Degradation of Triclosan in the Water Environment by Microorganisms: A Review
Abstract
:1. Introduction
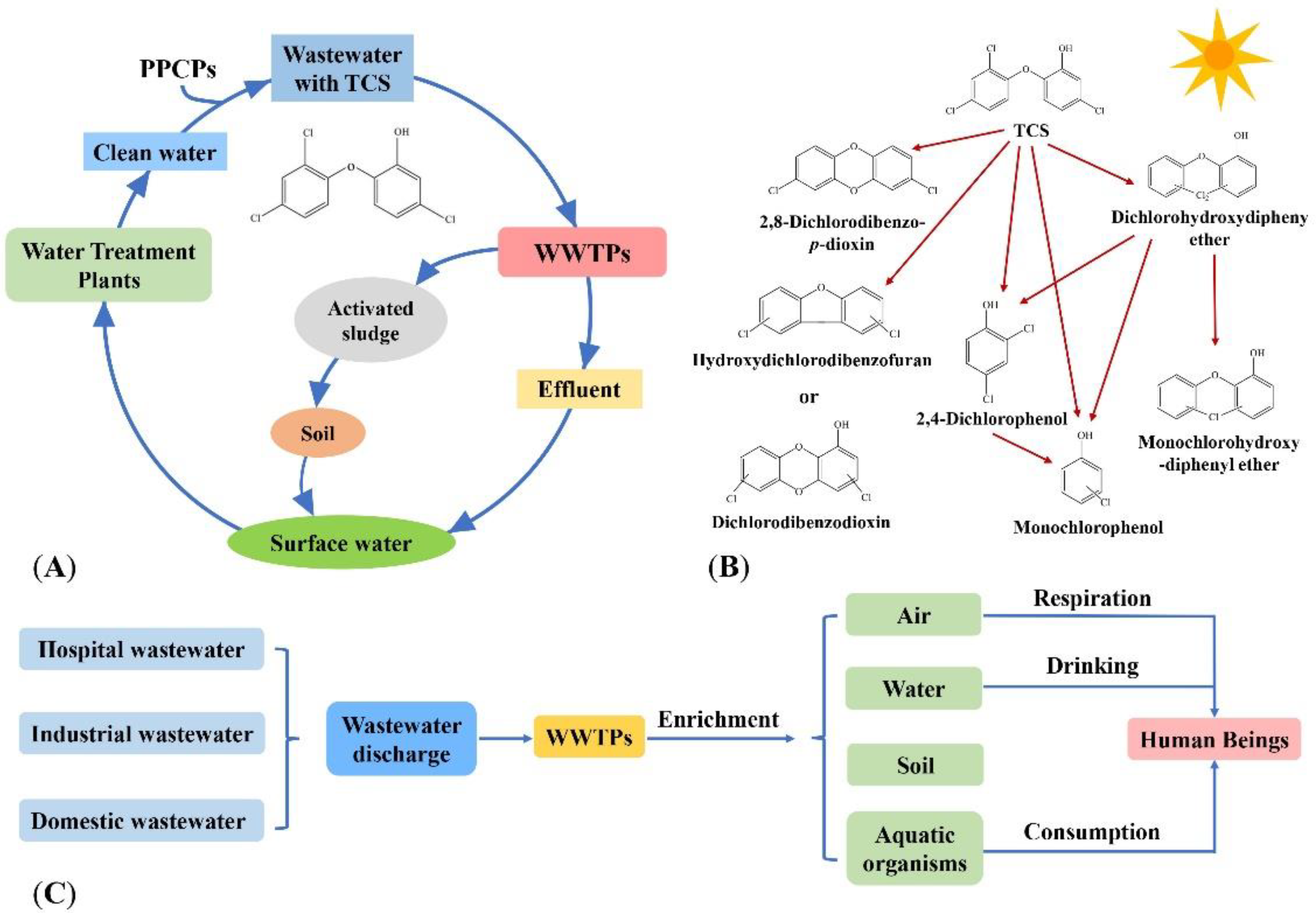
2. Occurrence of TCS in the Water Environment
3. TCS Biodegradation by Microorganisms
3.1. TCS Biodegradation by Isolates and Microbial Consortia
3.1.1. Species and Degradation Characteristics of TCS-Degrading Microbial Isolates
3.1.2. TCS Biodegradation by Microbial Consortia
3.2. Mechanisms of TCS-Degrading Microorganisms
3.2.1. Metabolic Pathways of TCS-Degrading Microorganisms
3.2.2. Enzymes Responsible for TCS Degradation
Specific Enzyme Responsible for TCS Biodegradation
Non-Specific Enzyme Responsible for TCS Biodegradation
- (1)
- Dioxygenase
- (2)
- Monooxygenase
- (3)
- Reductive dehalogenase
- (4)
- Others
4. Summary and Prospect
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roh, H.; Subramanya, N.; Zhao, F.M.; Yu, C.P.; Sandt, J.; Chu, K.H. Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere 2009, 77, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Macri, D. Worldwide Use of triclosan: Can dentistry do without this antimicrobial? Contemp. Clin. Dent. 2017, 8, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. On the need and speed of regulating triclosan and triclocarban in the United States. Environ. Sci. Technol. 2014, 48, 3603–3611. [Google Scholar] [CrossRef]
- Levy, C.W.; Roujeinikova, A.; Sedelnikova, S.; Baker, P.J.; Stuitje, A.R.; Slabas, A.R.; Rice, D.W.; Rafferty, J.B. Molecular basis of triclosan activity. Nature 1999, 398, 383–384. [Google Scholar] [CrossRef]
- Fujimoto, M.; Carey, D.E.; McNamara, P.J. Metagenomics reveal triclosan-induced changes in the antibiotic resistome of anaerobic digesters. Environ. Pollut. 2018, 241, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U.; Paull, D.H. Co-occurrence of triclocarban and triclosan in US water resources. Environ. Sci. Technol. 2005, 39, 1420–1426. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Pellegrino, M.; Saturnino, C.; Longo, P.; Aquaro, S. Triclosan: A small molecule with controversial roles. Antibiotics 2022, 11, 735. [Google Scholar] [CrossRef]
- Ates, G.; Goldberg, J.; Currais, A.; Maher, P. CMS121, a fatty acid synthase inhibitor, protects against excess lipid peroxidation and inflammation and alleviates cognitive loss in a transgenic mouse model of Alzheimer’s disease. Redox Biol. 2020, 36, 101648. [Google Scholar] [CrossRef]
- Contardo-Jara, V.; Meinecke, S.; Feibicke, M.; Berghahn, R.; Schmidt, R.; Mohr, S. Fate, bioaccumulation and toxic effects of triclosan on a freshwater community–A mesocosm study. Environ. Adv. 2021, 5, 100100. [Google Scholar] [CrossRef]
- Zhang, J.; Walker, M.E.; Sanidad, K.Z.; Zhang, H.N.; Liang, Y.S.; Zhao, E.M.; Chacon-Vargas, K.; Yeliseyev, V.; Parsonnet, J.; Haggerty, T.D.; et al. Microbial enzymes induce colitis by reactivating triclosan in the mouse gastrointestinal tract. Nat. Commun. 2022, 13, 136. [Google Scholar] [CrossRef]
- Yee, A.L.; Gilbert, J.A. Is triclosan harming your microbiome? Science 2016, 353, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Caporale, N.; Leemans, M.; Birgersson, L.; Germain, P.L.; Cheroni, C.; Borbely, G.; Engdahl, E.; Lindh, C.; Bressan, R.B.; Cavallo, F.; et al. From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures. Science 2022, 375, eabe8244. [Google Scholar] [CrossRef]
- Yuan, G.X.; Ma, Y.; Zeng, Y.X.; Pan, H.B.; Liu, P.Y.; Liu, Y.; Liu, G.H.; Cheng, J.Q.; Guo, Y.S. Associations between low-dose triclosan exposure and semen quality in a Chinese population. Environ. Pollut. 2022, 299, 118926. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, J.H. Disinfection spreads antimicrobial resistance. Science 2021, 371, 474. [Google Scholar] [CrossRef]
- Mulla, S.I.; Asefi, B.; Bharagava, R.N.; Saratale, G.D.; Li, J.W.; Huang, C.L.; Yu, C.P. Processes for the removal of triclosan in the environment and engineered systems: A review. Environ. Rev. 2020, 28, 55–66. [Google Scholar] [CrossRef]
- Sola-Gutierrez, C.; Schroder, S.; San-Roman, M.F.; Ortiz, I. Critical review on the mechanistic photolytic and photocatalytic degradation of triclosan. J. Environ. Manag. 2020, 260, 110101. [Google Scholar] [CrossRef]
