Effect of Calcium Ion Supplementation on Oral Microbial Composition and Biofilm Formation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Saliva Collection and Processing
2.2. Oral Microbial Community and Culture Conditions
2.3. Crystal Violet Assay
2.4. MTT Assay
2.5. DNA Extraction
2.6. PCR and Denaturing Gradient Gel Electrophoresis (DGGE)
2.7. 16S rRNA Gene Sequencing and Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Biofilm Biomass and DGGE Profile with CaCl2 Supplementation
3.2. Microbial Viability with CaCl2 Supplementation
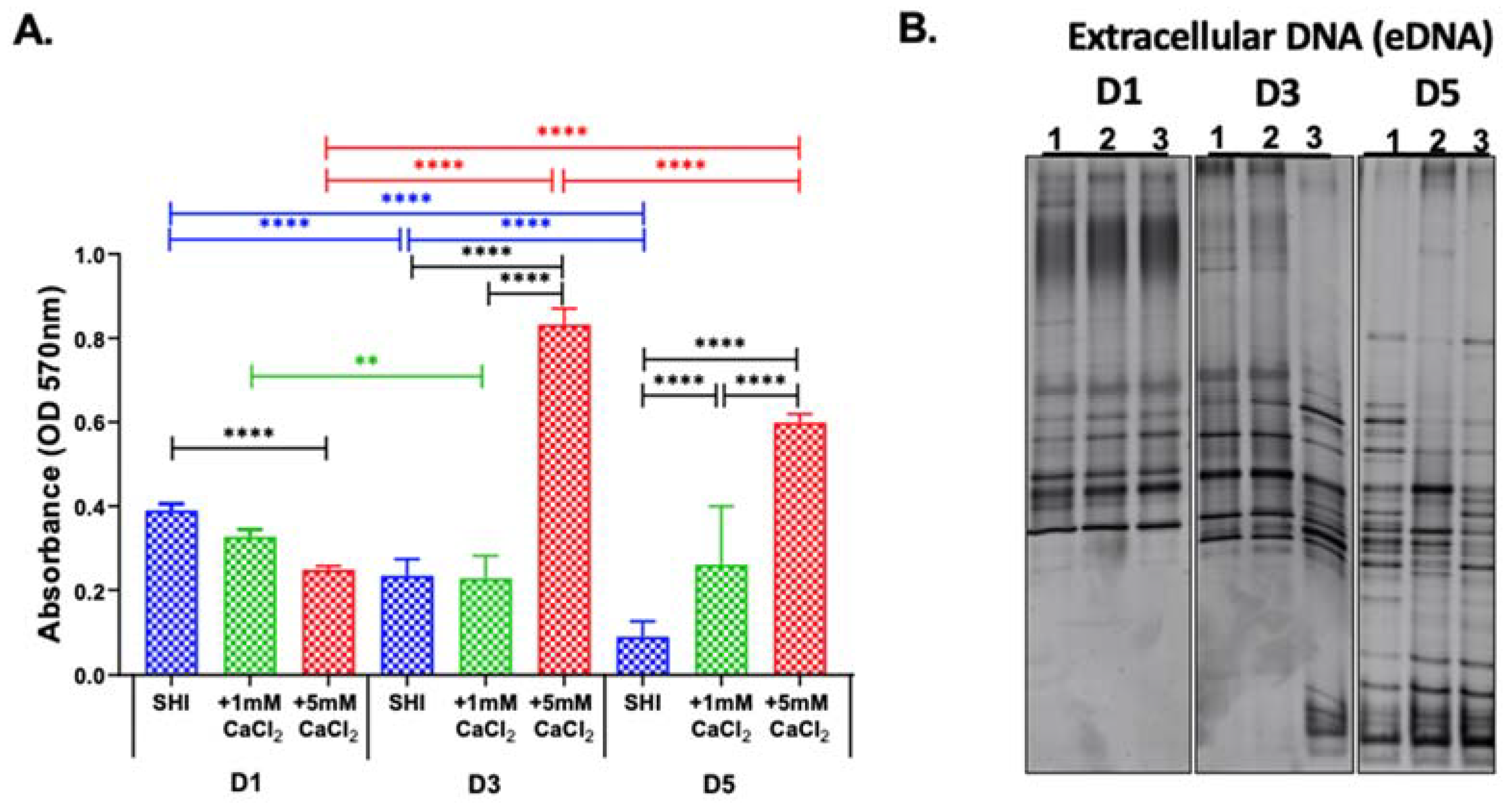
3.3. eDNA Profile
3.4. Biomass and Viability of Biofilms from Saliva of Healthy and Periodontitis Patients
3.5. 16S rRNA Sequencing of iDNA
3.6. Beta Diversity of iDNA and eDNA
3.7. Microbial Contribution to eDNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Iaderosa, G.; Corana, M.; Romano, F.; Citterio, F.; Giacomino, A.; Berta, G.N.; Aimetti, M. Macro and trace elements signature of periodontitis in saliva: A systematic review with quality assessment of ionomics studies. J. Periodontal. Res. 2022, 57, 30–40. [Google Scholar] [CrossRef]
- Sewon, L.; Makela, M. A study of the possible correlation of high salivary calcium levels with periodontal and dental conditions in young adults. Arch. Oral Biol. 1990, 35, 211S–212S. [Google Scholar] [CrossRef]
- Sewon, L.A.; Karjalainen, S.M.; Soderling, E.; Lapinleimu, H.; Simell, O. Associations between salivary calcium and oral health. J. Clin. Periodontol. 1998, 25, 915–919. [Google Scholar] [CrossRef]
- Sewon, L.A.; Karjalainen, S.M.; Sainio, M.; Seppa, O. Calcium and other salivary factors in periodontitis-affected subjects prior to treatment. J. Clin. Periodontol. 1995, 22, 267–270. [Google Scholar] [CrossRef]
- Subbarao, K.C.; Nattuthurai, G.S.; Sundararajan, S.K.; Sujith, I.; Joseph, J.; Syedshah, Y.P. Gingival Crevicular Fluid: An Overview. J. Pharm. Bioallied Sci. 2019, 11, S135–S139. [Google Scholar] [CrossRef]
- Biswas, S.; Duperon, D.F.; Chebib, F.S. Study of crevice fluid in relation to periodontal disease in children. II. Effect of age, sex and gingival inflammation on crevice fluid protein, carbohydrate, total calcium, phosphate and nitrogen. J. Periodontal. Res. 1977, 12, 265–278. [Google Scholar] [CrossRef]
