Acaricidal Activity and Field Efficacy Analysis of the Potential Biocontrol Agent Bacillus vallismortis NBIF-001 against Spider Mites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Acaricidal Assays
2.3. Optimisation of NBIF-001 Cultivation Conditions
2.4. Field Efficacy Trials of NBIF-001 Agents on Citrus
2.5. Whole-Genome Sequence Analysis and Annotation
2.6. Crude Protein Extraction from the Fermentation Broth Supernatants of NBIF-001
2.7. Protein Mass Spectrometry
2.8. Heterologous Expression of Potential Acaricidal Proteins from NBIF-001
2.9. Statistical Analysis
3. Results
3.1. Isolation of the Strain NBIF-001 That Exhibits Acaricidal Activity against Spider Mites
3.2. Raising Fermentation Level Increased Acaricidal Activity
3.3. Field Efficacy of NBIF-001 Wettable Powder against P. citri
3.4. Genomic Analysis and Special Features Suggesting Putative Acaricidal Compounds
3.5. Proteins in the Fermentation Broth Supernatants of NBIF-001 Show Toxicity against Spider Mites
3.6. Proteins of NBIF-001 Are Novel Acaricidal Compound against Spider Mites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldfield, G.N.; Proeseler, G. 1.4.9 Eriophyoid mites as vectors of plant pathogens. In World Crop Pests; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 6, pp. 259–275. [Google Scholar]
- Rincon, R.A.; Rodriguez, D.; Coy-Barrera, E. Botanicals Against Tetranychus urticae Koch Under Laboratory Conditions: A Survey of Alternatives for Controlling Pest Mites. Plants 2019, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Liang, X.; Lu, H.; Li, Q.; Chen, Q.; Zhang, P.; Li, K.; Liu, G.; Yan, W.; Song, J.; et al. Overproduction of superoxide dismutase and catalase confers cassava resistance to Tetranychus cinnabarinus. Sci. Rep. 2017, 7, 40179. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.J.; Tian, M.Y.; Pang, X.F.; Rae, D.J. Repellency, antifeeding effect and toxicity of a horticultural mineral oil against citrus red mite. In Spray Oils Beyond 2000; Beattie, G.A.C., Watson, D.M., Stevens, M.L., Eds.; University of Western Sydney: Sydney, NSW, Australia, 2002; pp. 134–141. [Google Scholar]