- Quan, B.Y.; Li, X.; Zhang, H.; Zhang, C.; Ming, Y.; Huang, Y.C.; Xi, Y.N.; Xu, W.H.; Liu, Y.G.; Tang, Y.Q. Technology and principle of removing triclosan from aqueous media: A review. Chem. Eng. J. 2019, 378, 122185. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Mohan, S. Treatment of triclosan through enhanced microbial biodegradation. J. Hazard. Mater. 2021, 420, 126430. [Google Scholar] [CrossRef] [PubMed]
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Comprehensive insight into triclosan—From widespread occurrence to health outcomes. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Shrestha, P.; Zhang, Y.M.; Chen, W.J.; Wong, T.Y. Triclosan: Antimicrobial mechanisms, antibiotics interactions, clinical applications, and human health. J. Environ. Sci. Health C Toxicol. Carcinog. 2020, 38, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Yee, A.L.; Gilbert, J.A.; Haider, A.; Jamal, S.B.; Muhammad, F. Triclosan-containing sutures: Safety and resistance issues need to be addressed prior to generalized use. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Kumar, S.; Paul, T.; Shukla, S.P.; Kumar, K.; Karmakar, S.; Bera, K.K.; Kumar, C.B. Biomarkers-based assessment of triclosan toxicity in aquatic environment: A mechanistic review. Environ. Pollut. 2021, 286, 117569. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhuang, J.; Bester, K. Degradation of triclosan by environmental microbial consortia and by axenic cultures of microorganisms with concerns to wastewater treatment. Appl. Microbiol. Biotechnol. 2018, 102, 5403–5417. [Google Scholar] [CrossRef]
- Chu, W.H.; Fang, C.; Deng, Y.; Xu, Z.X. Intensified disinfection amid COVID-19 pandemic poses potential risks to water quality and safety. Environ. Sci. Technol. 2021, 55, 4084–4086. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.R.; He, Y.Z.; Zhi, D.; Luo, L.; Sun, Y.Q.; Khan, E.; Wang, L.; Peng, Y.T.; Zhou, Y.Y.; Tsang, D.C.W. Current progress in treatment techniques of triclosan from wastewater: A review. Sci. Total Environ. 2019, 696, 133990. [Google Scholar] [CrossRef]
- Bedoux, G.; Roig, B.; Thomas, O.; Dupont, V.; Le Bot, B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. 2012, 19, 1044–1065. [Google Scholar] [CrossRef]
- Pashaei, R.; Dzingeleviciene, R.; Abbasi, S.; Szultka-Mlynska, M.; Buszewski, B. Determination of 15 human pharmaceutical residues in fish and shrimp tissues by high-performance liquid chromatography-tandem mass spectrometry. Environ. Monit. Assess 2022, 194, 325. [Google Scholar] [CrossRef]
- Roberts, J.; Price, O.R.; Bettles, N.; Rendal, C.; van Egmond, R. Accounting for dissociation and photolysis: A review of the algal toxicity of triclosan. Environ. Toxicol. Chem. 2014, 33, 2551–2559. [Google Scholar] [CrossRef]
- Wu, J.L.; Ji, F.F.; Zhang, H.N.; Hu, C.Q.; Wong, M.H.; Hu, D.; Cai, Z.W. Formation of dioxins from triclosan with active chlorine: A potential risk assessment. J. Hazard. Mater. 2019, 367, 128–136. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, J.T.; Li, S.Y.; Yu, M.Z. Occurrence, fate, and mass balance of selected pharmaceutical and personal care products (PPCPs) in an urbanized river. Environ. Pollut. 2020, 266, 115340. [Google Scholar] [CrossRef]
- Dar, O.I.; Raouf, A.; Deng, P.; Sunil, S.; Megha, A.; Kaur, A.; Jia, A.Q.; Faggio, C. Source, bioaccumulation, degradability and toxicity of triclosan in aquatic environments: A review. Environ. Technol. Innov. 2022, 25, 102122. [Google Scholar] [CrossRef]
- Sanchez-Prado, L.; Barro, R.; Garcia-Jares, C.; Llompart, M.; Lores, M.; Petrakis, C.; Kalogerakis, N.; Mantzavinos, D.; Psillakis, E. Sonochemical degradation of triclosan in water and wastewater. Ultrason. Sonochemistry 2008, 15, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Hu, B.Y.; Zhou, W.; Huang, K.; Fu, J.J.; Zhang, A.Q.; Jiang, G.B. Enhanced hand-to-mouth exposure from hand sanitizers during the COVID-19 pandemic: A case study of triclosan. Sci. Bullet 2022, 67, 995–998. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Hanna, K. Environmental side effects of the injudicious use of antimicrobials in the era of COVID-19. Sci. Total Environ. 2020, 745, 141053. [Google Scholar] [CrossRef]
