Long-Term Serological Investigations of Influenza A Virus in Free-Living Wild Boars (Sus scrofa) from Northern Italy (2007–2014)
Abstract
:1. Introduction
2. Materials and Methods
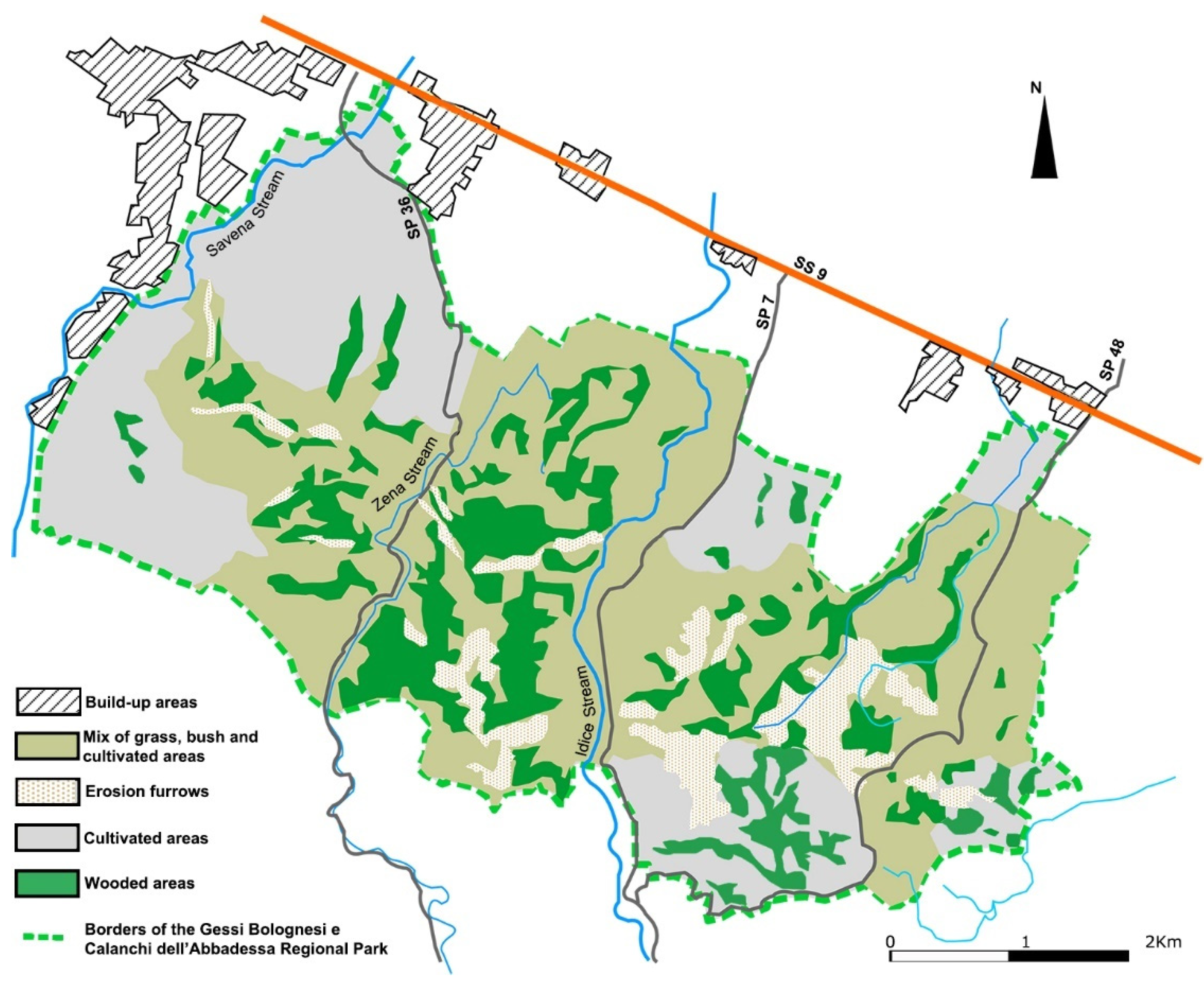
2.1. Study Area
2.2. Sample Collection
2.3. Serological Methods
2.4. Statistical Methods
2.5. Animal Rights Statement
3. Results
3.1. Study Populations
3.2. Serological Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.W.; Webby, R.J.; Webster, R.G. Evolution and ecology of influenza A viruses. Curr. Top Microbiol. Immunol. 2014, 385, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Scholtissek, C.; Bürger, H.; Kistner, O.; Shortridge, K.F. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 1985, 147, 287–294. [Google Scholar] [CrossRef]
- Castrucci, M.R.; Donatelli, I.; Sidoli, L.; Barigazzi, G.; Kawaoka, Y.; Webster, R.G. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology 1993, 193, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Bourret, V. Avian influenza viruses in pigs: An overview. Vet. J. 2018, 239, 7–14. [Google Scholar] [CrossRef]
- Donatelli, I.; Castrucci, M.R.; De Marco, M.A.; Delogu, M.; Webster, R.G. Human-Animal Interface: The Case for Influenza Interspecies Transmission. Adv. Exp. Med. Biol. 2017, 972, 17–33. [Google Scholar] [CrossRef]
- Ma, W. Swine influenza virus: Current status and challenge. Virus Res. 2020, 288, 198118. [Google Scholar] [CrossRef]
- Anderson, T.K.; Macken, C.A.; Lewis, N.S.; Scheuermann, R.H.; Van Reeth, K.; Brown, I.H.; Swenson, S.L.; Simon, G.; Saito, T.; Berhane, Y.; et al. A Phylogeny-Based Global Nomenclature System and Automated Annotation Tool for H1 Hemagglutinin Genes from Swine Influenza A Viruses. mSphere 2016, 14, e00275-16. [Google Scholar] [CrossRef]
- Nelson, M.I.; Vincent, A.L. Reverse zoonosis of influenza to swine: New perspectives on the human-animal interface. Trends Microbiol. 2015, 23, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Zell, R.; Groth, M.; Krumbholz, A.; Lange, J.; Philipps, A.; Dürrwald, R. Cocirculation of Swine H1N1 Influenza A Virus Lineages in Germany. Viruses 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Iacolina, L.; Pertoldi, C.; Amills, M.; Kusza, S.; Megens, H.J.; Bâlteanu, V.A.; Bakan, J.; Cubric-Curik, V.; Oja, R.; Saarma, U.; et al. Hotspots of recent hybridization between pigs and wild boars in Europe. Sci. Rep. 2018, 8, 17372, Erratum in: Sci. Rep. 2019, 9, 20187. [Google Scholar] [CrossRef] [PubMed]
- Touloudi, A.; Valiakos, G.; Athanasiou, L.V.; Birtsas, P.; Giannakopoulos, A.; Papaspyropoulos, K.; Kalaitzis, C.; Sokos, C.; Tsokana, C.N.; Spyrou, V.; et al. A serosurvey for selected pathogens in Greek European wild boar. Vet. Rec. Open. 2015, 28, e000077. [Google Scholar] [CrossRef]
