Hyalomma spp. in Austria—The Tick, the Climate, the Diseases and the Risk for Humans and Animals
Abstract
:1. Introduction
- i.
- Evaluate tick findings;
- ii.
- Evaluate and monitor the serological status of CCHF in sentinel animals;
- iii.
- Analyse retrospective and prevailing climatic conditions during the tick season in this certain area to estimate the potential for development of Hyalomma ticks;
- iv.
- Describe potential resting places for migratory birds and densities of hosts (large animals);
- v.
- Determine the risk for humans.
2. Materials and Methods
2.1. Tick Findings
2.2. Serology on CCHF Antibodies
2.3. Climate
2.4. Migratory Birds, Available Hosts
2.5. Risk
3. Results
3.1. Tick Findings
3.2. Serology in Cattle
3.3. Climate
3.4. Migratory Birds, Available Hosts
3.5. Risk
4. Discussion
4.1. Tick Findings
4.2. Serological Data
4.3. Climate
4.4. Migratory Birds, Available Hosts
4.5. Risk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duscher, G.G.; Hodžić, A.; Hufnagl, P.; Wille-Piazzai, W.; Schötta, A.M.; Markowicz, M.A.; Estrada-Peña, A.; Stanek, G.; Allerberger, F. Adult Hyalomma marginatum tick positive for Rickettsia aeschlimannii in Austria, October 2018. Eurosurveillance 2018, 23, 1800595. [Google Scholar] [CrossRef] [PubMed]
- Uiterwijk, M.; Ibanez-Justicia, A.; van de Vossenberg, B.; Jacobs, F.; Overgaauw, P.; Nijsse, R.; Dabekaussen, C.; Stroo, A.; Sprong, H. Imported Hyalomma ticks in the Netherlands 2018–2020. Parasit Vectors 2021, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Capek, M.; Literak, I.; Kocianova, E.; Sychra, O.; Najer, T.; Trnka, A.; Kverek, P. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick-Borne Dis. 2014, 5, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L.; Nava, S.; Bestehorn, M.; Dobler, G.; Wölfel, S. First detection of Hyalomma rufipes in Germany. Ticks Tick-Borne Dis. 2016, 7, 1135–1138. [Google Scholar] [CrossRef]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G.C. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick-Borne Dis. 2012, 3, 95–99. [Google Scholar] [CrossRef]
- Nijhof, A.M.; Bodaan, C.; Postigo, M.; Nieuwenhuijs, H.; Opsteegh, M.; Franssen, L.; Jebbink, F.; Jongejan, F. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector-Borne Zoonotic Dis. 2007, 7, 585–595. [Google Scholar] [CrossRef]
- Oehme, R.; Bestehorn, M.; Wölfel, S.; Chitimia-Dobler, L. Hyalomma marginatum in Tübingen, Germany. Syst. Appl. Acarol. 2017, 22, 1. [Google Scholar] [CrossRef]
- Hahn, S.; Bauer, S.; Liechti, F. The natural link between Europe and Africa—2.1 billion birds on migration. Oikos 2009, 118, 624–626. [Google Scholar] [CrossRef]
- Battisti, E.; Urach, K.; Hodžić, A.; Fusani, L.; Hufnagl, P.; Felsberger, G.; Ferroglio, E.; Duscher, G.G. Zoonotic Pathogens in Ticks from Migratory Birds, Italy. Emerg. Infect. Dis. 2020, 26, 2886–2988. [Google Scholar] [CrossRef]
- Wallménius, K.; Barboutis, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.E.; Nyström, F.; Olsen, B.; Salaneck, E.; Nilsson, K. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasites Vectors 2014, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Venzal, J.M. Climate niches of tick species in the mediterranean region: Modeling of occurrence data, distributional constraints, and impact of climate change. J. Med. Entomol. 2007, 44, 1130–1138. [Google Scholar] [CrossRef]
- Ticks of Europe and North Africa; Springer: Berlin/Heidelberg, Germany, 2017.
