracted from different clinical samples using PureLink™ Genomic DNA Mini Kit (Thermo Fisher Scientific K182002, Waltham, MA, USA) following the manufacturer’s instructions. The real-time PCR was performed out in a LightCycler® 96 (Roche Life Science, Upper Bavaria, Germany) and carried as previously described (sensitivity 0.97, specificity 1) [35].
2.4. Entomological Survey
A phlebotomine sandfly survey was carried out to confirm the presence of Leishmania competent vectors and assess possible variations in their distribution in Sicilian provinces. CDC miniature light traps (Hausherr’s Machine Works, Toms River, NJ, USA) were used for 6 months (from the first week of May to the last week of October) of 2013 and 2021. Collected sandflies were stored in 70% ethanol and identified by light microscope using specific morphological keys [36,37].
2.5. Ethical Statement
Samples were collected as part of routine diagnosis and/or post-treatment follow-up without any necessary or additional noninvasive procedures and written informed consent was obtained from the patient at the time of the clinical examination. Patient records and information was anonymized and de-identified before analysis. All adult subjects provided written informed consent and a parent or guardian of any child participant provided written informed consent on their behalf. All animal samples were collected for diagnostic use and no unnecessary invasive procedures were performed, including parasitological confirmation of FeL or CanL. At the time of clinical examination, oral informed consent was obtained from the owners of dogs and cats.
2.6. Statistical Analysis
Data were analyzed using GraphPad Prism 9 software. Descriptive analysis of the qualitative variables was evaluated considering percentages, the number of cases and annual incidence of Leishmaniasis per 100,000 inhabitants. Chi-square and Fisher exact tests were conducted to compare proportions. The differences were considered statistically significant when P-value was ≤0.05. Posteriorly, maps representing positive cases of CL, VL, CanL and FeL were constructed. The results were graphically depicted on thematic maps stratified according to the following positivity parameters for CanL: low (0.02 to 7.22%), medium (7.23 to 16.29%), high (16.3 to 29.31%), intense (29.32 to 51.88%), and very intense (51.89 to 100%) [36]. The study was conducted by geographic information system (GIS) technology (MapInfo® professional 17.0) software for generating the choroplethic maps. To estimate True Prevalence in dogs, cats and humans, the sensitivity and specificity estimates of each of qPCR and IFAT were imputed in the Rogan and Gladen formula: Tp = (Ap + CSps − 1)/(CSes + CSps − 1), where Tp = true prevalence; Ap = apparent prevalence; CSes = combined sensitivity of the test series (Se qPCR × Se IFAT), and CSps = combined specificity of the test series (1 − (1 − Sp qPCR) × (1 − Sp IFAT)) [38,39].
3. Results
3.1. Canine and Feline Data
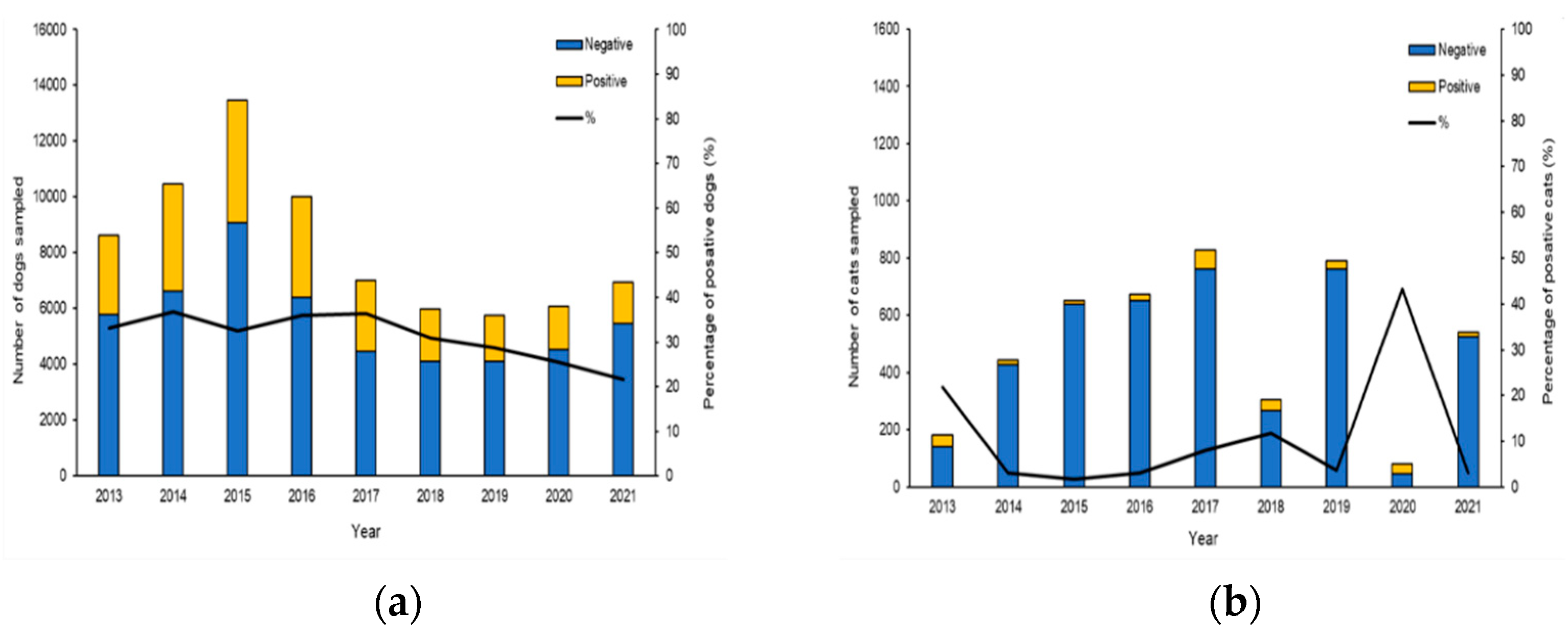
Investigations were initiated following the occurrence of autochthonous clinical cases reported by veterinarians, local medicals and confirmed by serological and molecular methods at The National Reference Center for Leishmaniasis (C.Re.Na.L). Analysis of epidemiological records from 2013 to 2021 with one or more diagnostic tests (Table S1) identified 74,349 tested dogs and 4774 tested cats living in Sicily (Figure 1). The results of our study revealed a true prevalence of 34.36% of CanL (adjusted Rogan-Gladen estimator) showing highest prevalence from 2013 to 2019, while in the years 2020 and 2021, we observed a slight decrease in prevalence with values of 25.4% and 21.6%, respectively (Figure 1a). The true prevalence of FeL obtained in our study period was 11.8% and the minimum number of feline samples was recorded in 2020 with 81, while the maximum number of samples was recorded in 2017 with 828 (Figure 1b).
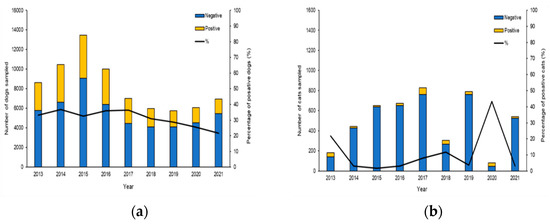
Figure 1.