- Vicente, J.; León-Vizcaíno, L.; Gortázar, C.; José Cubero, M.; González, M.; Martín-Atance, P. Antibodies to selected viral and bacterial pathogens in European wild boars from southcentral Spain. J. Wildl. Dis. 2002, 38, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Kaden, V.; Lange, E.; Starick, E.; Bruer, W.; Krakowski, W.; Klopries, M. Epidemiological survey of swine influenza A virus in selected wild boar populations in Germany. Vet. Microbiol. 2008, 131, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kaden, V.; Lange, E.; Hänel, A.; Hlinak, A.; Mewes, L.; Hergarten, G.; Irsch, B.; Dedek, J.; Bruer, W. Retrospective serological survey on selected viral pathogens in wild boar populations in Germany. Eur. J. Wildl. Res. 2009, 55, 153. [Google Scholar] [CrossRef]
- Roic, B.; Jemersic, L.; Terzic, S.; Keros, T.; Balatinec, J.; Florijancic, T. Prevalence of antibodies to selected viral pathogens in wild boars (Sus scrofa) in Croatia in 2005–2006 and 2009–2010. J. Wildl. Dis. 2012, 48, 131–137. [Google Scholar] [CrossRef]
- Sattler, T.; Sailer, E.; Wodak, E.; Schmoll, F. Serologische Erfassung von viralen Infektionskrankheiten in der Wildschweinpopulation verschiedener Jagdgebiete Süddeutschlands [Serological detection of emerging viral infections in wild boars from different hunting regions of Southern Germany]. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2012, 40, 27–32. [Google Scholar]
- Foni, E.; Garbarino, C.; Chiapponi, C.; Baioni, L.; Zanni, I.; Cordioli, P. Epidemiological survey of swine influenza A virus in the wild boar population of two Italian provinces. Influenza Other Respir. Viruses 2013, 7, 16–20. [Google Scholar] [CrossRef]
- Cano-Manuel, F.J.; López-Olvera, J.; Fandos, P.; Soriguer, R.C.; Pérez, J.M.; Granados, J.E. Long-term monitoring of 10 selected pathogens in wild boar (Sus scrofa) in Sierra Nevada National Park, southern Spain. Vet. Microbiol. 2014, 174, 148–154. [Google Scholar] [CrossRef]
- Risco, D.; Serrano, E.; Fernández-Llario, P.; Cuesta, J.M.; Gonçalves, P.; García-Jiménez, W.L.; Martínez, R.; Cerrato, R.; Velarde, R.; Gómez, L.; et al. Severity of bovine tuberculosis is associated with co-infection with common pathogens in wild boar. PLoS ONE 2014, 9, e110123. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Lim, S.I.; Jeoung, H.Y.; Kim, Y.K.; Song, J.Y.; Lee, J.B.; An, D.J. Serological evidence for influenza virus infection in Korean wild boars. J. Vet. Med. Sci. 2015, 77, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, G.; Molozhanova, A.; Halka, I.; Nychyk, S. Antibody Prevalence to Influenza Type A in Wild Boar of Northern Ukraine. Vector Borne Zoonotic Dis. 2017, 17, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Van Nguyen, D.; Yonemitsu, K.; Minami, S.; Nagata, N.; Hara, N.; Kuwata, R.; Murakami, S.; Kodera, Y.; Takeda, T.; et al. Influenza A virus infection in Japanese wild boars (Sus scrofa leucomystax). J. Vet. Med. Sci. 2017, 79, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Grech-Angelini, S.; Hervé, S.; Rose, N.; Barbier, N.; Casabianca, F.; Maestrini, O.; Falchi, A.; Simon, G. Serological survey of influenza A viruses in domestic and wild Suidae in Corsica (France), a Mediterranean island environment. Prev. Vet. Med. 2018, 157, 94–98. [Google Scholar] [CrossRef]
- Malmsten, A.; Magnusson, U.; Ruiz-Fons, F.; González-Barrio, D.; Dalin, A.M. A serologic survey of pathogens in wild boar (Sus scrofa) in Sweden. J. Wildl. Dis. 2018, 54, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Delogu, M.; Cotti, C.; Vaccari, G.; Raffini, E.; Frasnelli, M.; Nicoloso, S.; Biacchessi, V.; Boni, A.; Foni, E.; Castrucci, M.R.; et al. Serologic and Virologic Evidence of Influenza A Viruses in Wild Boars (Sus scrofa) from Two Different Locations in Italy. J. Wildl. Dis. 2019, 55, 158–163. [Google Scholar] [CrossRef]
- Schülein, A.; Ritzmann, M.; Christian, J.; Schneider, K.; Neubauer-Juric, A. Exposure of wild boar to Influenza A viruses in Bavaria: Analysis of seroprevalences and antibody subtype specificity before and after the panzootic of highly pathogenic avian influenza viruses A (H5N8). Zoonoses Public Health 2021, 68, 503–515. [Google Scholar] [CrossRef]
- Prosperi, A.; Soliani, L.; Canelli, E.; Baioni, L.; Gabbi, V.; Torreggiani, C.; Manfredi, R.; Calanchi, I.; Pupillo, G.; Barsi, F.; et al. Influenza A in Wild Boars: Viral Circulation in the Emilia-Romagna Region (Northern Italy) between 2017 and 2022. Animals 2022, 12, 1593. [Google Scholar] [CrossRef]
- Clavijo, A.; Nikooienejad, A.; Esfahani, M.S.; Metz, R.P.; Schwartz, S.; Atashpaz-Gargari, E.; Deliberto, T.J.; Lutman, M.W.; Pedersen, K.; Bazan, L.R.; et al. Identification and analysis of the first 2009 pandemic H1N1 influenza virus from U.S. feral swine. Zoonoses Public Health 2013, 60, 327–335. [Google Scholar] [CrossRef]
- Vittecoq, M.; Grandhomme, V.; Simon, G.; Herve, S.; Blanchon, T.; Renaud, F.; Thomas, F.; Gauthier-Clerc, M.; van der Werf, S. Study of influenza A virus in wild boars living in a major duck wintering site. Infect. Genet. Evol. 2012, 12, 483–486. [Google Scholar] [CrossRef] [PubMed]
- I.Stat–Consistenze Degli Allevamenti. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_CONSISTENZE (accessed on 15 July 2022).