- Herron, G.A.; Langfield, K.L.; Chen, Y.; Wilson, L.J. Development of abamectin resistance in Tetranychus urticae in Australian cotton and the establishment of discriminating doses for T. lambi. Exp. Appl. Acarol. 2021, 83, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, D.; Zhang, Y.; Wu, Q.; Xie, W.; Guo, Z.; Wang, S. Frequencies and mechanisms of pesticide resistance in Tetranychus urticae field populations in China. Insect Sci. 2022, 29, 827–839. [Google Scholar] [CrossRef]
- Kai, Z.; Tuizi, F.; Chao, X.; Zhao, Z. Top design and progress in research and development of synthesis technique for reduction and synergy of chemical fertilizers and pesticides in China. J. Plant Prot. 2019, 46, 943–953. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Roskam, M.M.; Timmer, R. Commercial Mass Production and Pricing of Organisms for Biological Control of Pests in Europe. Biol. Control. 1997, 10, 143–149. [Google Scholar] [CrossRef]
- Tsuchida, Y.; Masui, S. Biological control of the Japanese pear rust mite, Eriophyes chibaensis (Acari: Eriophyidae) and the Kanzawa spider mite, Tetranychus kanzawai (Acari: Tetranychidae) with Euseius sojaensis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2021, 84, 673–686. [Google Scholar] [CrossRef]
- Clifton, E.H.; Hajek, A.E.; Jenkins, N.E.; Roush, R.T.; Rost, J.P.; Biddinger, D.J. Applications of Beauveria bassiana (Hypocreales: Cordycipitaceae) to Control Populations of Spotted Lanternfly (Hemiptera: Fulgoridae), in Semi-Natural Landscapes and on Grapevines. Environ. Entomol. 2020, 49, 854–864. [Google Scholar] [CrossRef]
- Wekesa, V.W.; Maniania, N.K.; Knapp, M.; Boga, H.I. Pathogenicity of Beauveria bassiana and Metarhizium anisopliae to the tobacco spider mite Tetranychus evansi. Exp. Appl. Acarol. 2005, 36, 41–50. [Google Scholar] [CrossRef]
- Kheradmand, K.; Heidari, M.; Sedaratian-Jahromi, A.; Talaei-Hassanloui, R.; Havasi, M. Biological responses of Tetranychus urticae (Acari: Tetranychidae) to sub-lethal concentrations of the entomopathogenic fungus Beauveria bassiana. Bull. Entomol. Res. 2021, 112, 70–77. [Google Scholar] [CrossRef]
- Xue, Y.; Meats, A.; Beattie, G.A.; Spooner-Hart, R.; Herron, G.A. The influence of sublethal deposits of agricultural mineral oil on the functional and numerical responses of Phytoseiulus persimilis (Acari: Phytoseiidae) to its prey, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2009, 48, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Chalivendra, S. Microbial Toxins in Insect and Nematode Pest Biocontrol. Int. J. Mol. Sci. 2021, 22, 7657. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, D.; Xiong, Q.; Bao, B.; Zhang, W.; Dai, T.; Zhao, Y.; Borriss, R.; Fan, B. The Plant-Beneficial Rhizobacterium Bacillus velezensis FZB42 Controls the Soybean Pathogen Phytophthora sojae Due to Bacilysin Production. Appl. Environ. Microbiol. 2021, 87, e0160121. [Google Scholar] [CrossRef] [PubMed]