Annual trend and prevalence of CanL and FeL in Sicily, from 2013 to 2021. (a) Histogram showing the percentage of positive dogs between 2013 and 2021. (b) Histogram showing the percentage of positive cats between 2013 and 2021.
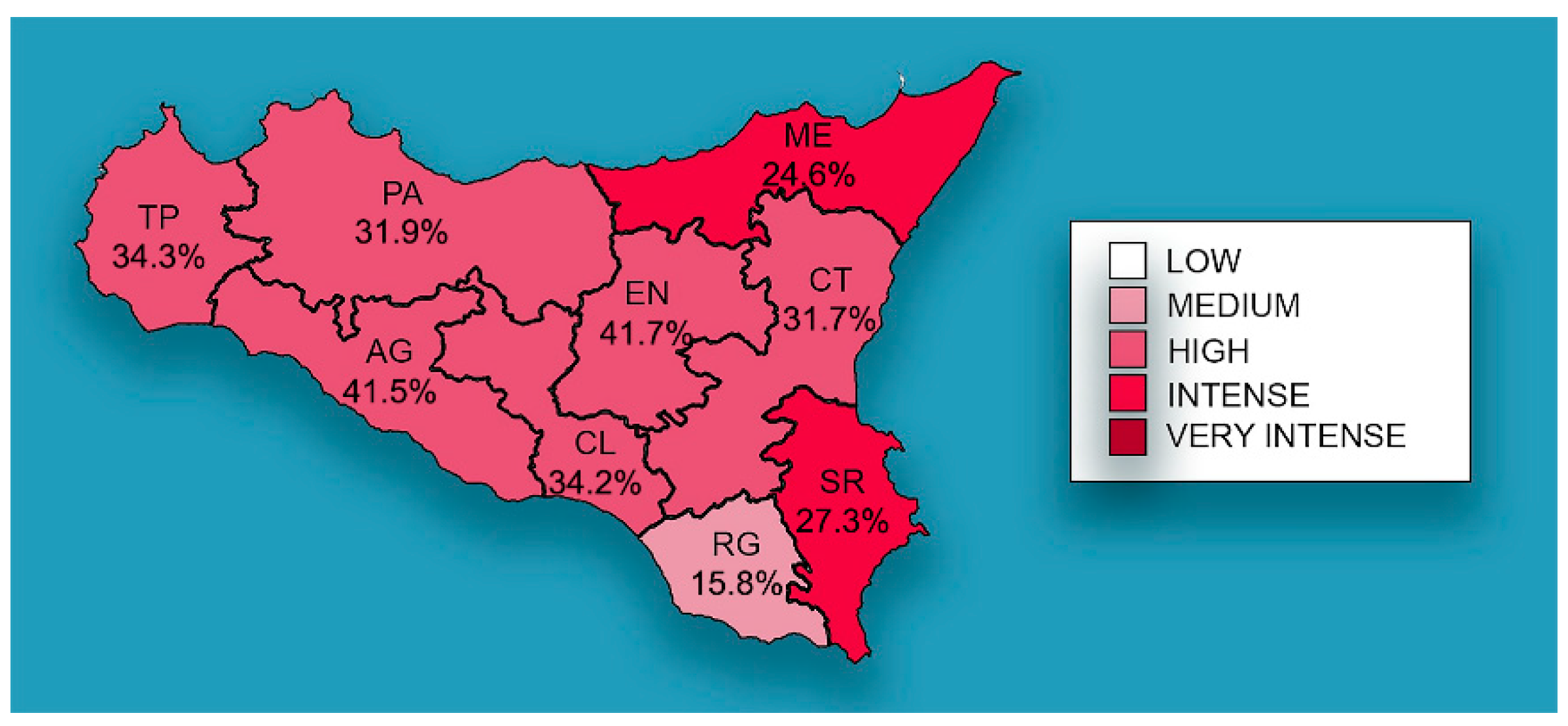
The distribution of mean prevalence of CanL in the Sicilian provinces is shown in Figure 2, with EN (41.7%) and AG (41.5%) recording the highest prevalence, followed by TP (34.3%), CL (34.2%), PA (31.9%), CT (31.7%), SR (27.3%) ME (24.6%), and RG (15.8%).
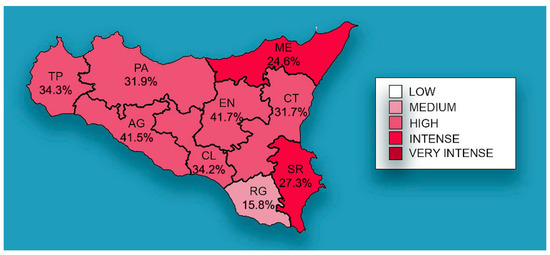
Figure 2.
Mean prevalence of CanL by Sicilian province. The spatial distribution of mean prevalence (%) from 2013 to 2021. The map generated with geographic information system (GIS) technology (MapInfo® professional 17.0) software.
3.2. Human Data
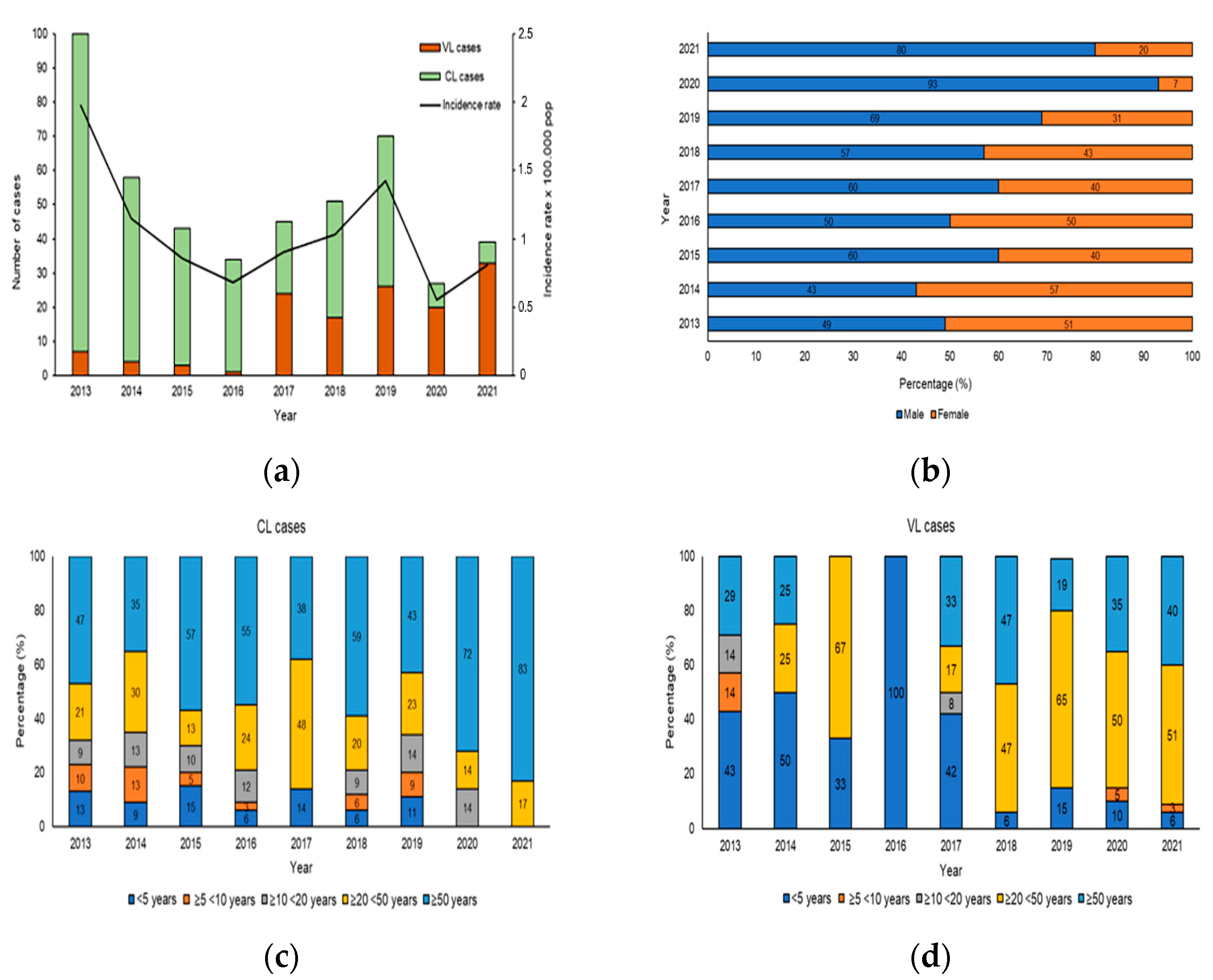
From 2013 to 2021 in Sicily, 467/1040 (true prevalence 48.2%) samples were positive for human Leishmaniasis, including 332 for CL and 135 for VL and the results distribution of molecular and serological analysis were shown in Table S1. The analysis of human Leishmaniasis included temporal distribution, sex and age. Figure 3a shows the evolution of the number of VL/CL cases and incidence rate reported in the study period from 2013 to 2021. The increase in the number of cases of CL and VL reported in 2013 and 2019 was responsible for the peaks observed in the overall Leishmaniasis case count in Figure 3a. During the study period, the cumulative incidence was 1.04 per 100,000 populations, with the highest incidence observed in 2013 (1.9 per 100,000 population) and the lowest in 2020 (0.5 per 100,000 population). During the first 4 years of the study, a higher number of CL cases was observed, whereas in the second period (2017–2021), there was a decrease in CL and an increase in VL cases. The