- Chiapponi, C.; Ebranati, E.; Pariani, E.; Faccini, S.; Luppi, A.; Baioni, L.; Manfredi, R.; Carta, V.; Merenda, M.; Affanni, P.; et al. Genetic analysis of human and swine influenza A viruses isolated in Northern Italy during 2010–2015. Zoonoses Public Health 2018, 65, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Chiapponi, C.; Prosperi, A.; Moreno, A.; Baioni, L.; Faccini, S.; Manfredi, R.; Zanni, I.; Gabbi, V.; Calanchi, I.; Fusaro, A.; et al. Genetic variability among swine Influenza viruses in Italy: Data analysis of the period 2017–2020. Viruses 2021, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Ente di Gestione per i Parchi e la Biodiversità–Emilia Orientale: Parco dei Gessi Bolognesi e Calanchi dell’Abbadessa. Available online: https://enteparchi.bo.it/parco-dei-gessi-bolognesi-e-calanchi-dellabbadessa/ (accessed on 14 July 2022).
- Piano di gestione e controllo del Cinghiale (Sus scrofa) nel Parco Regionale dei Gessi Bolognesi e Calanchi dell’Abbadessa (2020-2024). Available online: https://enteparchi.bo.it/Engine/RAServeFile.php/f/gestione_faunistica/Piano_di_Gestione_e_controllo_del_Cinghiale_Gessi_2020_2024_compressed.pdf (accessed on 14 July 2022).
- Monaco, A.; Carnevali, L.; Toso, S. Linee Guida per la Gestione del Cinghiale (Sus scrofa) Nelle Aree Protette, 2nd ed.; Quad. Cons. Natura, 34; Min. Ambiente–ISPRA: Roma, Italy, 2010. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/quaderni/conservazione-della-natura/linee-guida-per-la-gestione-del-cinghiale-sus-1 (accessed on 14 July 2022).
- Olsen, C.W.; Brown, I.H.; Easterday, B.C.; Van Reeth, K. Swine Influenza. In Diseases of Swine, 9th ed.; Straw, B.E., Zimmerman, J.J., D’Allaire, S., Taylor, D.J., Eds.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 469–482. [Google Scholar]
- Loeffen, W.L.; Heinen, P.P.; Bianchi, A.T.; Hunneman, W.A.; Verheijden, J.H. Effect of maternally derived antibodies on the clinical signs and immune response in pigs after primary and secondary infection with an influenza H1N1 virus. Vet. Immunol. Immunopathol. 2003, 92, 23–35. [Google Scholar] [CrossRef]
- Piccirillo, A.; Pasotto, D.; Martin, A.M.; Cordioli, P. Serological survey for influenza type A viruses in domestic dogs (Canis lupus familiaris) and cats (Felis catus) in north-eastern Italy. Zoonoses Public Health 2010, 57, 239–243. [Google Scholar] [CrossRef]
- De Boer, G.F.; Back, W.; Osterhaus, A.D. An ELISA for detection of antibodies against influenza A nucleoprotein in humans and various animal species. Arch. Virol. 1990, 115, 47–61. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2018. Influenza A Virus of Swine (Chapter 3.9.7). Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.09.07_INF_A_SWINE.pdf (accessed on 12 April 2022).