- Bilal, M.; Barcelo, D.; Iqbal, H.M.N. Persistence, ecological risks, and oxidoreductases-assisted biocatalytic removal of triclosan from the aquatic environment. Sci. Total Environ. 2020, 735, 139194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Liang, W. Occurrence, toxicity, and removal methods of triclosan: A timely review. Curr. Pollut. Rep. 2021, 7, 31–39. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Wang, L.; Zhang, X.M.; Zhang, X.; Li, Y.F.; Nikolaev, A.; Li, W.L. Fate processes of parabens, triclocarban and triclosan during wastewater treatment: Assessment via field measurements and model simulations. Environ. Sci. Pollut. Res. 2021, 28, 50602–50610. [Google Scholar] [CrossRef]
- Komolafe, O.; Mrozik, W.; Dolfing, J.; Acharya, K.; Vassalle, L.; Mota, C.R.; Davenport, R. Occurrence and removal of micropollutants in full-scale aerobic, anaerobic and facultative wastewater treatment plants in Brazil. J. Environ. Manag. 2021, 287, 112286. [Google Scholar] [CrossRef]
- Contreras, C.R.; Lopez, D.; Leiva, A.M.; Dominguez, C.; Bayona, J.M.; Vidal, G. Removal of organic micropollutants in wastewater treated by activated sludge and constructed wetlands: A comparative study. Water 2020, 11, 2515. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Balakrishnan, P. Triclosan in treated wastewater from a city wastewater treatment plant and its environmental risk assessment. Water Air Soil Pollut. 2019, 230, 69. [Google Scholar] [CrossRef]
- Li, W.L.; Zhang, Z.F.; Ma, W.L.; Liu, L.Y.; Song, W.W.; Li, Y.F. An evaluation on the intra-day dynamics, seasonal variations and removal of selected pharmaceuticals and personal care products from urban wastewater treatment plants. Sci. Total Environ. 2018, 640, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Heidler, J.; Halden, R.U. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 2007, 66, 362–369. [Google Scholar] [CrossRef]
- Zheng, G.D.; Yu, B.; Wang, Y.W.; Ma, C.; Chen, T.B. Removal of triclosan during wastewater treatment process and sewage sludge composting-A case study in the middle reaches of the Yellow River. Environ. Int. 2020, 134, 105300. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Chen, Z.F.; Ying, G.G.; Liu, Y.S.; Zhang, Q.Q.; Zhao, J.L.; Liu, S.S.; Chen, J.; Peng, F.J.; Lai, H.J.; Pan, C.G. Triclosan as a surrogate for household biocides: An investigation into biocides in aquatic environments of a highly urbanized region. Water Res. 2014, 58, 269–279. [Google Scholar] [CrossRef]
- Chen, P.; Zhong, Y.; Chen, K.C.; Guo, C.S.; Gong, J.; Wang, D.D.; Yang, Y.; Ma, S.T.; Yu, Y.X. The impact of discharge reduction activities on the occurrence of contaminants of emerging concern in surface water from the Pearl River. Environ. Sci. Pollut. Res. 2020, 27, 30378–30389. [Google Scholar] [CrossRef]
- Lu, S.; Wang, B.D.; Xin, M.; Wang, J.; Gu, X.; Lian, M.S.; Li, Y.; Lin, C.Y.; Ouyang, W.; Liu, X.T.; et al. Insights into the spatiotemporal occurrence and mixture risk assessment of household and personal care products in the waters from rivers to Laizhou Bay, southern Bohai Sea. Sci. Total Environ. 2022, 810, 152290. [Google Scholar] [CrossRef]
- Xie, J.H.; Zhao, N.; Zhang, Y.Y.; Hu, H.M.; Zhao, M.R.; Jin, H.B. Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from East China Sea. Chemosphere 2022, 287, 132218. [Google Scholar] [CrossRef]
- Liu, X.Y.; Tu, M.C.; Wang, S.P.; Wang, Y.Z.; Wang, J.; Hou, Y.; Zheng, X.; Yan, Z.G. Research on freshwater water quality criteria, sediment quality criteria and ecological risk assessment of triclosan in China. Sci. Total Environ. 2022, 816, 151616. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Narayanan, N.; Singh, N.; Gupta, S. Occurrence of endocrine disrupting chemicals (EDCs) in river water, ground water and agricultural soils of India. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Chafi, S.; Azzouz, A.; Ballesteros, E. Occurrence and distribution of endocrine disrupting chemicals and pharmaceuticals in the river Bouregreg (Rabat, Morocco). Chemosphere 2021, 287, 132202. [Google Scholar] [CrossRef]