- Negredo, A.; Sanchez-Ledesma, M.; Llorente, F.; Perez-Olmeda, M.; Belhassen-Garcia, M.; Gonzalez-Calle, D.; Sanchez-Seco, M.P.; Jimenez-Clavero, M.A. Retrospective Identification of Early Autochthonous Case of Crimean-Congo Hemorrhagic Fever, Spain, 2013. Emerg. Infect Dis. 2021, 27, 1754–1756. [Google Scholar] [CrossRef] [PubMed]
- Sherifi, K.; Rexhepi, A.; Berxholi, K.; Mehmedi, B.; Gecaj, R.M.; Hoxha, Z.; Joachim, A.; Duscher, G.G. Crimean-Congo hemorrhagic fever virus and Borrelia burgdorferi sensu lato in ticks from Kosovo and Albania. Front. Vet. Sci. 2018, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Negredo, A.; De La Calle-Prieto, F.; Palencia-Herrejón, E.; Mora-Rillo, M.; Astray-Mochales, J.; Sánchez-Seco, M.P.; Lopez, E.B.; Menárguez, J.; Fernández-Cruz, A.; Sánchez-Artola, B.; et al. Autochthonous crimean-congo hemorrhagic fever in Spain. N. Engl. J. Med. 2017, 377, 154–161. [Google Scholar] [CrossRef]
- Földvári, G.; Szabó, É.; Tóth, G.E.; Lanszki, Z.; Zana, B.; Varga, Z.; Kemenesi, G. Emergence of Hyalomma marginatum and Hyalomma rufipes adults revealed by citizen science tick monitoring in Hungary. Transbound. Emerg. Dis. 2022; In press. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. (In Press) [Google Scholar]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Summary for Policymakers Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- UNISDR (United Nations International Strategy for Disaster Reduction.) Sendai framework for disaster risk reduction 2015–2030. Available online: https://www.preventionweb.net/files/resolutions/N1516716.pdf (accessed on 1 April 2022).
- Kienberger, S.; Hagenlocher, M. Spatial-explicit modeling of social vulnerability to malaria in East Africa. Int. J. Health Geogr. 2014, 13, 29. [Google Scholar] [CrossRef]
- American Veterinary Medical Association. One Health: A New Professional Imperative. Available online: https://www.avma.org/sites/default/files/resources/onehealth_final.pdf (accessed on 1 April 2022).
- Atlas, R.M.; Maloy, S. (Eds.) One Health: People, Animals, and the Environment; American Society for Microbiology Press: Washington, DC, USA, 2014. [Google Scholar]
- Deem, S.L.; Lane-deGraaf, E.; Rayhel, A. (Eds.) Ntroduction to One Health: An Interdisciplinary Approach to Planetary Health; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Sas, M.A.; Comtet, L.; Donnet, F.; Mertens, M.; Vatansever, Z.; Tordo, N.; Pourquier, P.; Groschup, M.H. A novel double-antigen sandwich ELISA for the species-independent detection of Crimean-Congo hemorrhagic fever virus-specific antibodies. Antivir. Res. 2018, 151, 24–26. [Google Scholar] [CrossRef]
- Hiebl, J.; Frei, C. Daily precipitation grids for Austria since 1961—Development and evaluation of a spatial dataset for hydroclimatic monitoring and modelling. Theor. Appl. Climatol. 2018, 132, 327–345. [Google Scholar] [CrossRef]
- Hiebl, J.; Frei, C. Daily temperature grids for Austria since 1961—Concept, creation and applicability. Theor. Appl. Climatol. 2016, 124, 161–178. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; Lorenzo-Lacruz, J.; Camarero, J.J.; López-Moreno, J.I.; Azorin-Molina, C.; Revuelto, J.; Morán-Tejeda, E.; Sanchez-Lorenzo, A. Performance of drought indices for ecological, agricultural, and hydrological applications. Earth Interact. 2012, 16, 1–27. [Google Scholar] [CrossRef]
- Haslinger, K.; Koffler, D.; Schöner, W.; Laaha, G. Exploring the link between meteorological drought and streamflow: Effects of climate-catchment interaction. Water Resour. Res. 2014, 50, 2468–2487. [Google Scholar] [CrossRef]
- Haslinger, K.; Schöner, W.; Anders, I. Future drought probabilities in the Greater Alpine Region based on COSMO-CLM experiments—Spatial patterns and driving forces. Meteorol. Z. 2016, 25, 137–148. [Google Scholar] [CrossRef]
- Haslinger, K.; Bartsch, A. Creating long-term gridded fields of reference evapotranspiration in Alpine terrain based on a recalibrated Hargreaves method. Hydrol. Earth Syst. Sci. 2016, 20, 1211–1223. [Google Scholar] [CrossRef]
- BMSGPK: Avian Influenza risk map. Available online: https://www.verbrauchergesundheit.gv.at/tiere/krankheiten/ai.html (accessed on 2 May 2022).