- Dowdle, W.A.; Kendal, A.P.; Noble, G.R. Influenza viruses. In Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections, 5th ed.; Lennette, E.H., Schmidt, N.J., Eds.; American Public Health Association: Washington, DC, USA, 1979; pp. 585–609. [Google Scholar]
- Fowler, M.E. Restraint and Handling of Wild and Domestic Animals, 3rd ed.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 5–93, 149–159, 355–376. [Google Scholar]
- Kyriakis, C.S.; Olsen, C.W.; Carman, S.; Brown, I.H.; Brookes, S.M.; Doorsselaere, J.V.; Reeth, K.V. Serologic cross-reactivity with pandemic (H1N1) 2009 virus in pigs, Europe. Emerg. Infect. Dis. 2010, 16, 96–99. [Google Scholar] [CrossRef]
- De Marco, M.A.; Porru, S.; Cordioli, P.; Cesana, B.M.; Moreno, A.; Calzoletti, L.; Bonfanti, L.; Boni, A.; Di Carlo, A.S.; Arici, C.; et al. Evidence of cross-reactive immunity to 2009 pandemic influenza A virus in workers seropositive to swine H1N1 influenza viruses circulating in Italy. PLoS ONE 2013, 8, e57576. [Google Scholar] [CrossRef]
- Dürrwald, R.; Krumbholz, A.; Baumgarte, S.; Schlegel, M.; Vahlenkamp, T.W.; Selbitz, H.J.; Wutzler, P.; Zell, R. Swine influenza A vaccines, pandemic (H1N1) 2009 virus, and cross-reactivity. Emerg. Infect. Dis. 2010, 16, 1029–1030. [Google Scholar] [CrossRef]
- Kyriakis, C.S.; Brown, I.H.; Foni, E.; Kuntz-Simon, G.; Maldonado, J.; Madec, F.; Essen, S.C.; Chiapponi, C.; Van Reeth, K. Virological surveillance and preliminary antigenic characterization of influenza viruses in pigs in five European countries from 2006 to 2008. Zoonoses Public Health 2011, 58, 93–101. [Google Scholar] [CrossRef]
- Simon, G.; Larsen, L.E.; Dürrwald, R.; Foni, E.; Harder, T.; Van Reeth, K.; Markowska-Daniel, I.; Reid, S.M.; Dan, A.; Maldonado, J.; et al. European surveillance network for influenza in pigs: Surveillance programs, diagnostic tools and Swine influenza virus subtypes identified in 14 European countries from 2010 to 2013. PLoS ONE 2014, 9, e115815. [Google Scholar] [CrossRef] [PubMed]
- Banca Dati Nazionale dell’Anagrafe Zootecnica Istituita dal Ministero della Salute Presso il CSN dell’Istituto “G. Caporale” di Teramo”. Available online: https://www.vetinfo.it/j6_statistiche/#/ (accessed on 18 July 2022).
- Cannon, R.M.; Roe, R.T. Livestock Disease Surveys: A Field Manual for Veterinarians; Australian Bureau of Animal Health, Australian Government Publishing Service: Canberra, Australia, 1982; pp. 1–35. Available online: https://www.aphis.usda.gov/animal_health/surveillance_toolbox/docs/epi_surv/cannon_roe_1982_livestock_disease_surveys.pdf (accessed on 15 July 2022).