- van Frankenhuyzen, K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 2009, 101, 1–16. [Google Scholar] [CrossRef]
- Guo, Y.; Weng, M.; Sun, Y.; Carballar-Lejarazu, R.; Wu, S.; Lian, C. Bacillus thuringiensis toxins with nematocidal activity against the pinewood nematode Bursaphelenchus xylophilus. J. Invertebr. Pathol. 2022, 189, 107726. [Google Scholar] [CrossRef]
- Yu, Z.; Xiong, J.; Zhou, Q.; Luo, H.; Hu, S.; Xia, L.; Sun, M.; Li, L.; Yu, Z. The diverse nematicidal properties and biocontrol efficacy of Bacillus thuringiensis Cry6A against the root-knot nematode Meloidogyne hapla. J. Invertebr. Pathol. 2015, 125, 73–80. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, Y.H.; Yang, J.; Cui, W.Y.; He, P.J.; Munir, S.; He, P.F.; Wu, Y.X.; He, Y.Q. Acaricidal Activity of Cyclodipeptides from Bacillus amyloliquefaciens W1 against Tetranychus urticae. J. Agric. Food Chem. 2018, 66, 10163–10168. [Google Scholar] [CrossRef]
- Li, X.; Munir, S.; Xu, Y.; Wang, Y.; He, Y. Combined mass spectrometry-guided genome mining and virtual screening for acaricidal activity in secondary metabolites of Bacillus velezensis W1. RSC Adv. 2021, 11, 25441–25449. [Google Scholar] [CrossRef]
- Liu, X.; Min, Y.; Huang, D.; Zhou, R.; Fang, W.; Liu, C.; Rao, B.; Zhang, G.; Wang, K.; Yang, Z. Complete Genome Sequence of Bacillus vallismortis NBIF-001, a Novel Strain from Shangri-La, China, That Has High Activity against Fusarium oxysporum. Genome Announc. 2017, 5, e01305–e01317. [Google Scholar] [CrossRef]
- NY/T 1154.12-2008; Guideline for Laboratory Bioassay of Pesticides Part 12: Slide-Dip Method Im-Mersion. Institute for the Control of Agrochemicals: Beijing, China, 2018. (In Chinese)
- GB/T 1798011-2000; Pesticide-Guidelines for the Field Efficacy Trials (I)-Acaricides Against Spider Mites on Citrus. Institute for the Control of Agrochemicals: Beijing, China, 2000. (In Chinese)