distribution of CL and VL cases according to sex is shown in Figure 3b. The affected population consisted of 275 males (61.7%) and 192 females (38.3%), indicating an important and statistically significant sex bias (χ2 = 35.48, df = 8, p < 0.001). To reveal the association of CL and VL with age during the 9 years of the study, the data were stratified according to 5 age groups: <5 years, from 5 to 9 years, from 10 to 19 years, from 20 to 49 years and ≥50 years of age. While all age groups were affected by CL (Figure 3c), we observed significant, age-specific differences (χ2 = 169, df = 32, p < 0.001), with the highest CL prevalence seen in adults ≥20 < 50 (23.3%) and ≥50 years of age (54.3%). Likewise, statistically significant, age-specific differences were revealed for VL (χ2= 607, df = 32, p < 0.001), which mainly affected the age group ≥20 < 50 years between 2018–2021 (54.2%), while between 2013–2017, 66.6% patients were aged ≤5 years (Figure 3d).
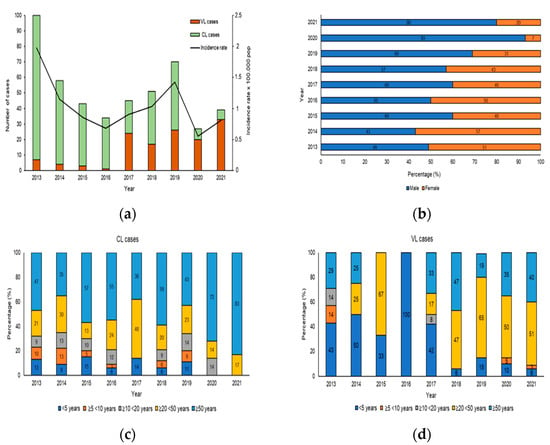
Figure 3.
Human Leishmaniasis in Sicily. (a) Number of patients and incidence rates of cutaneous and visceral Leishmaniasis in Sicily, from 2013 to 2021. The histogram shows the incidence rates of CL and VL cases (per 100,000 pop) between 2013 and 2021 (Source: ISTAT). (b) Proportion of cutaneous and visceral Leishmaniasis by sex from 2013 to 2021, p-value < 0.0001. (c) Proportion of CL by age group from 2013 to 2021. (d) Proportion of VL cases by age group, p-value < 0.0001.
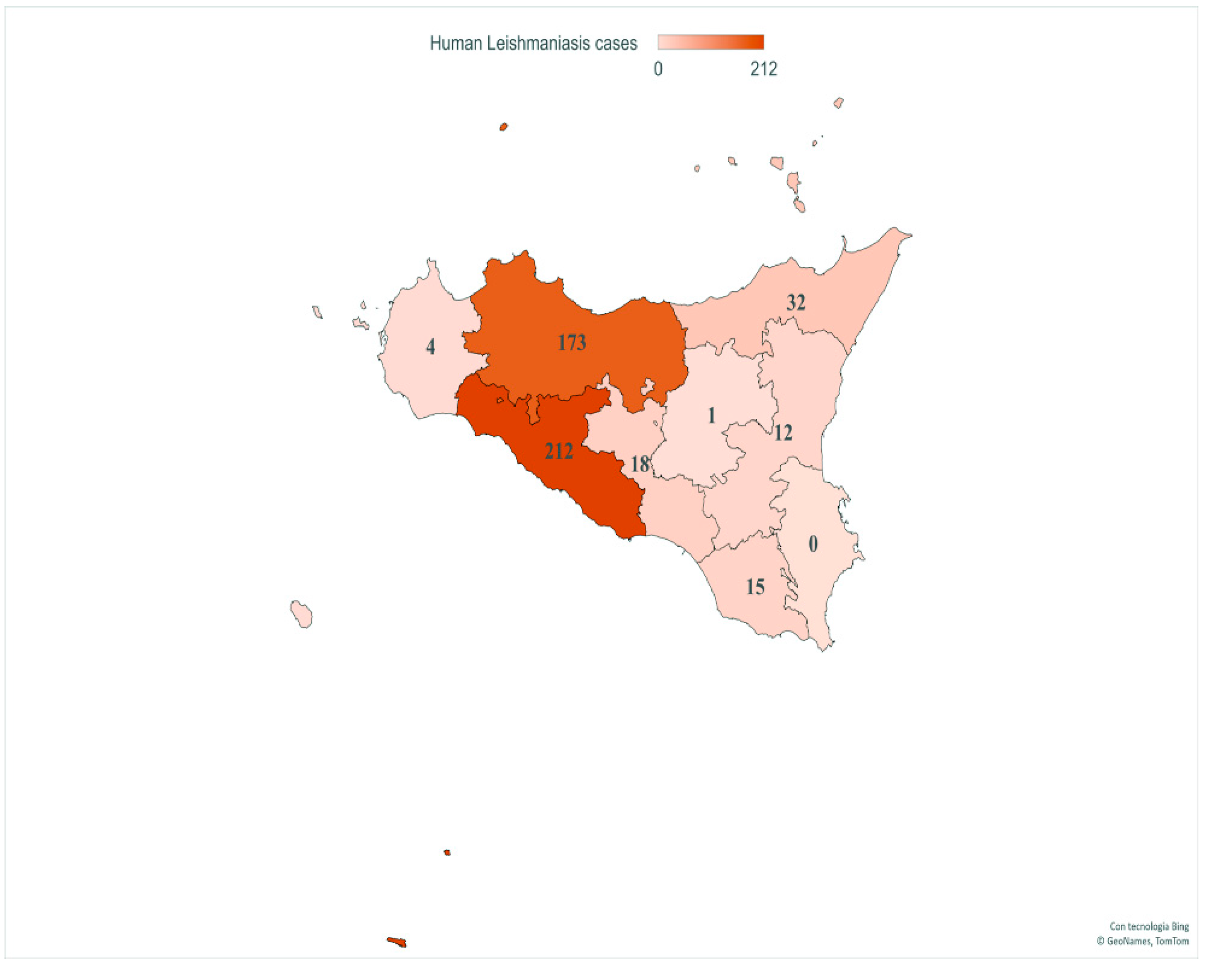
Finally, geospatial localization revealed CL and VL cases in all provinces of Sicily. The provinces with the highest number of patients were AG (212/467, 45.4%) and PA (173/467, 37%), followed by ME (32/467, 6.8%), CL (18/467, 3.85%), RG (15/467, 3.21%), CT (12/467, 2.56%), TP (4/467, 0.85%) and EN (1/467, 0.21%) (Figure 4).