- Pepin, K.M.; Pedersen, K.; Wan, X.F.; Cunningham, F.L.; Webb, C.T.; Wilber, M.Q. Individual-level antibody dynamics reveal potential drivers of Influenza A seasonality in wild pig populations. Integr. Comp. Biol. 2019, 59, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Di Trani, L.; Alborali, L.; Vaccari, G.; Barbieri, I.; Falcone, E.; Sozzi, E.; Puzelli, S.; Ferri, G.; Cordioli, P. First Pandemic H1N1 Outbreak from a Pig Farm in Italy. Open Virol. J. 2010, 4, 52–56. [Google Scholar] [CrossRef] [PubMed]


| Sampling Year | Sample Size | No. of Seropositive/Wild Boars Tested by NP-ELISA | NP-ELISA Seroprevalence% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Class * | Sex | |||||||||
| 1 ^ | 2 | 3 | 4 | U | F | M | U | |||
| 2007 | 469 | 30/188 | 5/88 | 4/62 | 3/16 | 9/115 | 20/175 | 23/199 | 8/95 | 10.9 (51/469) |
| 2008 | 76 | 0/13 | 0/27 | 0/13 | 0/3 | 0/20 | 0/32 | 0/42 | 0/2 | 0/76 |
| 2009 | 289 | 3/67 | 23/89 | 16/83 | 4/27 | 5/23 | 22/144 | 26/135 | 3/10 | 17.6 (51/289) |
| 2010 | 217 | 1/82 | 0/36 | 5/78 | 1/17 | 1/4 | 5/98 | 2/112 | 1/7 | 3.7 (8/217) |
| 2011 | 480 | 2/160 | 0/120 | 5/124 | 2/67 | 0/9 | 6/234 | 3/238 | 0/8 | 1.9 (9/480) |
| 2012 | 386 | 13/146 | 1/119 | 5/60 | 1/56 | 0/5 | 8/179 | 12/199 | 0/8 | 5.2 (20/386) |
| 2013 | 354 | 0/53 | 1/88 | 0/71 | 1/98 | 0/44 | 1/191 | 1/151 | 0/12 | 0.6 (2/354) |
| 2014 | 347 | 0/69 | 0/72 | 0/52 | 4/148 | 0/6 | 1/180 | 3/163 | 0/4 | 1.1 (4/347) |
| Total | 2618 | 778 | 639 | 543 | 432 | 226 | 1233 | 1239 | 146 | 5.5 (145/2618) |
| Sampling Date (No./IAV pos./HI pos.) | HI Titers from WB-Tested IAV-Positive by NP-ELISA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WB Serum ID | Age Class * | Sex | H1N1/98 | H1N1/13 | H1N1pdm/13 | H1N2/98 | H1N2/09 | H3N2/98 | H3N2/13 | |
| 9 March 2007 (1/1/1) | 1984 | U | U | - | - | - | - | - | - | 20 |
| 5 April 2007 (1/1/1) | 2035 | 2 | F | - | - | - | - | - | - | 20 |
| 22 May 2007 (11/4/1) | 2125 | 3 | M | - | - | - | - | - | - | 20 |
| 12 June 2007 (12/6/2) | 2175 | U | U | - | - | - | - | - | - | 40 |
| 2180 | 1 | M | - | - | - | - | - | - | 20 | |
| 14 June 2007 (18/14/2) | 2187 | 1 | F | - | - | - | - | - | - | 20 |
| 2197 | U | U | - | - | - | - | - | - | 20 | |
| 26 June 2007 (1/1/1) | 2222 | 1 | M | - | - | - | - | - | - | 20 |
| 3 July 2007 (2/2/2) | 2230 | 1 | M | - | - | - | - | - | - | 20 |
| 2231 | 1 | F | - | - | - | - | - | - | 20 | |
| 26 February 2009 (7/6/6) | 2600 | 2 | M | 320 | 160 | 80 | - | - | - | - |
| 2601 | 2 | F | 160 | 80 | 40 | - | - | - | - | |
| 2602 | 1 | M | 40 | 80 | 40 | - | - | - | - | |
| 2603 | 1 | F | 80 | 40 | 40 | - | - | - | - | |
| 2604 | 1 | M | 40 | 40 | 20 | - | - | - | 20 | |