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Galperin, M.Y.; Wolf, Y.I.; Makarova, K.S.; Vera Alvarez, R.; Landsman, D.; Koonin, E.V. COG database update: Focus on microbial diversity, model organisms, and widespread pathogens. Nucleic Acids Res. 2021, 49, D274–D281. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Burgess, R.R. Refolding solubilized inclusion body proteins. Methods Enzymol. 2009, 463, 259–282. [Google Scholar] [CrossRef]
- Liu, G.; Feng, K.; Guo, D.; Li, R. Overexpression and activities of 1-Cys peroxiredoxin from Pseudomonas fluorescens GcM5-1A carried by pine wood nematode. Folia Microbiol. 2015, 60, 443–450. [Google Scholar] [CrossRef]
- Nielsen-LeRoux, C.; Gaudriault, S.; Ramarao, N.; Lereclus, D.; Givaudan, A. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr. Opin. Microbiol. 2012, 15, 220–231. [Google Scholar] [CrossRef]
- Jeschke, P. Status and outlook for acaricide and insecticide discovery. Pest. Manag. Sci. 2021, 77, 64–76. [Google Scholar] [CrossRef]
- Koo, H.N.; Choi, J.; Shin, E.; Kang, W.; Cho, S.R.; Kim, H.; Park, B.; Kim, G.H. Susceptibility to Acaricides and the Frequencies of Point Mutations in Etoxazole- and Pyridaben-Resistant Strains and Field Populations of the Two-Spotted Spider Mite, Tetranychus urticae (Acari: Tetranychidae). Insects 2021, 12, 660. [Google Scholar] [CrossRef]
- Sun, J.; Li, C.; Jiang, J.; Song, C.; Wang, C.; Feng, K.; Wei, P.; He, L. Cross resistance, inheritance and fitness advantage of cyetpyrafen resistance in two-spotted spider mite, Tetranychus urticae. Pestic. Biochem. Physiol. 2022, 183, 105062. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Dermauw, W.; Grbic, M.; Tirry, L.; Feyereisen, R. Spider mite control and resistance management: Does a genome help? Pest. Manag. Sci. 2013, 69, 156–159. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lin, Y.H.; Yang, C.M.; Atlihan, R.; Saska, P.; Chi, H. Survival and Reproductive Strategies in Two-Spotted Spider Mites: Demographic Analysis of Arrhenotokous Parthenogenesis of Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2016, 109, 502–509. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Tirry, L. Mechanisms of Acaricide Resistance in the Two-Spotted Spider Mite Tetranychus urticae. In Biorational Control of Arthropod Pests: Application and Resistance Management; Ishaaya, I., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 347–393. [Google Scholar]
- Feyereisen, R.; Dermauw, W.; Van Leeuwen, T. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic. Biochem. Physiol. 2015, 121, 61–77. [Google Scholar] [CrossRef]