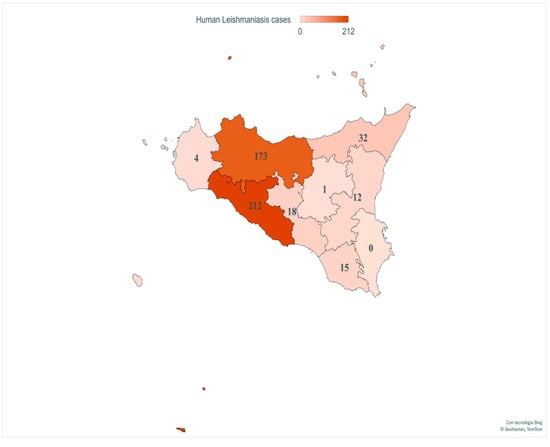
Figure 4.
Number of VL and CL patients in provinces of Sicily. This image presents the spatial distribution of human case counts from 2013 to 2021. Map generated with Excel Power Map.
3.3. Sandfly Data
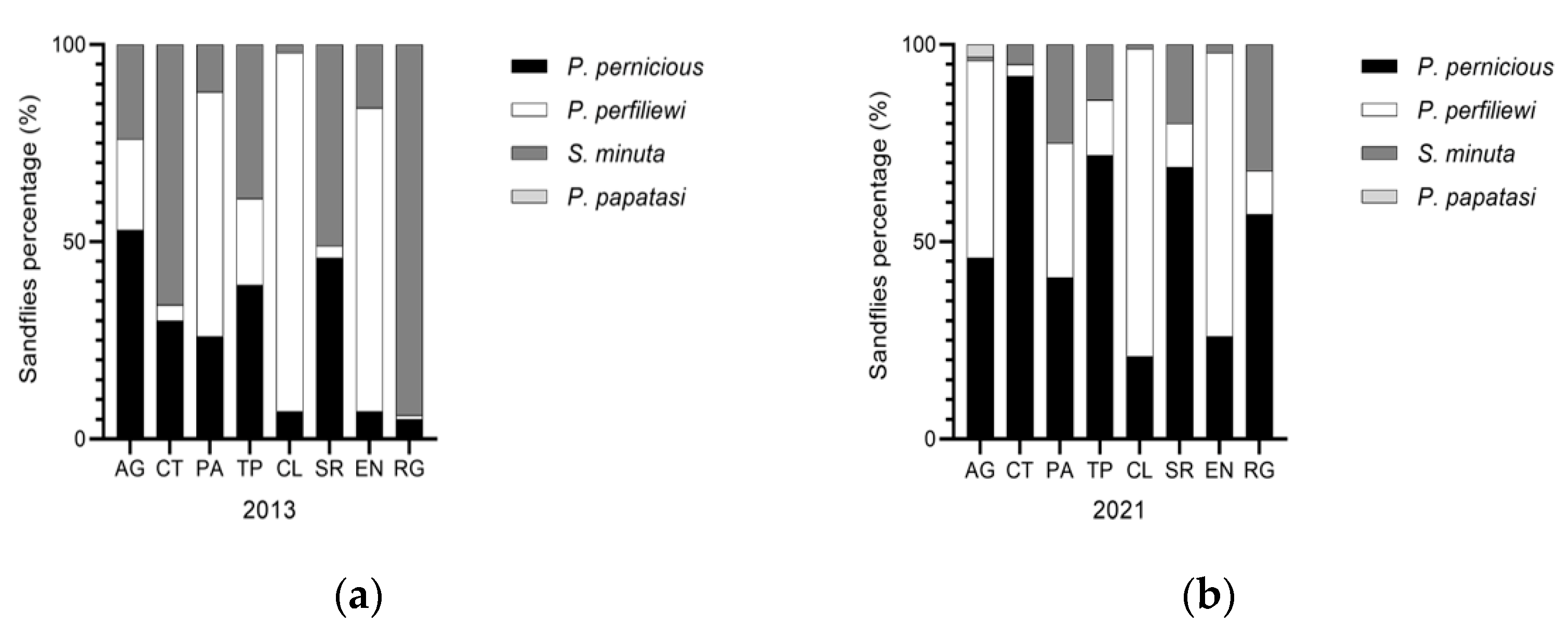
During 2013 in Sicily, 21,121 Phlebotomus were captured, identifying 3 species: P. perniciosus, P. perfiliewi, S. minuta. P. perfiliewi was the most abundant (68.7%), followed by P. perniciosus (17.2%) and S. minuta (14%), showing P. perfiliewi was the dominant species in Sicily [40,41]. In 2021, 21,716 Phlebotomus were captured identifying 4 species: P. perniciosus, P. perfiliewi, S. minuta and P. papatasi. P. perfiliewi was confirmed as the most common species in Sicily (61.6%), followed by perniciosus (with a percentage increased to 33.1%), and a reduction in S. minuta (4.7%). During the entomological analysis in 2021, an interesting finding was the identification of 90/21716 (0.41%) specimens of P. papatasi in the AG province (Table 1) and the proportion of sandfly species by Sicilian provinces was shown in Figure 5.

Table 1.
Phlebotomine sandfly species recorded in Sicilian provinces during 2013 and 2021.