| 2606 | 3 | F | 80 | 80 | 40 | - | - | - | 20 | |
| 27 February 2009 (1/1/1) | 2608 | 2 | M | 320 | 320 | 80 | - | - | - | 20 |
| 4 March 2009 (5/4/4) | 2611 | 2 | M | 40 | 40 | 20 | - | - | - | - |
| 2612 | 2 | M | 160 | 160 | 40 | - | - | - | 20 | |
| 2613 | 2 | F | 80 | 40 | 20 | - | - | - | 20 | |
| 2614 | 2 | M | 20 | 20 | - | - | - | - | 40 | |
| 10 March 2009 (7/2/2) | 2623 (§) | 2 | M | 80 | 40 | 40 | - | - | - | 20 |
| 2624 | 2 | F | 80 | 40 | 40 | - | - | 20 | 40 | |
| 12 March 2009 (3/3/3) | 2627 | 2 | M | 160 | 80 | 40 | - | - | - | 40 |
| 2628 | 4 | F | 640 | 640 | 160 | - | - | - | - | |
| 2629 | 2 | M | 40 | 20 | 20 | - | - | 20 | 40 | |
| 17 March 2009 (2/1/1) | 2632 | 3 | M | 40 | 40 | 20 | - | - | - | - |
| 18 March 2009 (1/1/1) | 2634 | 2 | M | 40 | 20 | 40 | - | - | - | 20 |
| 20 March 2009 (1/1/1) | 2635 | 3 | F | 40 | 40 | 20 | - | - | - | 40 |
| 24 March 2009 (4/3/3) | 2637 | 3 | F | 80 | 40 | 40 | - | - | - | 40 |
| 2638 | U | F | 40 | 40 | 40 | - | - | - | - | |
| 2640 | 2 | M | 80 | 40 | 20 | - | - | - | 20 | |
| 2 April 2009 (2/1/1) | 2646 | 3 | F | 40 | 40 | 20 | - | - | - | - |
| 7 April 2009 (4/2/2) | 2651 | U | U | 20 | 20 | 20 | - | - | - | 80 |
| 2652 | U | U | 40 | 40 | 20 | - | - | - | 20 | |
| 8 April 2009 (2/2/2) | 2654 | 2 | M | 40 | 40 | - | - | - | - | 20 |
| 2656 | 2 | M | 40 | 40 | 40 | - | - | - | 20 | |
| 21 April 2009 (2/1/1) | 2660 | 3 | F | 80 | 80 | 40 | - | - | - | 20 |
| 24 April 2009 (4/1/1) | 2669 | U | U | 20 | 40 | 20 | - | - | - | 40 |
| 29 April 2009 (7/5/5) | 2671 | 2 | M | 40 | 40 | 80 | - | - | - | - |
| 2672 | 2 | F | 80 | 40 | 40 | - | - | - | - | |
| 2673 | 2 | F | 80 | 80 | 40 | - | - | - | - | |
| 2674 | 3 | F | 160 | 80 | 80 | - | - | - | - | |
| 2675 | 3 | M | 80 | 80 | 40 | - | - | - | - | |
| 7 May 2009 (3/1/1) | 2690 | 4 | F | 40 | 20 | - | - | - | - | - |
| 12 May 2009 (2/1/1) | 2707 | 3 | M | 20 | 20 | 20 | - | - | - | 40 |
| 19 May 2009 (4/1/1) | 2711 | 2 | M | 80 | 40 | 40 | - | - | - | - |
| 30 June 2009 (8/1/1) | 2754 | 2 | M | 320 | 80 | 40 | - | - | - | - |
| 2 July 2009 (8/2/2) | 2756 | 3 | M | 80 | 40 | 40 | - | - | - | - |
| 2757 | 2 | F | 160 | 80 | 80 | - | - | - | - | |
| 16 July 2009 (2/1/1) | 2781 | 3 | F | 80 | 80 | 40 | - | - | - | - |
| 30 September 2009 (1/1/1) | 2809 | 4 | M | 160 | 80 | 160 | - | - | - | - |
| 20 October 2009 (12/3/3) | 2829 | 3 | F | 320 | 160 | 80 | - | - | - | 20 |
| 2832 | 3 | F | 160 | 80 | 40 | - | - | - | - | |
| 2833 | 3 | F | 160 | 80 | 40 | - | - | - | - | |
| 11 December 2009 (8/1/1) | 2894 | 3 | M | 320 | 80 | 40 | - | - | - | - |
| 7 May 2010 (3/1/1) | 2940 (§) | 3 | M | 640 | 640 | 320 | - | - | - | - |
| 18 May 2010 (3/2/2) | 2955 (§) | 4 | M | 160 | 80 | 40 | - | - | - | - |