- Kachhawaha, A.S.; Nagarnaik, P.M.; Labhasetwar, P.K.; Banerjee, K. Pharmaceuticals and personal care products in aqueous urban environment of western India. Water Environ. J. 2021, 35, 1302–1312. [Google Scholar] [CrossRef]
- Gopal, C.M.; Bhat, K.; Ramaswamy, B.R.; Kumar, V.; Singhal, R.K.; Basu, H.; Udayashankar, H.N.; Vasantharaju, S.G.; Praveenkumarreddy, Y.; Shailesh; et al. Seasonal occurrence and risk assessment of pharmaceutical and personal care products in Bengaluru rivers and lakes, India. J. Environ. Chem. Eng. 2021, 9, 105610. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, H.H.; Li, L.; Ren, M.; Qie, H.T.; Lin, A.J. A review of distribution and risk of pharmaceuticals and personal care products in the aquatic environment in China. Ecotoxicol. Environ. Saf. 2021, 213, 112044. [Google Scholar] [CrossRef]
- Lalonde, B.; Garron, C.; Dove, A.; Struger, J.; Farmer, K.; Sekela, M.; Gledhill, M.; Backus, S. Investigation of spatial distributions and temporal trends of triclosan in Canadian surface waters. Arch. Environ. Contam. Toxicol. 2019, 76, 231–245. [Google Scholar] [CrossRef]
- Ma, X.Q.; Wan, Y.J.; Wu, M.Y.; Xu, Y.; Xu, Q.; He, Z.Y.; Xia, W. Occurrence of benzophenones, parabens and triclosan in the Yangtze River of China, and the implications for human exposure. Chemosphere 2018, 213, 517–525. [Google Scholar] [CrossRef]
- Esteban, S.; Gorga, M.; Petrovic, M.; Gonzalez-Alonso, S.; Barcelo, D.; Valcarcel, Y. Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci. Total Environ. 2014, 466–467, 939–951. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Muthwa, S.F.; Chimuka, L. Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high performance liquid chromatography. S. Afr. J. Chem. 2014, 67, 143–150. [Google Scholar]
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-residue analysis of 90 emerging contaminants in liquid and solid environmental matrices by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1431, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Arias, C.A.; Nguyen, L.X.; Salvado, V.; Brix, H. Occurrence and behavior of emerging contaminants in surface water and a restored wetland. Chemosphere 2012, 88, 1083–1089. [Google Scholar] [CrossRef]
- Rusiniak, P.; Kmiecik, E.; Wator, K.; Duda, R.; Bugno, R. Pharmaceuticals and personal care products in the urban groundwater—Preliminary monitoring (case study: Krakow, Southern Poland). Urban Water J. 2021, 18, 364–374. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Occurrence of multiclass endocrine disrupting compounds in a drinking water supply system and associated risks. Sci. Rep. 2020, 10, 17755. [Google Scholar] [CrossRef]
- Li, X.; Ying, G.G.; Su, H.C.; Yang, X.B.; Wang, L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ. Int. 2010, 36, 557–562. [Google Scholar] [CrossRef]
- Tang, N.; Fan, P.P.; Yu, X.G.; Ma, R.; Tao, Y.X.; Wang, W.Y.; Ouyang, F.X. Effects of long-term triclosan exposure on microbiota in zebrafish. Front. Microbiol. 2021, 12, 604313. [Google Scholar] [CrossRef] [PubMed]
- Rüdela, H.; Böhmera, W.; Müller, M. Retrospective study of triclosan and methyl-triclosan residues in fish and suspended particulate matter: Results from the German Environmental Specimen Bank. Chemosphere 2013, 91, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Liang, B.; Kong, D.; Li, X.K.; Wang, A.J. Fate, risk and removal of triclocarban: A critical review. J. Hazard. Mater. 2020, 387, 121944. [Google Scholar] [CrossRef]
- Sherburne, J.J.; Anaya, A.M.; Fernie, K.J.; Forbey, J.S.; Furlong, E.T.; Kolpin, D.W.; Dufty, A.M.; Kinney, C.A. Occurrence of triclocarban and triclosan in an agro-ecosystem following application of biosolids. Environ. Sci. Technol. 2016, 50, 13206–13214. [Google Scholar] [CrossRef]
- Iyer, A.P.; Xue, J.C.; Honda, M.; Robinson, M.; Kumosani, T.A.; Abulnaja, K.; Kannan, K. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ. Res. 2018, 160, 91–96. [Google Scholar] [CrossRef]