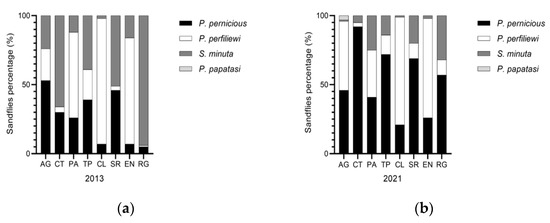
Figure 5.
Proportion of sandfly species by Sicilian provinces. (a) Phlebotomus species collected during 2013 year; and (b) Phlebotomus species collected during 2021 year.
4. Discussion
One-Health is a concept rooted in the history of medicine and healthcare. It proposes a holistic view of the distinct disciplines of veterinary medicine, human medicine, wildlife conservation and environmental science [42,43]. This concept is especially important for vector-borne zoonoses, such as Leishmaniasis. The dynamics and temporal–spatial evolution of Leishmaniasis in Europe depends on various factors, including global warming that affects the ecology and distribution of phlebotomine vectors, and anthropogenic risk factors such as migration, travel, animal trade and environmental modifications [44]. The region of Sicily, an island in southern Italy, is considered endemic for L. infantum [31,33,45,46]. With the current study, we used a One-Health approach, integrating human and animal data, to assess and map the risk of endemic transmission of Leishmania in region Sicily, covering a time span of 9 years. We found positive associations between human cases, infected reservoir hosts and vector spatial distribution. Our study revealed a mean true prevalence of 34.4% of CanL during the years under investigation, confirming Leishmaniosis as an endemic public health threat in Sicily [33,45]. The highest prevalence of CanL in Sicily was recorded from 2013 to 2019, since the sampling of dogs included in the study was carried out mainly for diagnostic purposes, following a clinical suspicion. From 2020, the establishment of the “Regional monitoring plan for Leishmaniasis in the territory of the region of Sicily” (decree 473/2020), which required an active surveillance of dogs in health shelters and public kennels, resulted in a slight decrease in prevalence in the years 2020 and 2021 with values of 25.4% and 21.6%, respectively.
Diagnosis based only on the presence of clinical signs related to the disease could overestimate the prevalence of leishmaniasis in this area, and since the data were collected only in the context of routine diagnosis, following a diagnostic suspicion, the data obtained certainly led to a selection BIAS, since the selection of individuals for analysis was not randomized. The risk of collecting biased samples that do not represent the entire population represents a limitation. This study therefore underlined the importance of a surveillance plan by random sampling, as it allows (i) identification of asymptomatic carriers in endemic areas, (ii) valid estimation of the disease prevalence, and (iii) control programs for the prevention and treatment of disease.
The sensitivity and specificity of IFAT and qPCR are singularly high and close to 100%, but the use of an integrated diagnostic approach of these techniques can try to solve the diagnostic problems the low predictive values of serology, whose results can be affected by either persistent antibodies (false positive) or immunosuppression (false negative) and the inability to detect parasitic DNA in some samples, thus considerably increasing sensitivity and specificity. The prevalence of CanL in the Sicilian provinces showed a homogeneous distribution pattern in all provinces, with the exception of EN and AG, although the data for the province of EN is due to a low sample number, overestimating disease prevalence. Unlike dogs, cats have been considered as incidental hosts resistant to Leishmaniasis for many years. However, this feline now appears to be an important part of the ecological system in which Leishmania parasites can persist indefinitely [47]. Several studies on Leishmania infection in cats have been conducted in recent years [48]. Feline Leishmaniasis has gained importance, being considered today as an emerging disease. Recent epidemiological studies suggest that cats living in Leishmania endemic areas have similar prevalence data and exposure risk as dogs in the same area, although they are generally subclinical or asymptomatic [46,49]. When associated with disease, FeL can include a variety of signs, with skin or muco-cutaneous lesions and enlarged lymph nodes are being the most common [16,49]. Indeed, dermatologic lesions predominate in the clinical presentation of FeL, representing more than half of the clinical manifestations [16,49]. From 2013 to 2021, the true prevalence of FeL obtained in our study in Sicily was 11.8%. It is important to discuss some potential limitations of the data obtained: such as the low sample number and an unequal distribution of cases in the Sicilian provinces, as the cats are primarily from Messina and Palermo provinces. However, our study may suffer from underestimation. First, immunopathogenesis of FeL is poorly understood. This protozoonosis shows a wide range of clinical signs and clinicopathologic abnormalities, which coupled with the lack of standardized protocols, make diagnosis even more difficult for veterinarians [48]. Second, the diagnosis of FeL is usually based on the results of histopathology, cytology, immunohistochemistry, culture, PCR and serology. In addition to the inherent limitations and advantages of each of these methods, their diagnostic value depends on many conditions, including the reagents, the biological sample used and the particular technique used. The most common laboratory method for diagnosing FeL is the IFA test [47]. However, care should be taken before confirming FeL with IFAT; in fact, many present with clinical symptoms of Leishmaniasis, but are negative on IFAT and should be subjected to other additional diagnostic tools such as cytology and PCR [47]. Our surveillance was not limited to canine and feline Leishmaniosis, but it extends to infections of human patients showing clinical signs of VL and CL. The cumulative incidence during these 9 years of observation was 1.04 per 100,000 inhabitants, with the highest incidence found in 2013 (1.9 per 100,000 inhabitants) and the lowest in 2020 (0.5 per 100,000 inhabitants). During the study period, a change in the type of Leishmaniasis was observed with an increase in CL cases from 2013 to 2019, and an increase in VL cases from 2020 to 2021, probably due to the coronavirus pandemic in Italy changing the way of practicing dermatology and because most of the departments are closed. Between 1989 and 2009, Italy reported an increase in the number of VL cases from a baseline of about 10–30 cases annually notified since the 1950s [50]. However, there is an increasing trend in Italy, which could be associated with two factors: first, the reporting performance of the VL system in Italy, although acceptable, varies from region to region, while CL is certainly under-notified nationally because it often does not necessitate hospitalization; and second, several health centers specializing in infectious diseases are available regionally, consequently the diagnosis of Leishmaniasis is not centralized. Thus, the knowledge about the epidemiology of L. infantum is still insufficient, and our surveillance data in the Sicily region are discrepant with those in Italy, which may be affected by underestimation. Therefore, C.Re.Na.L. in a One-Health perspective, supporting the diagnosis of the main Sicilian nosocomial showed a high incidence of human Leishmaniasis in Sicily. Our study has shown that the percentage of