- Zebisch, M.; Schneiderbauer, S.; Fritzsche, K.; Bubeck, P.; Kienberger, S.; Kahlenborn, W.; Schwan, S.; Below, T. The vulnerability sourcebook and climate impact chains—A standardised framework for a climate vulnerability and risk assessment. Int. J. Clim. Chang. Strateg. Manag. 2021, 13, 35–59. [Google Scholar] [CrossRef]
- Auer, I.; Böhm, R.; Jurkovic, A.; Lipa, W.; Orlik, A.; Potzmann, R.; Schöner, W.; Ungersböck, M.; Matulla, C.; Briffa, K.; et al. HISTALP—Historical instrumental climatological surface time series of the Greater Alpine Region. Int. J. Climatol. 2007, 27, 17–46. [Google Scholar] [CrossRef]

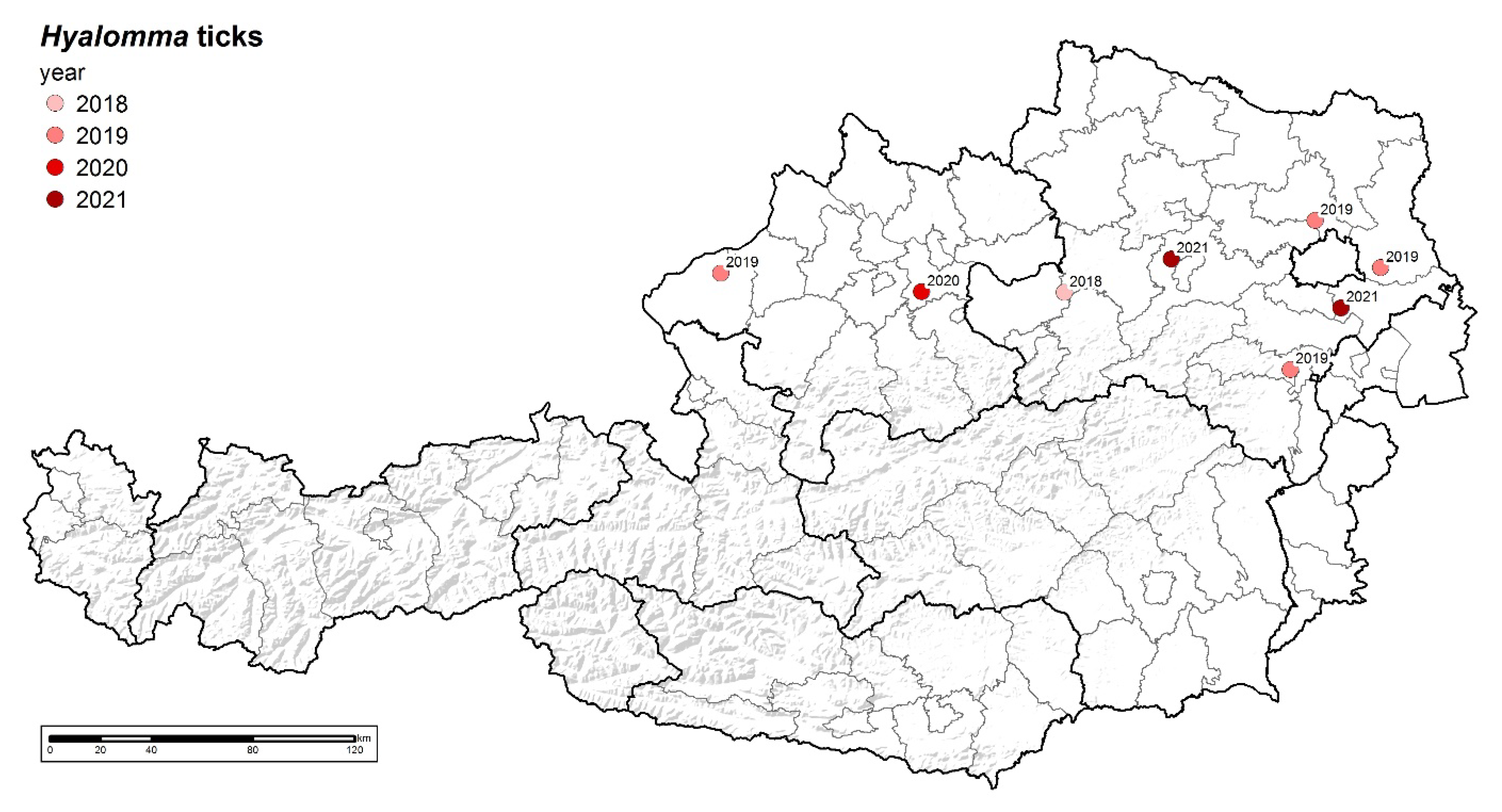

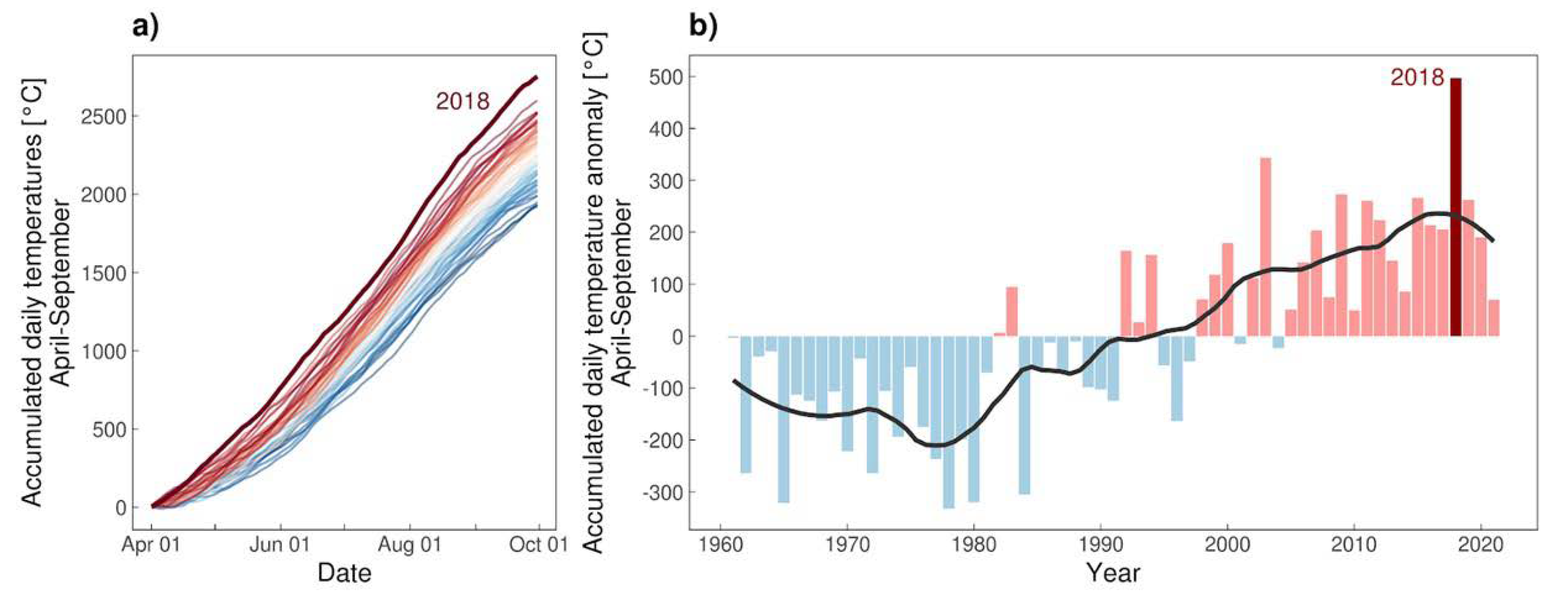
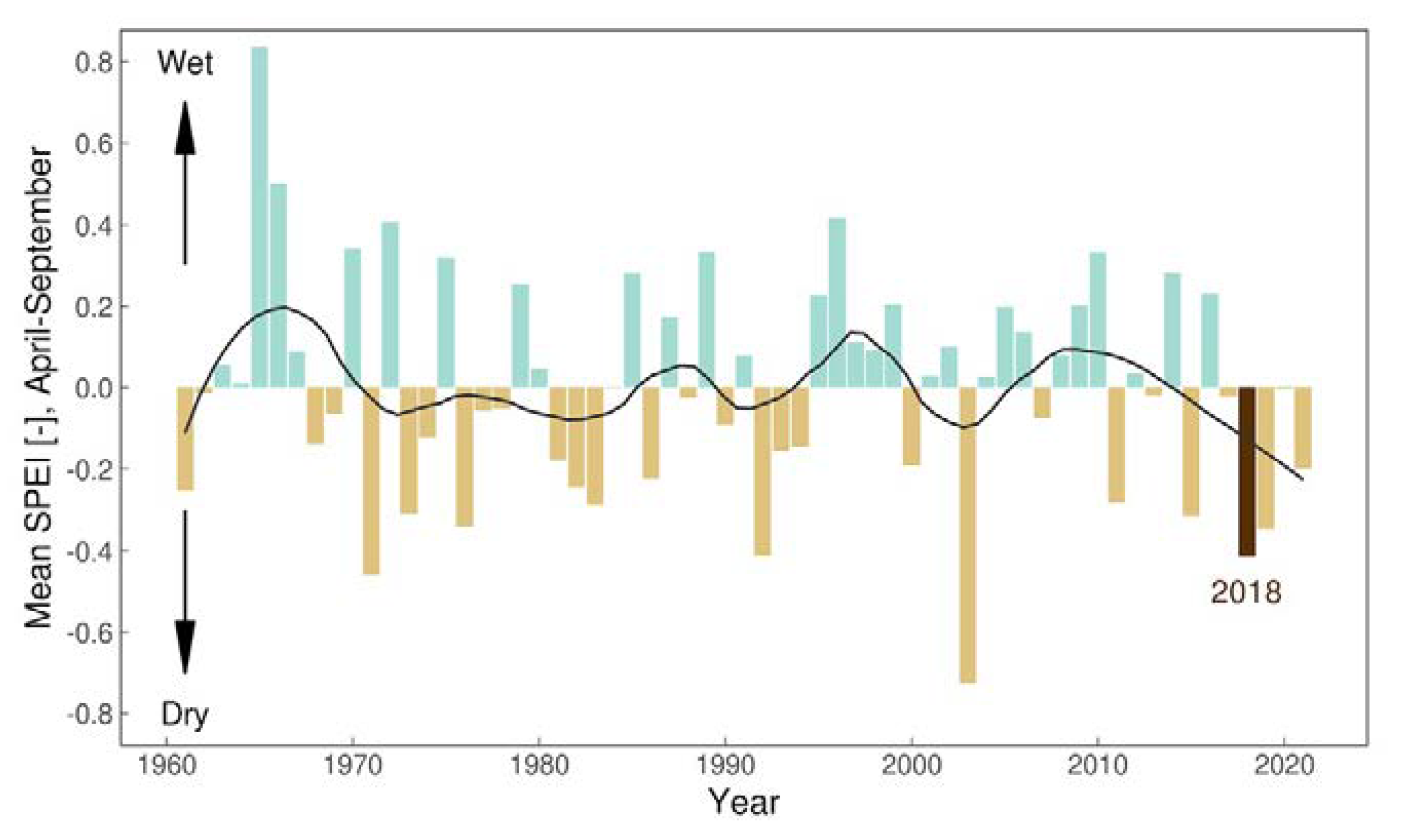
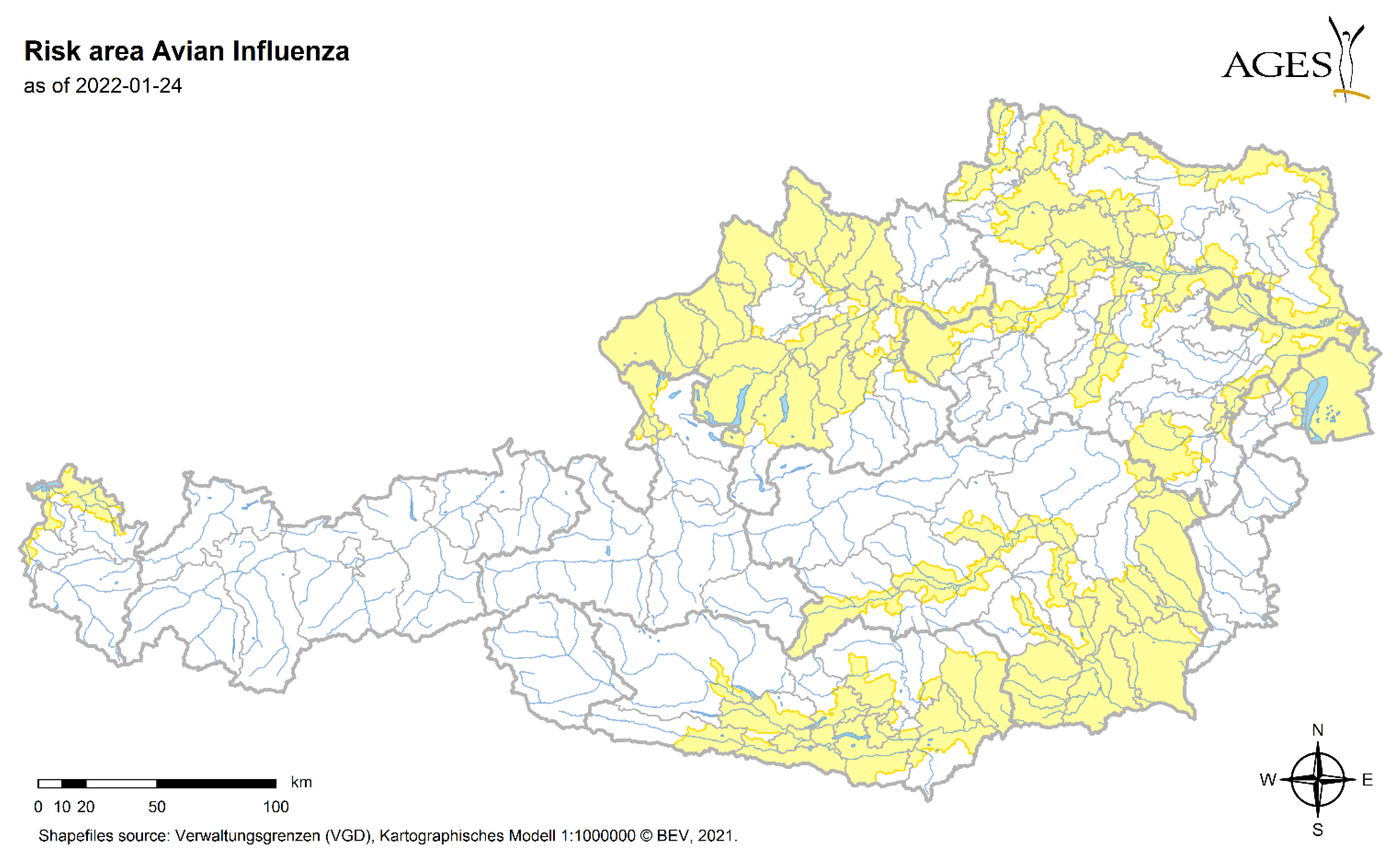
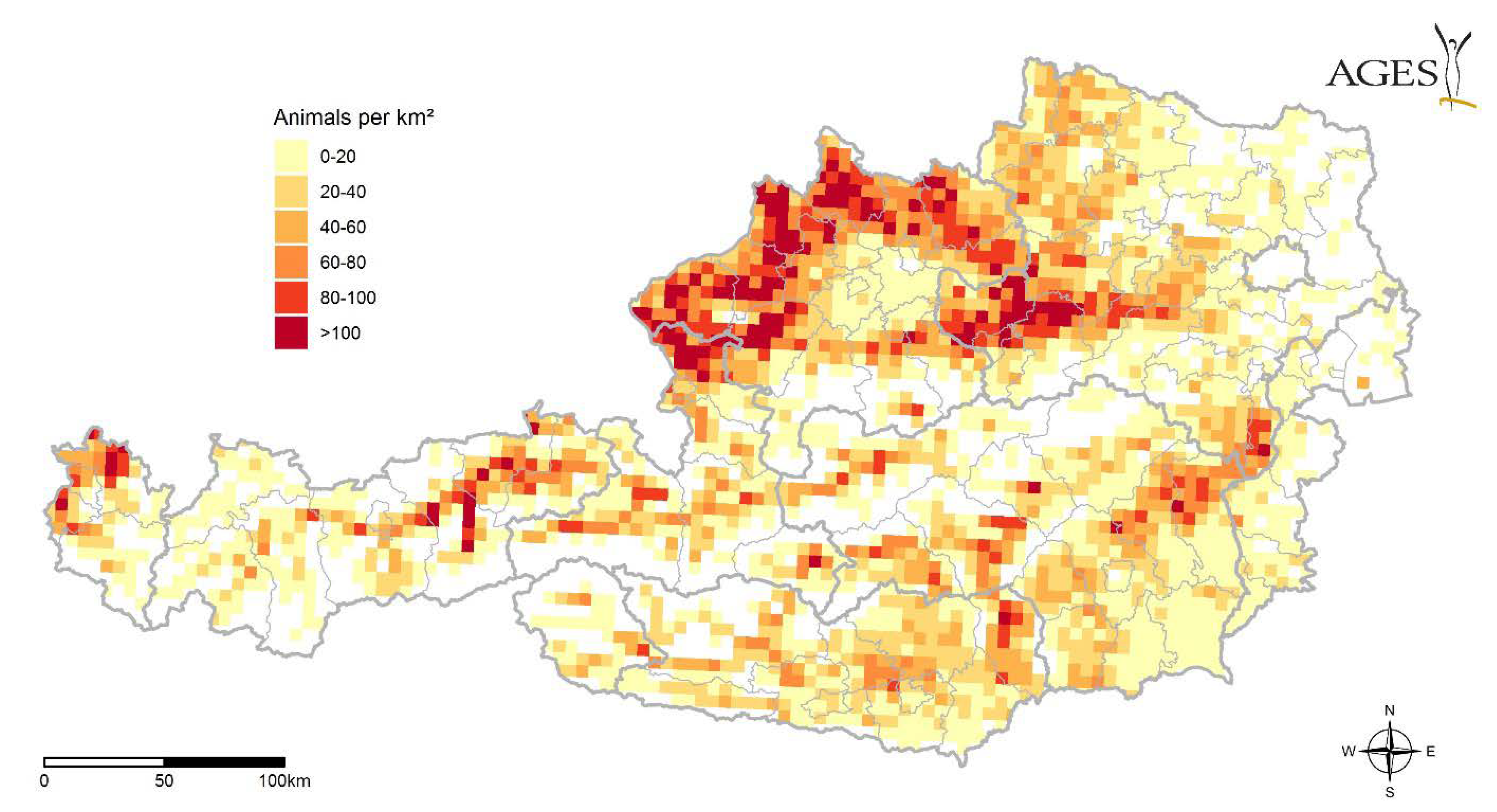

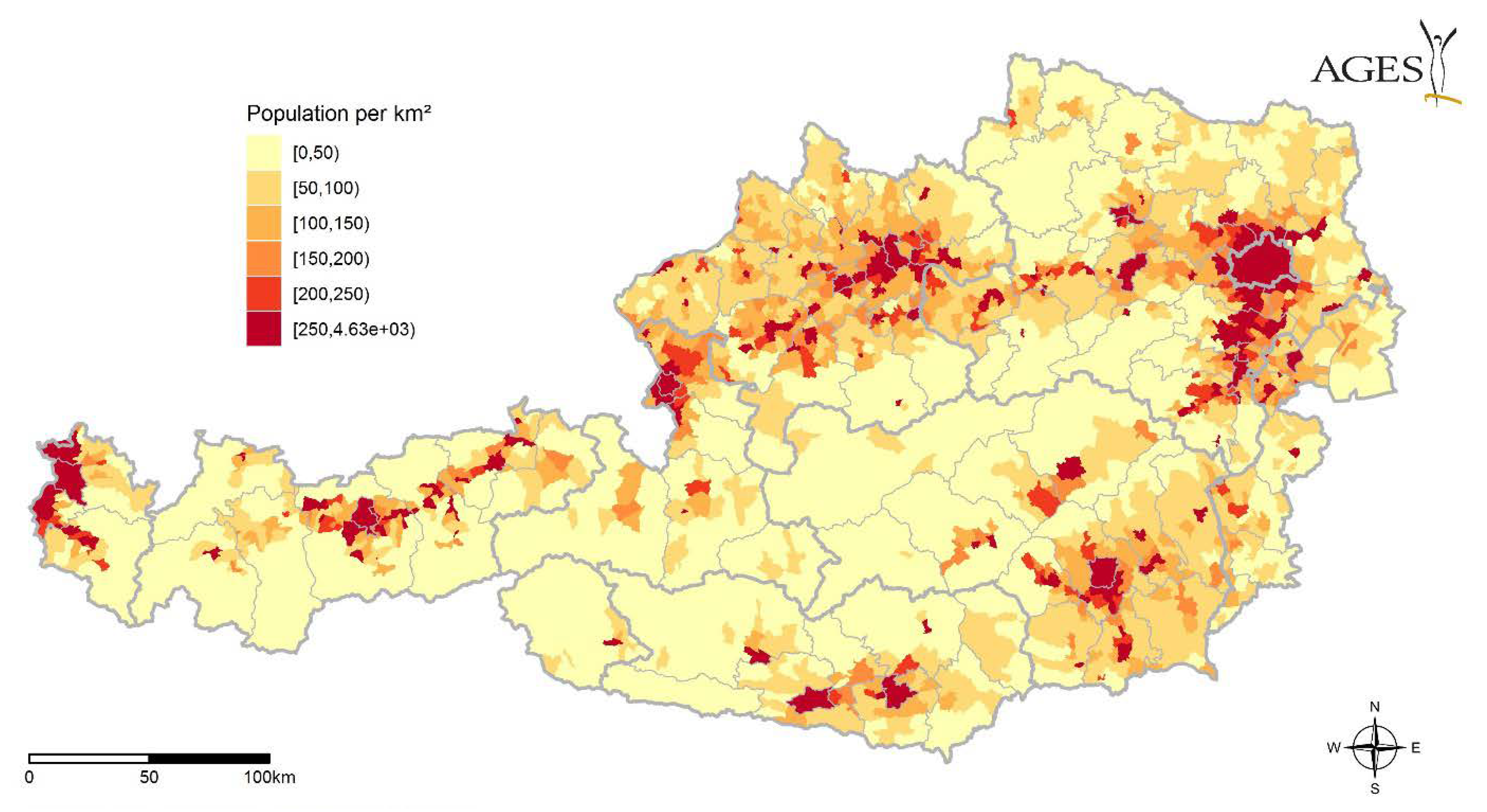