- Ren, H.; Li, H.B.; Wang, H.X.; Huang, H.; Lu, Z.M. Biodegradation of tetrahydrofuran by the newly isolated filamentous fungus Pseudallescheria boydii ZM01. Microorganisms 2020, 8, 1190. [Google Scholar] [CrossRef]
- Huang, H.; Yu, H.X.; Qi, M.B.; Liu, Z.B.; Wang, H.X.; Lu, Z.M. Enrichment and characterization of a highly efficient tetrahydrofuran-degrading bacterial culture. Biodegradation 2019, 30, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.G.; Dees, P.M.; Sayler, G.S. Growth of a bacterial consortium on triclosan. FEMS Microbiol. Ecol. 2001, 36, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sachan, S.G.; Sachan, A. Exploring triclosan degradation potential of Citrobacter freundii KS2003. Int. J. Environ. Sci. Technol. 2022, 19, 3565–3580. [Google Scholar] [CrossRef]
- Wang, L.; Xu, S.N.; Pan, B.; Yang, Y. Emerging investigator series: Dual role of organic matter in the anaerobic degradation of triclosan. Environ. Sci. Processes Impacts 2017, 19, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sachan, S.G. Bioconversion of toxic micropollutant triclosan to 2,4-dichlorophenol using a wastewater isolate Pseudomonas aeruginosa KS2002. Int. J. Environ. Sci. Technol. 2019, 16, 7663–7672. [Google Scholar] [CrossRef]
- Meade, M.J.; Waddell, R.L.; Callahan, T.M. Soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp. denitrificans inactivate triclosan in liquid and solid substrates. FEMS Microbiol. Lett. 2001, 204, 45–48. [Google Scholar] [CrossRef]
- Devatha, C.P.; Pavithra, N. Isolation and identification of Pseudomonas from wastewater, its immobilization in cellulose biopolymer and performance in degrading Triclosan. J. Environ. Manag. 2019, 232, 584–591. [Google Scholar] [CrossRef]
- Wang, S.Z.; Yin, Y.A.; Wang, J.L. Microbial degradation of triclosan by a novel strain of Dyella sp. Appl. Microbiol. Biotechnol. 2018, 102, 1997–2006. [Google Scholar] [CrossRef]
- Zhao, F.M. Biodegradation of Triclosan by a Triclosan-Degrading Isolate and an Ammonia-Oxidizing Bacterium. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2006. [Google Scholar]
- Lee, D.G.; Zhao, F.M.; Rezenom, Y.H.; Russell, D.H.; Chu, K.H. Biodegradation of triclosan by a wastewater microorganism. Water Res. 2012, 46, 4226–4234. [Google Scholar] [CrossRef]
- Kim, Y.M.; Murugesan, K.; Schmidt, S.; Bokare, V.; Jeon, J.R.; Kim, E.J.; Chang, Y.S. Triclosan susceptibility and co-metabolism—A comparison for three aerobic pollutant-degrading bacteria. Bioresour. Technol. 2011, 102, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Mulla, S.I.; Hu, A.Y.; Wang, Y.W.; Sun, Q.; Huang, S.L.; Wang, H.; Yu, C.P. Degradation of triclocarban by a triclosan-degrading Sphingomonas sp. strain YL-JM2C. Chemosphere 2016, 144, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Mulla, S.I.; Wang, H.; Sun, Q.; Hu, A.Y.; Yu, C.P. Characterization of triclosan metabolism in Sphingomonas sp. strain YL-JM2C. Sci. Rep. 2016, 6, 21965. [Google Scholar] [CrossRef] [PubMed]
- Jayalatha, N.A.; Devatha, C.P. Degradation of triclosan from domestic wastewater by biosurfactant produced from Bacillus licheniformis. Mol. Biotechnol. 2019, 61, 674–680. [Google Scholar] [CrossRef]
- Lee, D.G.; Chu, K.H. Effects of growth substrate on triclosan biodegradation potential of oxygenase-expressing bacteria. Chemosphere 2013, 93, 1904–1911. [Google Scholar] [CrossRef]
- Tastan, B.E.; Ozdemir, C.; Tekinay, T. Effects of different culture media on biodegradation of triclosan by Rhodotorula mucilaginosa and Penicillium sp. Water Sci. Technol. 2016, 74, 473–481. [Google Scholar] [CrossRef]
- Garcia, A.M.; Carlo, P.P.; Mendez, M.O. Draft genome sequence of Paenibacillus sp. strain OT2-17, a triclosan-degrading rhizobacterium. Microbiol. Resour. Announc. 2020, 9, e01596-19. [Google Scholar] [CrossRef]
- Lopez, C.; Nnorom, M.A.; Tsang, Y.F.; Knapp, C.W. Pharmaceuticals and personal care products’ (PPCPs) impact on enriched nitrifying cultures. Environ. Sci. Pollut. Res. 2021, 28, 60968–60980. [Google Scholar] [CrossRef]