affected men in Sicily was higher as compared to women in Sicily, from 2013 to 2021. Recent studies have described a male bias in certain infectious diseases [51], including Leishmaniasis [52], suggesting behavioral and physiological theories. However, we cannot exclude that men are more exposed to infected vectors because of their social or working activities [53]. By age groups in individuals with CL, our results obtained found the support of another study that examined epidemiological aspects of CL by age, observing higher Leishmaniasis infection in people older than 50 years of age and correlating it with occupation and increased outdoor living [52]. VL patients were in the largest percentage in the age group ≥20 < 50 years from 2018 to 2021, while from 2013 to 2017, the age group ≤5 was the largest. In the younger population, the main causes are related with a lack of an efficient immune response [51,53]. This is in line with findings in other similar studies [54,55]. In adults, the disease is associated with states of immunosuppression, due to treatment or diseases such as HIV, whereas in children it could be due to lack of an effective immune response [56]. According to our data, 467 people have been infected with Leishmania in the past 9 years, and considering the geospatial location of infected humans, CL and VL cases were observed in all provinces of the island except SR (Figure 4). The reasons for this absence and/or reduced number of cases are unclear, but may be related to a lack of a surveillance system, a diagnosis system, and a possible underestimation of clinical suspicion at the primary care level. We found positive associations between VL and CL cases and the geographic distribution of CanL, in the provinces of AG and PA, indicating that strains of L. infantum were circulating in some areas of the region during the study period. While CanL, FeL, CL, and VL cases referred to a time span up to 2013 to 2021, the entomological samples were based on monitoring activities carried out only in 2013 and 2021. Our entomological results supported that P. perfiliewi represented the principal vector of L. infantum in some areas of VL endemicity in the Mediterranean basin according to available entomological investigations on L. infantum vectors in southern Sicily [57,58]. The presence of P. papatasi on the island was to be considered non-threatening to people and dogs, given its specificity in transmitting Leishmania major [59]. L. major caused CL, is endemic in Africa, thus potentially importable by the migration phenomenon; however, the risk of local transmission was reduced due to the absence in Sicily of gerbils, natural reservoir hosts [45]. However, the fact that Leishmania spp. can undergo genetic exchange by forming L. major/L. infantum hybrids, efficiently transmitted by the specific vector P. papatasi, could result in increased transmission potential [59,60]. These data highlight the potential epidemiological impact of genetic switch in parasites; in fact, hybrids may have the potential risk to expand disease transmission and invade new territories [59]. This suggests that in the wild and especially in Sicily, hybrid strains may spread using P. papatasi sandfly vectors, thus increasing the risk of their spreading into new foci. The presence of P. papatasi in AG could be correlated with the high number (212/467, 45.4%) of human cases found in this province, but further investigation is needed. Regarding the distribution of phlebotomine species in the provinces of the island, it was found that the species S. minuta in 2013 was predominantly found in the eastern part of Sicily (RG, CT, SR and EN), while in 2021, the distribution remains the same although with a significant reduction. Climate change is allegedly affecting the seasonality of many species, including several insect vectors, geographic distribution and increased connectivity has facilitated the expansion of sandflies into new geographic areas [7,61]. Even though in Sicily Leishmaniasis is endemic, this region will continue to be a highly transited island, with international tourism, a higher flow of migration, that can facilitate the occurrence of imported cases. Thus, trying to work on a One-Health concept that integrates the different fields involved in transmission processes, including entomological, animal, and human data, is of fundamental importance to know the risk of transmission and spread of the parasite. The One-Health approach is the perfect concept to apply on studying Leishmaniasis by a holistic view of the previously various professions belonging to human and veterinary medicine, environmental science and wildlife conservation. It is a parasitosis of great human and veterinary medical relevance that involves a complicated interaction between a pathogenic protozoan, sandflies vectors, environmental influence on vector distribution, a small reservoir of companion animal (dog) infection, and susceptible human populations. An interdisciplinary team of human physicians, microbiologists, veterinarians, parasitologists, entomologists, ecologists, epidemiologists, immunologists, and public health officials are essential for effective control against Leishmaniasis. In addition, the One Health approach is well suited to the surveillance and control requirements of this infection. This study examines the current state of Leishmaniasis prevention, control, and surveillance in Sicily, but it also reveals gaps in the islands under study that could be remedied through the application of the One-Health principles we have been discussing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10091704/s1, Table S1: Results distribution of molecular and serological analysis in humans, canines and felines population.
Author Contributions
Conceptualization, G.C., F.B. and F.V.; methodology, G.C., F.B., S.R., F.L.R., E.O., F.F., V.V., G.M., V.G., S.G. and A.G.; software, F.B. and G.F.S.; validation, G.C., F.B., F.V. and G.F.S.; formal analysis, G.C., F.B. and F.V.; investigation, G.C., F.B., F.L.R. and E.O.; resources, F.V.; data curation, S.R., F.L.R., E.O., F.F., V.V., G.M., V.G., S.G. and A.G.; writing—original draft, G.C., F.B. and G.F.S.; writing—review and editing, G.C. and F.B.; visualization, F.V. and G.F.S.; supervision, F.V. and G.F.S.; project administration, F.V.; funding acquisition, G.C., F.B. and F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministero della Salute grants number: RC IZS SI 04/21, GR-2019- 12369134 “Leishmaniasis in Agrigento, Caltanissetta and Palermo provinces: human outbreaks and animal reservoirs”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Samples were collected as part of routine diagnosis and/or post-treatment follow-up without any necessary or additional noninvasive procedures and written informed consent was obtained from the patient at the time of the clinical examination. Patient records and information was anonymized and de-identified before analysis. All adult subjects provided written informed consent and a parent or guardian of any child participant provided written informed consent on their behalf. All animal samples were collected for di-agnostic use and no unnecessary invasive procedures were performed, including parasitological confirmation of FeL or CanL. At the time of clinical examination, oral informed consent was obtained from the owners of dogs and cats.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Valeria Vitale Badaco, Michele Di Gesaro and Carmelo Bongiorno for technical, graphical and administrative supports.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Health Sci. J. 2017, 11. [Google Scholar] [CrossRef]