| 2957 | U | U | 160 | 80 | 40 | - | - | - | 20 | |
| 19 May 2010 (6/1/1) | 2959 | 3 | F | 160 | 160 | 160 | - | - | - | - |
| 31 May 2010 (3/1/1) | 3002 | 3 | F | 160 | 160 | 80 | - | - | - | - |
| 4 June 2010 (14/2/2) | 3007 | 3 | F | 640 | 320 | 320 | - | - | - | - |
| 3019 | 1 | F | 40 | 20 | 20 | - | - | - | - | |
| 18 June 2010 (9/1/1) | 3044 | 3 | M | 1280 | 320 | 320 | - | - | - | - |
| 27 July 2010 (1/1/1) | 3092 | 4 | M | 40 | 40 | 20 | - | - | - | - |
| 3 September 2010 (1/1/1) | 3104 | 3 | F | 80 | 40 | 40 | - | - | - | 40 |
| 6 June 2011 (2/1/1) | 3303 | 3 | M | - | - | - | - | - | - | 20 |
| 14 June 2011 (4/1/1) | 3322 | 3 | F | 20 | - | - | - | - | - | - |
| 15 July 2011 (3/1/1) | 3361 | 1 | F | - | - | - | - | - | - | 20 |
| 23 July 2011 (2/1/1) | 3376 | 3 | F | 320 | 160 | 40 | - | - | - | - |
| 7 October 2011 (2/1/1) | 3507 | 4 | F | 80 | 40 | 40 | - | - | - | - |
| 11 November 2011 (1/1/1) | 3576 | 4 | M | 80 | 20 | 20 | - | - | - | - |
| 1 June 2012 (1/1/1) | 3803 | 4 | F | 160 | 80 | 40 | - | - | - | - |
| 21 June 2012 (2/1/1) | 3806 | 3 | M | - | - | - | - | 40 | 20 | 80 |
| 20 September 2012 (10/2/1) | 3852 | 3 | F | - | - | - | - | 20 | - | - |
| 26 September 2012 (6/1/1) | 3874 | 3 | M | - | - | - | - | - | - | 20 |
| 5 October 2012 (3/2/2) | 3903 | 1 | M | - | - | - | - | 20 | - | - |
| 3904 | 1 | M | - | - | - | - | 20 | - | - | |
| 4 March 2013 (4/1/1) | 4091 | 4 | F | 20 | 20 | 20 | - | - | - | - |
| 12 June 2014 (6/2/1) | 4716 | 4 | M | - | - | - | - | - | - | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marco, M.A.; Cotti, C.; Raffini, E.; Frasnelli, M.; Prosperi, A.; Zanni, I.; Romanini, C.; Castrucci, M.R.; Chiapponi, C.; Delogu, M. Long-Term Serological Investigations of Influenza A Virus in Free-Living Wild Boars (Sus scrofa) from Northern Italy (2007–2014). Microorganisms 2022, 10, 1768. https://doi.org/10.3390/microorganisms10091768
De Marco MA, Cotti C, Raffini E, Frasnelli M, Prosperi A, Zanni I, Romanini C, Castrucci MR, Chiapponi C, Delogu M. Long-Term Serological Investigations of Influenza A Virus in Free-Living Wild Boars (Sus scrofa) from Northern Italy (2007–2014). Microorganisms. 2022; 10(9):1768. https://doi.org/10.3390/microorganisms10091768
Chicago/Turabian StyleDe Marco, Maria Alessandra, Claudia Cotti, Elisabetta Raffini, Matteo Frasnelli, Alice Prosperi, Irene Zanni, Chiara Romanini, Maria Rita Castrucci, Chiara Chiapponi, and Mauro Delogu. 2022. "Long-Term Serological Investigations of Influenza A Virus in Free-Living Wild Boars (Sus scrofa) from Northern Italy (2007–2014)" Microorganisms 10, no. 9: 1768. https://doi.org/10.3390/microorganisms10091768