- Wang, X.J.; Wu, H.; Dai, C.H.; Wang, X.Y.; Wang, L.J.; Xu, J.M.; Lu, Z.M. Microbial interactions enhanced environmental fitness and expanded ecological niches under dibutyl phthalate and cadmium co-contamination. Environ. Pollut. 2022, 306, 119362. [Google Scholar] [CrossRef]
- Yu, K.; Yi, S.; Li, B.; Guo, F.; Peng, X.X.; Wang, Z.P.; Wu, Y.; Alvarez-Cohen, L.; Zhang, T. An integrated meta-omics approach reveals substrates involved in synergistic interactions in a bisphenol A (BPA)-degrading microbial community. Microbiome 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Romero, I.; van Dillewijn, P.; Nesme, J.; Sorensen, S.J.; Nogales, R.; Delgado-Moreno, L.; Romero, E. A novel and affordable bioaugmentation strategy with microbial extracts to accelerate the biodegradation of emerging contaminants in different media. Sci. Total Environ. 2022, 834, 155234. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.H.; Gao, J.F.; Li, D.C.; Wang, Z.Q.; Cui, Y.C.; Zhao, Y.F. Family Sphingomonadaceae as the key executor of triclosan degradation in both nitrification and denitrification systems. Chem. Eng. J. 2022, 442, 136202. [Google Scholar] [CrossRef]
- Dai, H.H.; Gao, J.F.; Li, D.C.; Wang, Z.Q.; Duan, W.J. DNA-based stable isotope probing deciphered the active denitrifying bacteria and triclosan-degrading bacteria participating in granule-based partial denitrification process under triclosan pressure. Water Res. 2021, 210, 118011. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Gao, J.F.; Zhang, W.Z.; Wang, Z.Q.; Cui, Y.C.; Dai, H.H.; Li, D.C.; Zhang, Y. Robustness of the partial nitrification-anammox system exposing to triclosan wastewater: Stress relieved by extracellular polymeric substances and resistance genes. Environ. Res. 2021, 206, 112606. [Google Scholar] [CrossRef]
- Navrozidou, E.; Remmas, N.; Melidis, P.; Sylaios, G.; Ntougias, S. Biotreatment efficiency, degradation mechanism and bacterial community structure in an immobilized cell bioreactor treating triclosan-rich wastewater. Environ. Technol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.H.; Gao, J.F.; Li, D.C.; Wang, Z.Q.; Duan, W.J. Metagenomics combined with DNA-based stable isotope probing provide comprehensive insights of active triclosan-degrading bacteria in wastewater treatment. J. Hazard. Mater. 2021, 404, 124192. [Google Scholar] [CrossRef]
- Tan, Q.Y.; Chen, J.M.; Chu, Y.F.; Liu, W.; Yang, L.L.; Ma, L.; Zhang, Y.; Qiu, D.R.; Wu, Z.B.; He, F. Triclosan weakens the nitrification process of activated sludge and increases the risk of the spread of antibiotic resistance genes. J. Hazard. Mater. 2021, 416, 126085. [Google Scholar] [CrossRef]
- Granatto, C.F.; Grosseli, G.M.; Sakamoto, I.K.; Fadini, P.S.; Varesche, M.B.A. Influence of metabolic cosubstrates on methanogenic potential and degradation of triclosan and propranolol in sanitary sewage. Environ. Res. 2021, 199, 111220. [Google Scholar] [CrossRef]
- Dong, X.Q.; He, Y.Z.; Peng, X.X.; Jia, X.S. Triclosan in contact with activated sludge and its impact on phosphate removal and microbial community. Bioresour. Technol. 2021, 319, 124134. [Google Scholar] [CrossRef]
- Dai, H.H.; Gao, J.F.; Wang, S.J.; Li, D.C.; Wang, Z.Q. The key active degrader, metabolic pathway and microbial ecology of triclosan biodegradation in an anoxic/oxic system. Bioresour. Technol. 2020, 317, 124014. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, F.; Truper, H.G.; Klein, J.; Schacht, S. Degradation of diphenylether by Pseudomonas cepacia Et4: Enzymatic release of phenol from 2,3-dihydroxydiphenylether. Arch. Microbiol. 1993, 159, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kagle, J.M.; Paxson, C.; Johnstone, P. Identification of a gene cluster associated with triclosan catabolism. Biodegradation 2015, 26, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Y.; Rogers, M.J.; He, J.Z. Abundance of organohalide respiring bacteria and their role in dehalogenating antimicrobials in wastewater treatment plants. Water Res. 2020, 181, 115893. [Google Scholar] [CrossRef]