- Tedla, D.G.; Bariagabr, F.H.; Abreha, H.H. Incidence and Trends of Leishmaniasis and Its Risk Factors in Humera, Western Tigray. J. Parasitol. Res. 2018, 2018, 8463097. [Google Scholar] [CrossRef]
- Leta, S.; Dao, T.H.T.; Mesele, F.; Alemayehu, G. Visceral Leishmaniasis in Ethiopia: An Evolving Disease. PLoS Negl. Trop. Dis. 2014, 8, e3131. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Leishmaniasis emergence in Europe. Eurosurveillance 2010, 15, 19505. [Google Scholar] [CrossRef]
- Spear, R.C. Review of “Mathematical Models for Neglected Tropical Diseases: Essential Tools for Control and Elimination, Part B” Edited by Maria-Gloria Basáñez and Roy M. Anderson. Parasites Vectors 2017, 10, 38. [Google Scholar] [CrossRef][Green Version]
- Oryan, A.; Alidadi, S.; Akbari, M. Risk Factors Associated with Leishmaniasis. Trop. Med. Surg. 2014, 2, e118. [Google Scholar] [CrossRef]
- Ready, P.D. Leishmaniasis Emergence and Climate Change. Rev. Sci. Tech. 2008, 27, 399–412. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Brandão-Filho, S.P. Visceral Leishmaniasis in Brazil: Revisiting Paradigms of Epidemiology and Control. Rev. Inst. Med. Trop. Sao Paulo 2006, 48, 151–156. [Google Scholar] [CrossRef]
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef]
- Lemma, W.; Tekie, H.; Balkew, M.; Gebre-Michael, T.; Warburg, A.; Hailu, A. Population dynamics and habitat preferences of Phlebotomus orientalis in extra-domestic habitats of Kafta Humera lowlands—Kala azar endemic areas in Northwest Ethiopia. Parasites Vectors 2014, 7, 359. [Google Scholar] [CrossRef][Green Version]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- Alvar, J.; Yactayo, S.; Bern, C. Leishmaniasis and poverty. Trends Parasitol. 2006, 22, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO. Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Cardenas, R.; Sandoval, C.M.; Rodriguez-Morales, A.J.; Franco-Paredes, C. Impact of climate variability in the occurrence of leishmaniasis in northeastern Colombia. Am. J. Trop. Med. Hyg. 2006, 75, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Alwassouf, S.; Cristóvão, J.M.; Ayhan, N.; Pereira, A.; Charrel, R.N.; Campino, L. Serological association between Leishmania infantum and sand fly fever Sicilian (but not Toscana) virus in sheltered dogs from southern Portugal. Parasites Vectors 2017, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G. Leishmaniosis of companion animals in Europe: An update. Vet. Parasitol. 2015, 208, 35–47. [Google Scholar] [CrossRef]
- Alvar, J.; Cañavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine Leishmaniasis. In Advances in Parasitology; Academic Press: Cambridge, MA, USA, 2004; Volume 57, pp. 1–88. [Google Scholar]
- Iatta, R.; Furlanello, T.; Colella, V.; Tarallo, V.D.; Latrofa, M.S.; Brianti, E.; Trerotoli, P.; Decaro, N.; Lorusso, E.; Schunack, B.; et al. A nationwide survey of Leishmania infantum infection in cats and associated risk factors in Italy. PLoS Negl. Trop. Dis. 2019, 13, e0007594. [Google Scholar] [CrossRef]
- Iatta, R.; Mendoza-Roldan, J.A.; Latrofa, M.S.; Cascio, A.; Brianti, E.; Pombi, M.; Gabrielli, S.; Otranto, D. Leishmania tarentolae and Leishmania infantum in humans, dogs and cats in the Pelagie archipelago, southern Italy. PLoS Negl. Trop. Dis. 2021, 15, e0009817. [Google Scholar] [CrossRef]
- Koch, L.K.; Kochmann, J.; Klimpel, S.; Cunze, S. Modeling the climatic suitability of leishmaniasis vector species in Europe. Sci. Rep. 2017, 7, 13325. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Signorini, M.; Cassini, R.; Drigo, M.; DI Regalbono, A.F.; Pietrobelli, M.; Montarsi, F.; Stensgaard, A.-S. Ecological niche model of Phlebotomus perniciosus, the main vector of canine leishmaniasis in north-eastern Italy. Geospat. Health 2014, 9, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B. Sampling methods for phlebotomine sandflies. Med. Vet. Entomol. 2000, 14, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Van Bortel, W.; Zeller, H.; Alten, B. A summary of the evidence for the change in European distribution of phlebotomine sand flies (Diptera: Psychodidae) of public health importance. J. Vector Ecol. 2014, 39, 72–77. [Google Scholar] [CrossRef]
- Alten, B.; Maia, C.; Afonso, M.O.; Campino, L.; Jiménez, M.; González, E.; Molina, R.; Bañuls, A.L.; Prudhomme, J.; Vergnes, B.; et al. Seasonal Dynamics of Phlebotomine Sand Fly Species Proven Vectors of Mediterranean Leishmaniasis Caused by Leishmania infantum. PLoS Negl. Trop. Dis. 2016, 10, e0004458. [Google Scholar] [CrossRef]
- Gradoni, L.; Gramiccia, M.; Scalone, A. Visceral Leishmaniasis Treatment, Italy. Emerg. Infect. Dis. 2003, 9, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Moirano, G.; Zanet, S.; Giorgi, E.; Battisti, E.; Falzoi, S.; Acquaotta, F.; Fratianni, S.; Richiardi, L.; Ferroglio, E.; Maule, M. Integrating environmental, entomological, animal, and human data to model the Leishmania infantum transmission risk in a newly endemic area in Northern Italy. One Health 2020, 10, 100159. [Google Scholar] [CrossRef]
- Hailu, T. One Health Approach Prospect for Integrated Control and Elimination of Visceral Leishmaniasis in Ethiopia: A Narrative Review Article. Iran. J. Parasitol. 2016, 11, 1–9. [Google Scholar] [PubMed]
- Vilas, V.J.D.R.; Maia-Elkhoury, A.N.S.; Yadon, Z.E.; Cosivi, O.; Sanchez-Vazquez, M.J. Visceral leishmaniasis: A One Health approach. Vet. Rec. 2014, 175, 42–44. [Google Scholar] [CrossRef]
- Castelli, G.; Bruno, F.; Caputo, V.; Fiorella, S.; Sammarco, I.; Lupo, T.; Migliazzo, A.; Vitale, F.; Reale, S. Genetic tools discriminate strains of Leishmania infantum isolated from humans and dogs in Sicily, Italy. PLoS Negl. Trop. Dis. 2020, 14, e0008465. [Google Scholar] [CrossRef]
- Orndorff, G.R.; Cooper, B.A.; Smith, W.; Ryan, J.R. Canine Visceral Leishmaniasis in Sicily. Mil. Med. 2000, 165, 29–32. [Google Scholar] [CrossRef][Green Version]
- Verso, M.G.; Vitale, F.; Castelli, G.; Bruno, F.; Migliazzo, A.; Bongiorno, M.R.; Santi, F.; Pistone, G.; Amodio, E.; Picciotto, D. Suspected Cutaneous Leishmaniasis in a Sample of Westerns Sicily Residents: What Correlation with Occupation? Med. Lav. 2017, 108, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Vitale, F.; Bruno, F.; Migliazzo, A.; Galante, A.; Vullo, A.; Graziano, R.; D’Avola, S.; Caputo, V.; Castelli, G. Cross-Sectional Survey of Canine Leishmaniasis in Pantelleria Island in Sicily. Vet. Ital. 2020, 56, 103–107. [Google Scholar] [CrossRef] [PubMed]
- International Office of Epizootics. Aquatic Animal Health Standards Commission. In Manual of Diagnostic Tests for Aquatic Animals, 8th ed.; Chapter 3.1.11 Leishmaniosis; OIE: Paris, France, 2021; ISBN 978-92-95115-59-0. [Google Scholar]
- Castelli, G.; Bruno, F.; Reale, S.; Catanzaro, S.; Valenza, V.; Vitale, F. Molecular Diagnosis of Leishmaniasis: Quantification of Parasite Load by a Real-Time PCR Assay with High Sensitivity. Pathogens 2021, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Romi, R.; Khoury, C.; Bigliocchi, F.; Maroli, M. Fact Sheet on Mites and Insects of Medical Importance. Schede Guida Su Acari e Insetti Di Interesse Sanitario; Istituto Superiore Di Sanità: Rome, Italy, 1994.