- Herron, G.A.; Rophail, J.; Holloway, J.; Barchia, I. Potentiation of a propargite and fenpyroximate mixture against two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2003, 29, 115–119. [Google Scholar] [CrossRef]
- Luo, Y.J.; Yang, Z.G.; Xie, D.Y.; Ding, W.; Da, A.S.; Ni, J.; Chai, J.P.; Huang, P.; Jiang, X.J.; Li, S.X. Molecular cloning and expression of glutathione S-transferases involved in propargite resistance of the carmine spider mite, Tetranychus cinnabarinus (Boisduval). Pestic. Biochem. Physiol. 2014, 114, 44–51. [Google Scholar] [CrossRef]
- Wang, Z.; Cang, T.; Wu, S.; Wang, X.; Qi, P.; Wang, X.; Zhao, X. Screening for suitable chemical acaricides against two-spotted spider mites, Tetranychus urticae, on greenhouse strawberries in China. Ecotoxicol. Environ. Saf. 2018, 163, 63–68. [Google Scholar] [CrossRef]
- Lasota, J.A.; Dybas, R.A. Abamectin as a pesticide for agricultural use. Acta Leiden 1990, 59, 217–225. [Google Scholar]
- Bergh, J.C.; Rugg, D.; Jansson, R.K.; McCoy, C.W.; Robertson, J.L. Monitoring the susceptibility of citrus rust mite (Acari: Eriophyidae) populations to abamectin. J. Econ. Entomol. 1999, 92, 781–787. [Google Scholar] [CrossRef]
- Dermauw, W.; Ilias, A.; Riga, M.; Tsagkarakou, A.; Grbic, M.; Tirry, L.; Van Leeuwen, T.; Vontas, J. The cys-loop ligand-gated ion channel gene family of Tetranychus urticae: Implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochem. Mol. Biol. 2012, 42, 455–465. [Google Scholar] [CrossRef]
- Ouyang, J.; Tian, Y.; Jiang, C.; Yang, Q.; Wang, H.; Li, Q. Laboratory assays on the effects of a novel acaricide, SYP-9625 on Tetranychus cinnabarinus (Boisduval) and its natural enemy, Neoseiulus californicus (McGregor). PLoS ONE 2018, 13, e0199269. [Google Scholar] [CrossRef]
- Chen, J.C.; Gong, Y.J.; Shi, P.; Wang, Z.H.; Cao, L.J.; Wang, P.; Wei, S.J. Field-evolved resistance and cross-resistance of the two-spotted spider mite, Tetranychus urticae, to bifenazate, cyenopyrafen and SYP-9625. Exp. Appl. Acarol. 2019, 77, 545–554. [Google Scholar] [CrossRef] [PubMed]