- Mason, J.R.; Cammack, R. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu. Rev. Microbiol. 1992, 46, 277–305. [Google Scholar] [CrossRef] [PubMed]
- Bünz, P.V.; Cook, A.M. Dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1: Angular dioxygenation by a three-component enzyme system. J. Bacteriol. 1993, 175, 6467–6475. [Google Scholar] [CrossRef]
- Batie, C.J.; LaHaie, E.; Ballou, D.P. Purification and characterization of phthalate oxygenase and phthalate oxygenase reductase from Pseudomonas cepacia. J. Biol. Chem. 1987, 262, 1510–1518. [Google Scholar] [CrossRef]
- Kasuga, K.; Nitta, A.; Kobayashi, M.; Habe, H.; Nojiri, H.; Yamane, H.; Omori, T.; Kojima, I. Cloning of dfdA genes from Terrabacter sp. strain DBF63 encoding dibenzofuran 4,4a-dioxygenase and heterologous expression in Streptomyces lividans. Appl. Microbiol. Biotechnol. 2013, 97, 4485–4498. [Google Scholar] [CrossRef]
- Ohmori, T.; Morita, H.; Tanaka, M.; Tomoi, M.; Miyauchi, K.; Kasai, D.; Furukawa, K.; Masai, E.; Fukuda, E. Expression in Escherichia coli of biphenyl 2,3-dioxygenase genes from a gram-positive polychlorinated biphenyl degrader, Rhodococcus jostii RHA1. Biosci. Biotechnol. Biochem. 2011, 75, 26–33. [Google Scholar] [CrossRef]
- Li, J.D.; Min, J.; Wang, Y.; Chen, W.W.; Kong, Y.C.; Guo, T.Y.; Mahto, J.K.; Sylvestre, M.; Hu, X.K. Engineering Burkholderia xenovorans LB400 BphA through site-directed mutagenesis at position 283. Appl. Environ. Microbiol. 2020, 86, e01040-20. [Google Scholar] [CrossRef]



| Country | Name of the WWTP | Processing Technology | Concentration of TCS in Wastewater | Concentration of TCS in Sludge | Reference | ||
|---|---|---|---|---|---|---|---|
| Influent | Treated Effluent | Removal Rate/% | |||||
| China | Northern China WWTP | Anoxic-aerobic (A/O) | 295 ± 4.2 ng/L | 39 ± 2.7 ng/L | 86.77 | 1801 ng/g | [38] |
| Brazil | WWTP A | Activated sludge (AS) | 1.30 ± 0.22 μg/L | 0.55 ± 0.02 μg/L | 57.69% | 0.94 μg/L | [39] |
| WWTP B | Upflow anaerobic sludge blanket | 1.26 ± 0.09 μg/L | 0.78 ± 0.05 μg/L | 38.10% | 2.79 μg/L | ||
| WWTP C | Waste stabilization pond | 1.42 ± 0.04 μg/L | 0.39 ± 0.02 μg/L | 72.54% | 0.53 μg/L | ||
| Chile | WWTP | AS and a pilot plant of horizontal subsurface flow | 0.20 ± 0.06 μg/L | 0.02 ± 0.01 μg/L | 90.00% | 0.01 ± 0.01 μg/L | [40] |
| India | WWTP 1 | AS | N.A. 1 | N.A. 1 | 39–62% | N.A. 1 | [41] |
| WWTP 2 | 45–55% | ||||||
| China | WWTP#1 | A/O | 59–1100 ng/L Mean 274 ng/L | 13–110 ng/L Mean 83 ng/L | 69.71% | N.A. 1 | [42] |
| WWTP#2 | Hydrolytic acidification and cyclic activated sludge technology | 230–2900 ng/L Mean 389 ng/L | 9–180 ng/L Mean 17 ng/L | 95.63% | |||
| Environment | Method | TCS Concentration | Country | Year of the TCS Determination | Reference |
|---|---|---|---|---|---|
| River water | Liquid chromatography-tandem mass spectrometry (LC-MS/MS) | N.D. 1 −62.124 µg/L | India | 2019–2020 | [52] |
| River water | LC-MS/MS | N.D. 1 −135 ng/L Mean 25.4 ng/L | China | 2018, 2019 and 2021 | [49] |
| River water | Gas chromatography-mass spectrometer (GC-MS) | 0.06–500 ng/L Mean 176.2 ng/L | Morocco | 2019 | [53] |
| River water | LC-MS/MS | Up to 74.3 µg/L | India | / 2 | [54] |
| River water | High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) | N.D. 1 −1761 ng/L Mean 942 ng/L in monsoon season | India | 2018–2019 | [55] |
| River water | Literature data collection | N.D. 1 −293.64 ng/L | China | 2010–2019 | [56] |
| River water | LC-MS/MS | 0.69–17.5 ng/L | China | 2019 | [48] |
| River water | LC-MS/MS | 5.1–874 ng/L Mean 0.06 nM | Canada | 2012–2013 | [57] |
| River water | HPLC-MS/MS | N.D. −65.6 ng/L Mean 0.02 nM | China | 2015 | [58] |
| River water | LC-LC-MS/MS | N.D. 1 −0.77 nM | Spain | 2012 | [59] |
| River water | High performance liquid chromatography with photo diode array detection | N.D. 1 −3.87 nM | South Africa | / 2 | [60] |
| River water | HPLC-MS/MS | 0.349 ± 0.032 nM | UK | / 2 | [61] |
| River water | GC-MS | 0.01–0.207 nM | Denmark | 2010 | [62] |
| Sea water | LC-MS/MS | N.D. 1 −58.3 ng/L Mean 22.3 ng/L | China | 2018, 2019 and 2021 | [49] |