- Dantas-Torres, F.; Tarallo, V.D.; Otranto, D. Morphological keys for the identification of Italian phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae). Parasites Vectors 2014, 7, 479. [Google Scholar] [CrossRef]
- Rogan, W.J.; Gladen, B. Estimating Prevalence from the Results of a Screening Test. Am. J. Epidemiol. 1978, 107, 71–76. [Google Scholar] [CrossRef]
- Tadesse, G. Brucellosis Seropositivity in Animals and Humans in Ethiopia: A Meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0005006. [Google Scholar] [CrossRef]
- Maroli, M.; Pampiglione, S.; Tosti, A. Cutaneous Leishmaniasis in Western Sicily (Italy) and Preliminary Survey of Phlebotomine Sandflies (Diptera: Psychodidae). Parassitologia 1988, 30, 211–217. [Google Scholar]
- Lisi, O.; D’Urso, V.; Vaccalluzzo, V.; Bongiorno, G.; Khoury, C.; Severini, F.; Di Muccio, T.; Gramiccia, M.; Gradoni, L.; Maroli, M. Persistence of phlebotomine Leishmania vectors in urban sites of Catania (Sicily, Italy). Parasites Vectors 2014, 7, 560. [Google Scholar] [CrossRef][Green Version]
- Palatnik-De-Sousa, C.B.; Day, M.J. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasites Vectors 2011, 4, 197. [Google Scholar] [CrossRef]
- Day, M.J. One health: The importance of companion animal vector-borne diseases. Parasites Vectors 2011, 4, 49. [Google Scholar] [CrossRef]
- Dujardin, J.-C.; Campino, L.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Soteriadou, K.; Mazeris, A.; Ozbel, Y.; Boelaert, M. Spread of Vector-Borne Diseases and Neglect of Leishmaniasis, Europe. Emerg. Infect. Dis. J. 2008, 14, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Manzillo, V.F.; Gizzarelli, M.; Vitale, F.; Montagnaro, S.; Torina, A.; Sotera, S.; Oliva, G. Serological and entomological survey of canine leishmaniasis in Lampedusa island, Italy. BMC Vet. Res. 2018, 14, 286. [Google Scholar] [CrossRef]
- Otranto, D.; Napoli, E.; Latrofa, M.S.; Annoscia, G.; Tarallo, V.D.; Greco, G.; Lorusso, E.; Gulotta, L.; Falsone, L.; Basano, F.S.; et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: Pathogen and vector circulation in a confined environment. Vet. Parasitol. 2017, 236, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, S.; Fakhar, M.; Teshnizi, S.H. Is the cat an important reservoir host for visceral leishmaniasis? A systematic review with meta-analysis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190012. [Google Scholar] [CrossRef]
- Pereira, A.; Maia, C. Leishmania infection in cats and feline leishmaniosis: An updated review with a proposal of a diagnosis algorithm and prevention guidelines. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100035. [Google Scholar] [CrossRef]
- Abramo, F.; Albanese, F.; Gattuso, S.; Randone, A.; Fileccia, I.; Dedola, C.; Ibba, F.; Ottaiano, P.; Brianti, E. Skin Lesions in Feline Leishmaniosis: A Systematic Review. Pathogens 2021, 10, 472. [Google Scholar] [CrossRef]
- Gramiccia, M.; Scalone, A.; Di Muccio, T.; Orsini, S.; Fiorentino, E.; Gradoni, L. The Burden of Visceral Leishmaniasis in Italy from 1982 to 2012: A Retrospective Analysis of the Multi-Annual Epidemic That Occurred from 1989 to 2009. Eurosurveillance 2013, 18, 20535. [Google Scholar] [CrossRef]
- Guerra-Silveira, F.; Abad-Franch, F. Sex Bias in Infectious Disease Epidemiology: Patterns and Processes. PLoS ONE 2013, 8, e62390. [Google Scholar] [CrossRef]
- Cloots, K.; Burza, S.; Malaviya, P.; Hasker, E.; Kansal, S.; Mollett, G.; Chakravarty, J.; Roy, N.; Lal, B.K.; Rijal, S.; et al. Male predominance in reported Visceral Leishmaniasis cases: Nature or nurture? A comparison of population-based with health facility-reported data. PLoS Negl. Trop. Dis. 2020, 14, e0007995. [Google Scholar] [CrossRef]
- Palma, D.; Mercuriali, L.; Figuerola, J.; Montalvo, T.; Bueno-Marí, R.; Millet, J.-P.; Simón, P.; Masdeu, E.; Rius, C. Trends in the Epidemiology of Leishmaniasis in the City of Barcelona (1996–2019). Front. Vet. Sci. 2021, 8, 653999. [Google Scholar] [CrossRef]
- Herrador, Z.; Gherasim, A.; Jimenez, B.C.; Granados, M.; Martín, J.V.S.; Aparicio, P. Epidemiological Changes in Leishmaniasis in Spain According to Hospitalization-Based Records, 1997–2011: Raising Awareness towards Leishmaniasis in Non-HIV Patients. PLoS Negl. Trop. Dis. 2015, 9, e0003594. [Google Scholar] [CrossRef] [PubMed]
- Suárez Rodríguez, B.; Isidoro Fernández, B.; Santos Sanz, S.; Sierra Moros, M.J.; Molina Moreno, R.; Astray Mochales, J.; Amela Heras, C. Situación Epidemiológica y de Los Factores de Riesgo de Transmisión de Leishmania Infantum En España. Rev. Esp. Salud Pública 2012, 86, 555–564. [Google Scholar]
- Riera, C.; Napp, S.; Manzanares, S. Epidemiología de la leishmaniasis humana en la ciudad de Barcelona (1997–2014). Rev. Enf. Emerg. 2016, 15, 68–76. [Google Scholar]
- Abbate, J.M.; Maia, C.; Pereira, A.; Arfuso, F.; Gaglio, G.; Rizzo, M.; Caracappa, G.; Marino, G.; Pollmeier, M.; Giannetto, S.; et al. Identification of trypanosomatids and blood feeding preferences of phlebotomine sand fly species common in Sicily, Southern Italy. PLoS ONE 2020, 15, e0229536. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, G.; Brianti, E.; Napoli, E.; Falsone, L.; Dantas-Torres, F.; Tarallo, V.D.; Otranto, D.; Giannetto, S. Effect of night time-intervals, height of traps and lunar phases on sand fly collection in a highly endemic area for canine leishmaniasis. Acta Trop. 2014, 133, 73–77. [Google Scholar] [CrossRef]
- Volf, P.; Benkova, I.; Myskova, J.; Sadlova, J.; Campino, L.; Ravel, C. Increased transmission potential of Leishmania major/Leishmania infantum hybrids. Int. J. Parasitol. 2007, 37, 589–593. [Google Scholar] [CrossRef]
- Gibson, W.; Stevens, J. Genetic Exchange in the Trypanosomatidae. In Advances in Parasitology; Baker, J.R., Muller, R., Rollinson, D., Eds.; Academic Press: Cambridge, MA, USA, 1999; Volume 43, pp. 1–46. [Google Scholar]
- Chalghaf, B.; Chemkhi, J.; Mayala, B.; Harrabi, M.; Benie, G.B.; Michael, E.; Ben Salah, A. Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: Impact of climate change. Parasites Vectors 2018, 11, 461. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).