- van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Wu, L.; Huo, X.; Zhou, X.; Zhao, D.; He, W.; Liu, S.; Liu, H.; Feng, T.; Wang, C. Acaricidal Activity and Synergistic Effect of Thyme Oil Constituents against Carmine Spider Mite (Tetranychus Cinnabarinus (Boisduval)). Molecules 2017, 22, 1873. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.P.; Venzon, M.; das Dores, R.G.R.; Franzin, M.L.; Martins, E.F.; de Araujo, G.J.; Fonseca, M.C.M. Toxicity of Varronia curassavica Jacq. Essential Oil to Two Arthropod Pests and Their Natural Enemy. Neotrop. Entomol. 2021, 50, 835–845. [Google Scholar] [CrossRef]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef]
- Rodriguez, M.; Marin, A.; Torres, M.; Bejar, V.; Campos, M.; Sampedro, I. Aphicidal Activity of Surfactants Produced by Bacillus atrophaeus L193. Front. Microbiol. 2018, 9, 3114. [Google Scholar] [CrossRef] [Green Version]
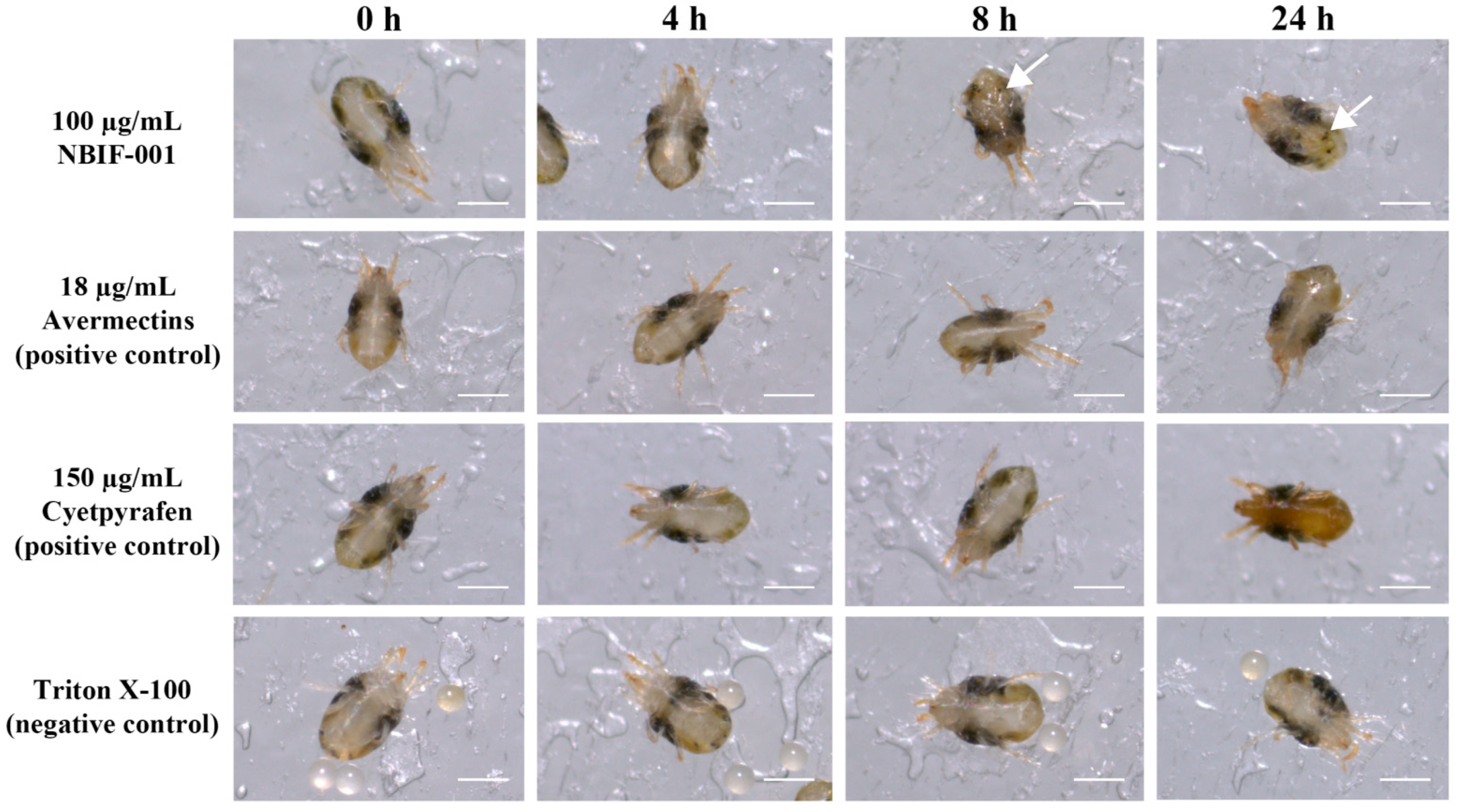
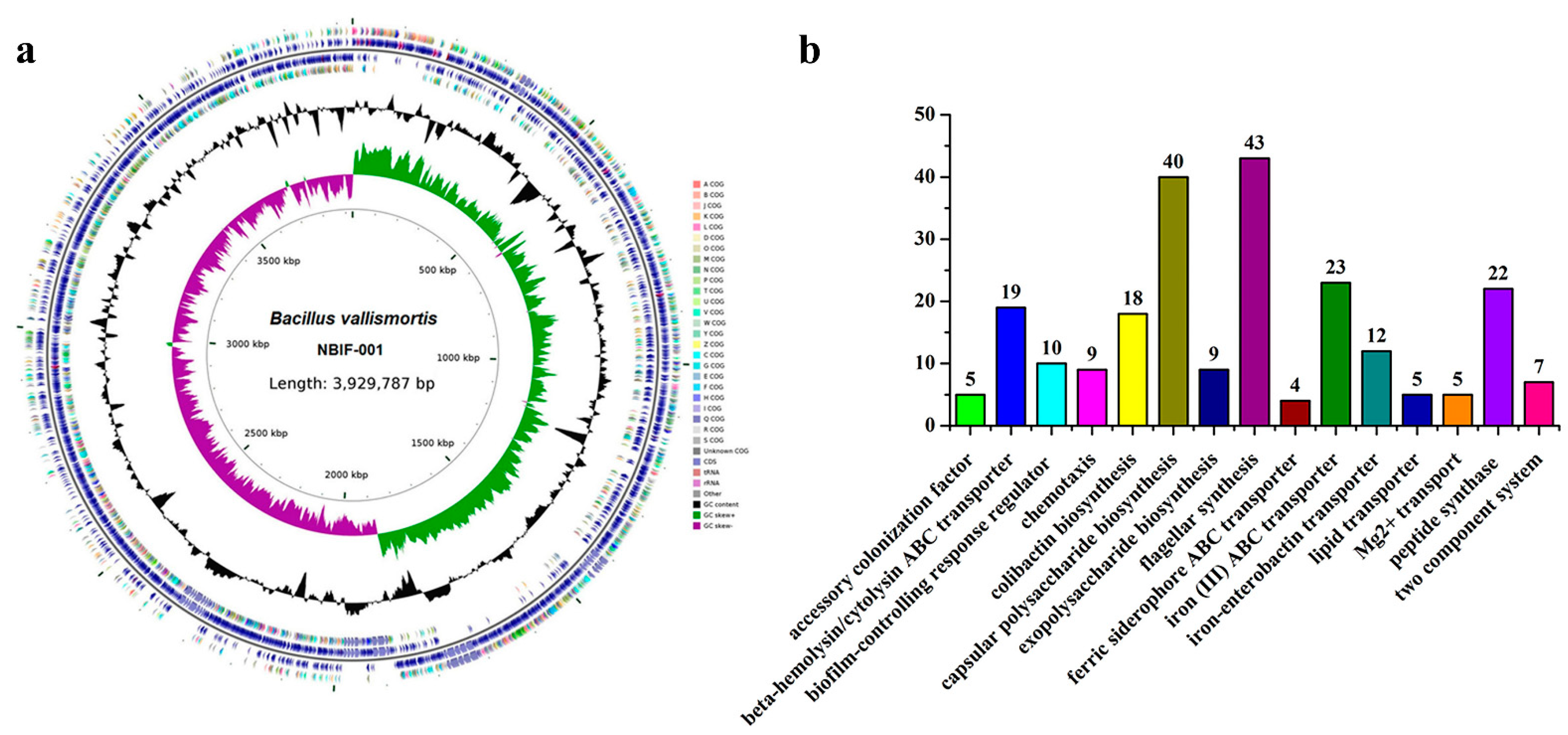
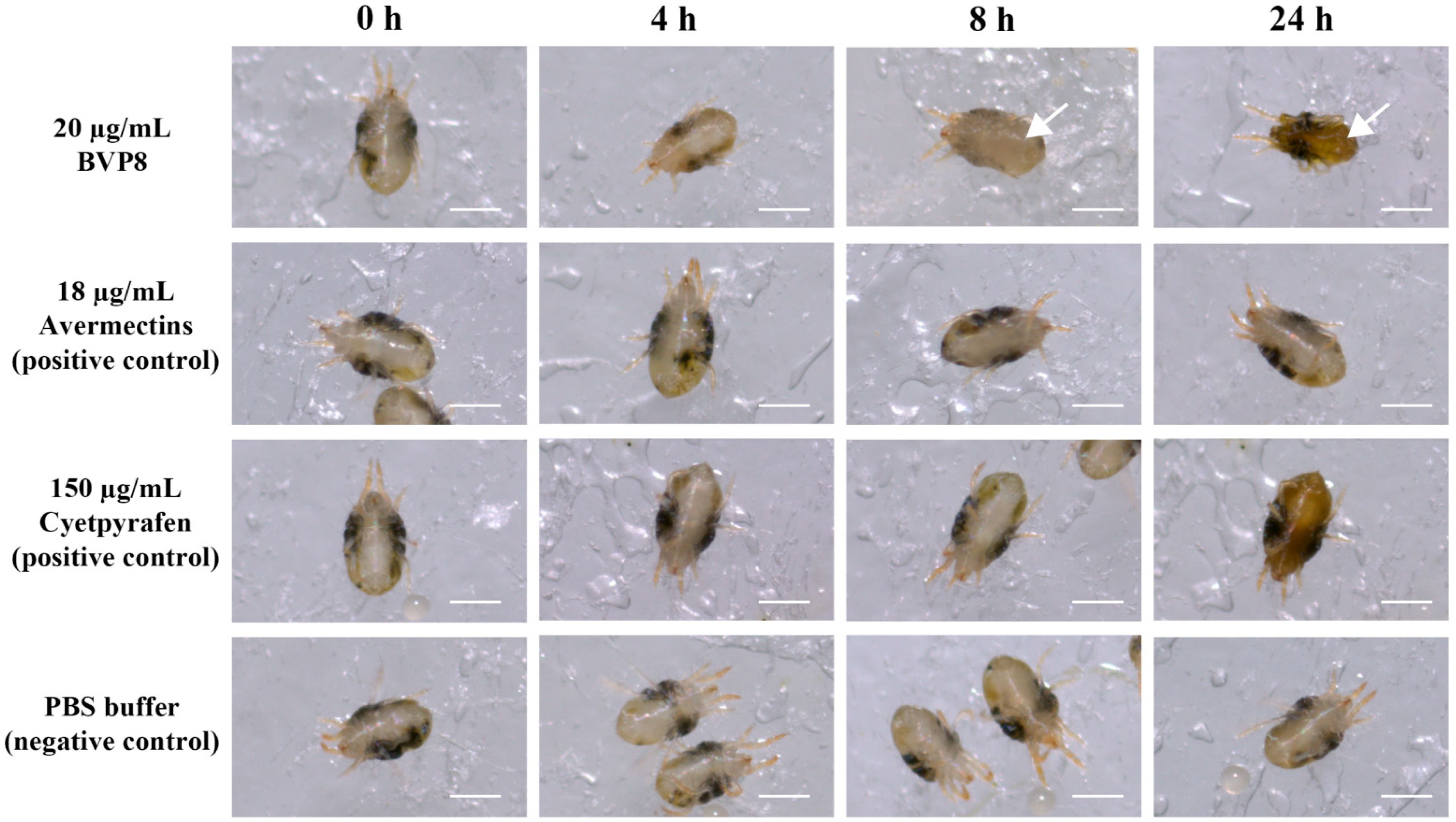
| Strains | Concentration (μg/mL) | Concentration (CFU/mL) | Corrected Mortality (%) and LC50 (95% Confidence Interval) a (μg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| T. urticae (green) | T. urticae (red) | P. citri | ||||||
| NBIF-001 | 1.0 | 3.2 × 104 | 6.5 ± 3.3 | 50.2 (45.6–55.4) | 16.6 ± 5.3 | 18.0 (15.2–20.8) | 13.2 ± 4.5 | 15.7 (3.2–28.6) |
| 10.0 | 3.2 × 105 | 10.2 ± 3.8 | 39.1 ± 7.9 | 45.1 ± 5.4 | ||||
| 25.0 | 8.1 × 105 | 22.9 ± 2.8 | 60.1 ± 7.5 | 71.3 ± 4.4 | ||||
| 50.0 | 1.6 × 106 | 46.9 ± 4.2 | 96.4 ± 2.5 | 96.9 ± 3.1 | ||||
| 100.0 | 3.2 × 106 | 94.5 ± 4.8 | 99.1 ± 1.4 | 98.0 ± 1.7 | ||||
| 200.0 | 6.4 × 106 | 100.0 ± 0.0 | 96.6 ± 5.9 | 100.0 ± 0.0 | ||||
| HD-1 (negative control) | 200.0 | - | 3.0 ± 3.6 | - | 1.1 ± 3.3 | - | 1.8 ± 6.7 | - |
| Avermectins (positive control) | 18.0 | - | 91.9 ± 5.0 | - | - | - | - | - |
| Cyetpyrafen (positive control) | 150.0 | - | 98.9 ± 1.6 | - | - | - | - | - |
| Concentration (μg/mL) | Concentration (CFU/mL) | Corrected Mortality (%) and LC50 (95% Confidence Interval) a (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| T. urticae (green) | T. urticae (red) | P. citri | |||||
| 0.1 | 7.5 × 103 | 4.8 ± 2.9 | 28.8 (21.8–39.0) | 13.9 ± 7.2 | 8.3 (6.8–10.4) | 10.0 ± 6.2 | 6.2 (5.0–7.6) |
| 1.0 | 7.5 × 104 | 12.0 ± 4.8 | 22.0 ± 9.1 | 26.3 ± 5.3 | |||
| 10.0 | 7.5 × 105 | 26.6 ± 6.2 | 57.6 ± 15.3 | 70.4 ± 7.9 | |||
| 25.0 | 1.9 × 106 | 39.8 ± 3.1 | 78.4 ± 1.5 | 95.4 ± 2.7 | |||
| 50.0 | 3.7 × 106 | 83.2 ± 7.9 | 97.0 ± 1.5 | 97.3 ± 2.7 | |||
| 100.0 | 7.5 × 106 | 99.0 ± 1.4 | 100.0 ± 0.0 | 100.0 ± 0.0 | |||