- Bang, J.; Cimasoni, G.; Rosenbusch, C.; Duckert, A. Sodium, potassium and calcium contents of crevicular exudate: Their relations to gingivitis and periodontitis. J. Periodontol. 1973, 44, 770–774. [Google Scholar] [CrossRef]
- Koregol, A.C.; More, S.P.; Nainegali, S.; Kalburgi, N.; Verma, S. Analysis of inorganic ions in gingival crevicular fluid as indicators of periodontal disease activity: A clinico-biochemical study. Contemp. Clin. Dent. 2011, 2, 278–282. [Google Scholar] [CrossRef]
- Kaslick, R.S.; Chasens, A.I.; Mandel, I.D.; Weinstein, D.; Waldman, R.; Pluhar, T.; Lazzara, R. Sodium, potassium and calcium in gingival fluid: A study of the relationship of the ions to one another, to circadian rhythms, gingival bleeding, purulence, and to conservative periodontal therapy. J. Periodontol. 1970, 41, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Is Acid-Precipitation of Salivary Proteins a Factor in Plaque Formation? Arch. Oral Biol. 1964, 9, 375–376. [Google Scholar] [CrossRef]
- Sewon, L.; Soderling, E.; Karjalainen, S. Comparative study on mineralization-related intraoral parameters in periodontitis-affected and periodontitis-free adults. Scand. J. Dent. Res. 1990, 98, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Sewon, L.; Soderling, E.; Karjalainen, S. A mineral-related feature of young plaque characteristic to periodontitis-affected adults. J. Periodontol. 1990, 61, 42–44. [Google Scholar] [CrossRef]
- Sewon, L.A.; Soderling, E. Calcium concentrations in dental plaque of patients with juvenile and adult periodontitis. J. Periodontol. 1987, 58, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; He, X.; Torralba, M.; Yooseph, S.; Nelson, K.E.; Lux, R.; McLean, J.S.; Yu, G.; Shi, W. Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol. Oral Microbiol. 2010, 25, 357–367. [Google Scholar] [CrossRef]
- Edlund, A.; Yang, Y.; Hall, A.P.; Guo, L.; Lux, R.; He, X.; Nelson, K.E.; Nealson, K.H.; Yooseph, S.; Shi, W.; et al. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome 2013, 1, 25. [Google Scholar] [CrossRef]
- Li, B.; Zhou, X.; Zhou, X.; Wu, P.; Li, M.; Feng, M.; Peng, X.; Ren, B.; Cheng, L. Effects of different substrates/growth media on microbial community of saliva-derived biofilm. FEMS Microbiol. Lett. 2017, 364, fnx123. [Google Scholar] [CrossRef]