| Sea water | Ultra-performance liquid chromatography coupled to a triple quadrupole mass spectrometry | N.D. 1 −8.7 ng/L Mean 4.2 ng/L | China | 2019 | [50] |
| Underground water | LC-MS/MS | 0.5–13.1 ng/L Mean 2.9 µg/L | Poland | 2019 | [63] |
| Drinking water | LC-MS | Up to 9.74 ng/L | Malaysia | 2018 | [64] |
| Drinking water | GC-MS | 0.6–9.7 ng/L | China | / 2 | [65] |
| Environmental Matrices | TCS Initial Concentration | Potential TCS-Degrading Taxa | Community Analysis Approaches | Reference |
|---|---|---|---|---|
| Bioreactor | 20 μg/g | Flavobacterium, Thermomicrobia, Nonomuraea and Fluviicola | 16S rRNA amplicon sequencing and metagenomic sequencing | [92] |
| Nitrifying sequencing batch reactors (SBRs) and denitrifying SBRs | 4 mg/L | Sphingomonadaceae | DNA stable isotope detection (DNA-SIP), 16S rRNA amplicon sequencing and oligotyping analysis | [93] |
| SBR | 3 mg/L | Thauera | DNA-SIP and 16S rRNA amplicon sequencing | [94] |
| Partial nitrification-anammox process | 0.5 mg/L | Alicycliphilus and Sphingopyxis | DNA-SIP and 16S rRNA amplicon sequencing | [95] |
| Immobilized cell bioreactor | 400 mg/L | Bradyrhizobiaceae, Ferruginibacter, Thermomonas, Lysobacter and Gordonia | 16S rRNA amplicon sequencing | [96] |
| Sludge | 4 mg/L | Sphingobium | DNA-SIP, 16S rRNA amplicon sequencing, oligotyping analysis and metagenomic sequencing | [97] |
| SBR | 100 μg/L | Flavobacterium | 16S rRNA amplicon sequencing | [98] |
| Sludge and wastewater | 5.8 g/L | Chloroflexi, Smithella and Pseudomonas | 16S rRNA amplicon sequencing | [99] |
| SBR | 100 μg/L | Unidentified Xanthomonadaceae, unidentified Rhizobiales, Lacibacter, and Dechloromonas | 16S rRNA amplicon sequencing and fluorescent in situ hybridization | [100] |
| A lab-scale A/O System | 2, 4, 6 and 8 mg/L | Candidatus Microthrix, Flavobacterium, Thiothrix | DNA-SIP and 16S rRNA amplicon sequencing | [101] |
| AS | 500 mg/L | Sphingomonas | Genomic fingerprinting, Ribosomal intergenic spacer analysis (RISA) | [73] |
| Classification | Enzyme | Method of Identification | Strain | Catabolic Pathways | Reference | |
|---|---|---|---|---|---|---|
| Specific enzyme | Oxygenase | TCS oxygenase (TcsA) | Constructing TCS-degrading fosmid clone | Sphingomonas sp. RD1 | Converting TCS into 2,4-dichlorophenol and 4-chlorocatechol | [103] |
| Non-specific enzyme | Dioxygenase | Catechol 2,3-dioxygenase (C23O) | Adding inhibitors of C23O; detecting enzyme activity | Sphingopyxis sp. KCY1 | Meta-cleavage pathway | [81] |
| Adding inhibitors of C23O; detecting enzyme activity | Pseudomonas aeruginosa KS2002 | [76] | ||||
| C12O | Adding inhibitors of C12O; detecting enzyme activity | Citrobacter freundii KS2003 | Ortho-cleavage pathway | [74] | ||
| Monooxygenase | Propane monooxygenase (PMO) | Adding inhibitors of PMO | Mycobacterium vaccae JOB5 | / 1 | [86] | |
| Alkane monooxygenase (AlkMO) | Adding inhibitors of AlkMO | Rhodococcus jostii RHA1 | / 1 | |||
| AMO | Adding inhibitors of AMO | Nitrosomonas europaea ATCC 19178 | / 1 | [1] | ||
| Peroxidase | Manganese peroxidase (MnP) | Detecting enzyme activity | Providencia rettgeri MB-IIT | Ether bond cleavage | [18] | |
| LAC | LAC | Detecting enzyme activity | Transferring molecular oxygen | |||
| Reductive dehalogenase | Reductive dehalogenase (PcbA1) | Proteome; detecting enzyme activity in vitro | Dehalococcoides mccartyi CG1 | Dechlorination (convert TCS into diclosan) | [104] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Wu, H.; Jiang, Z.; Jiang, J.; Lu, Z. Degradation of Triclosan in the Water Environment by Microorganisms: A Review. Microorganisms 2022, 10, 1713. https://doi.org/10.3390/microorganisms10091713
Yin Y, Wu H, Jiang Z, Jiang J, Lu Z. Degradation of Triclosan in the Water Environment by Microorganisms: A Review. Microorganisms. 2022; 10(9):1713. https://doi.org/10.3390/microorganisms10091713
Chicago/Turabian StyleYin, Yiran, Hao Wu, Zhenghai Jiang, Jingwei Jiang, and Zhenmei Lu. 2022. "Degradation of Triclosan in the Water Environment by Microorganisms: A Review" Microorganisms 10, no. 9: 1713. https://doi.org/10.3390/microorganisms10091713