| Avermectins (18.0, positive control) | - | 91.6 ± 10.9 | - | - | - | - | - |
| Cyetpyrafen (150.0, positive control) | - | 100.0 ± 0.0 | - | - | - | - | - |
| Treatments | 1 Day after Treatment | 3 Days after Treatment | 7 Days after Treatment | 14 Days after Treatment | ||||
|---|---|---|---|---|---|---|---|---|
| Mites Reduced Rate (%) | Corrected Field Efficacy (%) | Mites Reduced Rate (%) | Corrected Field Efficacy (%) | Mites Reduced Rate (%) | Corrected Field Efficacy (%) | Mites Reduced Rate (%) | Corrected Field Efficacy (%) | |
| NBIF-001 50× dilution | 94.4 ± 1.6 a | 95.8 ± 1.0 a | 88.3 ± 4.4 a | 91.9 ± 3.2 a | 82.0 ± 1.3 a | 88.3 ± 1.7 a | 65.9 ± 6.4 a | 67.1 ± 7.9 a |
| NBIF-001 100× dilution | 90.7 ± 4.0 a | 93.2 ± 2.5 ab | 84.5 ± 6.3 ab | 88.9 ± 5.2 a | 83.6 ± 6.7 ab | 88.9 ± 5.2 a | 61.3 ± 14.4 a | 59.5 ± 20.0 a |
| NBIF-001 200× dilution | 89.0 ± 5.3 a | 92.0 ± 3.4 ab | 61.1 ± 3.5 b | 73.1 ± 3.3 b | 38.3 ± 9.7 b | 59.9 ± 9.2 b | 42.2 ± 3.8 a | 43.3 ± 13.6 a |
| Cyetpyrafen 30% SC 2000× dilution | 83.1 ± 2.6 a | 87.5 ± 1.4 b | 75.6 ± 5.8 ab | 83.0 ± 4.8 ab | 61.5 ± 4.4 ab | 75.1 ± 3.9 ab | 69.7 ± 6.4 a | 71.1 ± 6.4 a |
| Water control | –34.3 ± 8.1 b | N/A | –46.0 ± 14.8 c | N/A | –57.1 ± 22.1 c | N/A | –7.0 ± 23.1 b | N/A |
| Genes in NBIF-001 | Gene Names | Bacteriocins | Similarity |
|---|---|---|---|
| B9C48_01805-B9C48_01910 | yciC, yx01, yckC-E, nin, nuc, hxlB, hxlA, hxlR, xy02, srfAA, srfAB, comS, srfAC, srfAD, aat, ycxC, ycxD, sfp, yczE, yckI, yckJ | Surfactin | 86% |
| B9C48_07335-B9C48_07380 | pdhA, pks2A-pks2I | Macrolactin | 100% |
| B9C48_08635-B9C48_08700 | acpK, baeB-baeS | Bacillaene | 100% |
| B9C48_09260-B9C48_09440 | yngE-yngL, fenA-fenE, dacC | Fengycin | 100% |
| B9C48_11155-B9C48_11225 | difA-difO | Difficidin | 100% |
| B9C48_14735-B9C48_14955 | essA-essC, entC | Bacillibactin | 100% |
| B9C48_17850-B9C48_17880 | ywfA, bacA-bacE, ywfG | Bacilysin | 100% |
| Treatments | Dilutions | Corrected Mortality (%) |
|---|---|---|
| Fermentation broth | 1× | 100.0 ± 0.0 |
| 10× | 96.3 ± 2.6 | |
| 50× | 78.4 ± 4.0 | |
| 100× | 67.4 ± 7.8 | |
| 500× | 28.1 ± 4.0 | |
| 1000× | 11.9 ± 2.7 | |
| Supernatants | 1× | 100.0 ± 0.0 |
| 10× | 90.5 ± 1.1 | |
| 50× | 72.5 ± 5.4 | |
| 100× | 62.7 ± 4.5 | |
| 500× | 21.7 ± 3.1 | |
| 1000× | 7.1 ± 5.9 | |
| Cell resuspension | 1× | 90.1 ± 4.8 |
| 10× | 57.0 ± 13.4 | |