| Year | Date | District | Species | Sex | Host | CCHFV | Rickettsia sp. | Babesia sp. |
|---|---|---|---|---|---|---|---|---|
| 2018 | 02.10. | Melk | H. marginatum | male | horse | neg | pos [1] | neg |
| 2019 | 26.04. | Braunau | Hyalomma sp. | female | human | na | na | na |
| 2019 | 15.06. | Korneuburg | Hyalomma sp. | male | human | na | na | na |
| 2019 | 28.08. | Wiener Neustadt | Hyalomma sp. | na | horse | neg | na | na |
| 2019 | 05.10. | Gänserndorf | Hyalomma sp. | male | horse | na | na | na |
| 2020 | 03.09. | Linz Land | Hyalomma marginatum | male | horse | neg | na | na |
| 2021 | 30.06. | Bruck/Leitha | Hyalomma sp. | na | horse | na | na | na |
| 2021 | 25.07. | St. Pölten Land | Hyalomma sp. | female | horse | na | na | na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duscher, G.G.; Kienberger, S.; Haslinger, K.; Holzer, B.; Zimpernik, I.; Fuchs, R.; Schwarz, M.; Hufnagl, P.; Schiefer, P.; Schmoll, F. Hyalomma spp. in Austria—The Tick, the Climate, the Diseases and the Risk for Humans and Animals. Microorganisms 2022, 10, 1761. https://doi.org/10.3390/microorganisms10091761
Duscher GG, Kienberger S, Haslinger K, Holzer B, Zimpernik I, Fuchs R, Schwarz M, Hufnagl P, Schiefer P, Schmoll F. Hyalomma spp. in Austria—The Tick, the Climate, the Diseases and the Risk for Humans and Animals. Microorganisms. 2022; 10(9):1761. https://doi.org/10.3390/microorganisms10091761
Chicago/Turabian StyleDuscher, Georg Gerhard, Stefan Kienberger, Klaus Haslinger, Barbara Holzer, Irene Zimpernik, Reinhard Fuchs, Michael Schwarz, Peter Hufnagl, Peter Schiefer, and Friedrich Schmoll. 2022. "Hyalomma spp. in Austria—The Tick, the Climate, the Diseases and the Risk for Humans and Animals" Microorganisms 10, no. 9: 1761. https://doi.org/10.3390/microorganisms10091761