| 50× | 25.3 ± 5.0 | |
| 100× | 10.3 ± 4.3 | |
| 500× | 1.3 ± 5.9 | |
| 1000× | 2.3 ± 3.3 | |
| Avermectins (positive control) | 1000× | 92.2 ± 1.3 |
| Cyetpyrafen (positive control) | 2000× | 100.0 ± 0.0 |
| Proteins | Accession No. | Description | Concentration (μg/mL) | Corrected Mortality (%) | LC50 (95% Confidence Interval) a (μg/mL) |
|---|---|---|---|---|---|
| BV02315 | ARM26736.1 | hypothetical protein | 276.4 | 30.9 ± 15.9 | 425.1 (327.3–678.5) |
| 138.2 | 26.8 ± 6.7 | ||||
| 69.1 | 15.3 ± 13.0 | ||||
| 34.6 | 8.0 ± 1.5 | ||||
| BV05580 | ARM27309.1 | proteinase inhibitor | 366.0 | 50.9 ± 9.7 | 361.2 (325.5–408.0) |
| 183.0 | 33.4 ± 12.3 | ||||
| 91.5 | 5.9 ± 2.0 | ||||
| 45.8 | 2.1 ± 0.8 | ||||
| BV07375 | ARM27646.1 | serine hydrolase | 225.6 | 44.4 ± 8.4 | 247.0 (207.3–329.8) |
| 112.8 | 28.4 ± 3.2 | ||||
| 56.4 | 13.3 ± 4.2 | ||||
| 28.2 | 4.0 ± 3.0 | ||||
| BV18385 (BVP8) | ARM29686.1 | hypothetical protein | 26.9 | 95.5 ± 4.4 | 12.4 (10.9–13.9) |
| 13.4 | 52.8 ± 6.6 | ||||
| 6.7 | 26.7 ± 5.7 | ||||
| 3.4 | 15.5 ± 2.5 | ||||
| BV18675 | ARM29738.1 | hypothetical protein | 262.0 | 38.3 ± 1.9 | 302.0 (268.1–354.7) |
| 131.0 | 11.4 ± 2.9 | ||||
| 65.5 | 3.6 ± 1.1 | ||||
| 32.7 | 1.9 ± 1.8 | ||||
| Avermectins (positive control) | - | - | 18.0 | 91.6 ± 1.9 | - |
| Cyetpyrafen (positive control) | - | - | 150.0 | 100.0 ± 0.0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Chen, L.; Min, Y.; Liu, F.; Liao, X.; Rao, B.; Qiu, Y.; Chen, W.; Wang, K.; Yang, Z.; et al. Acaricidal Activity and Field Efficacy Analysis of the Potential Biocontrol Agent Bacillus vallismortis NBIF-001 against Spider Mites. Microorganisms 2022, 10, 1750. https://doi.org/10.3390/microorganisms10091750
Zhu L, Chen L, Min Y, Liu F, Liao X, Rao B, Qiu Y, Chen W, Wang K, Yang Z, et al. Acaricidal Activity and Field Efficacy Analysis of the Potential Biocontrol Agent Bacillus vallismortis NBIF-001 against Spider Mites. Microorganisms. 2022; 10(9):1750. https://doi.org/10.3390/microorganisms10091750
Chicago/Turabian StyleZhu, Lei, Ling Chen, Yong Min, Fang Liu, Xianqing Liao, Ben Rao, Yimin Qiu, Wei Chen, Kaimei Wang, Ziwen Yang, and et al. 2022. "Acaricidal Activity and Field Efficacy Analysis of the Potential Biocontrol Agent Bacillus vallismortis NBIF-001 against Spider Mites" Microorganisms 10, no. 9: 1750. https://doi.org/10.3390/microorganisms10091750