- Wang, D.; Nambu, T.; Tanimoto, H.; Iwata, N.; Yoshikawa, K.; Okinaga, T.; Yamamoto, K. Interdental Plaque Microbial Community Changes under In Vitro Violet LED Irradiation. Antibiotics 2021, 10, 1348. [Google Scholar] [CrossRef]
- Lamont, E.I.; Gadkari, A.; Kerns, K.A.; To, T.T.; Daubert, D.; Kotsakis, G.; Bor, B.; He, X.; McLean, J.S. Modified SHI medium supports growth of a disease-state subgingival polymicrobial community in vitro. Mol. Oral Microbiol. 2021, 36, 37–49. [Google Scholar] [CrossRef]
- Zmantar, T.; Kouidhi, B.; Miladi, H.; Mahdouani, K.; Bakhrouf, A. A microtiter plate assay for Staphylococcus aureus biofilm quantification at various pH levels and hydrogen peroxide supplementation. New Microbiol. 2010, 33, 137–145. [Google Scholar] [PubMed]
- Agnello, M.; Marques, J.; Cen, L.; Mittermuller, B.; Huang, A.; Chaichanasakul Tran, N.; Shi, W.; He, X.; Schroth, R.J. Microbiome Associated with Severe Caries in Canadian First Nations Children. J. Dent. Res. 2017, 96, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Rupf, S.; Merte, K.; Eschrich, K. Quantification of bacteria in oral samples by competitive polymerase chain reaction. J. Dent. Res. 1999, 78, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010, 2010, baq013. [Google Scholar] [CrossRef]
- Rabiei, M.; Asli, H.N.; Mohamadi, M.H. Comparison of Salivary Calcium Level in Dentulous and Edentulous Patients. Eur. J. Dent. 2019, 13, 36–41. [Google Scholar] [CrossRef]
- Inonu, E.; Hakki, S.S.; Kayis, S.A.; Nielsen, F.H. The Association Between Some Macro and Trace Elements in Saliva and Periodontal Status. Biol. Trace Elem. Res. 2020, 197, 35–42. [Google Scholar] [CrossRef]
- Tanaka, M.; Margolis, H.C. Release of mineral ions in dental plaque following acid production. Arch. Oral Biol. 1999, 44, 253–258. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Serrage, H.J.; Jepson, M.A.; Rostami, N.; Jakubovics, N.S.; Nobbs, A.H. Understanding the Matrix: The Role of Extracellular DNA in Oral Biofilms. Front. Oral Health 2021, 2, 640129. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ou, Y.; Yang, L.; Zhu, Y.; Tolker-Nielsen, T.; Molin, S.; Qu, D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 2007, 153, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sharma, P.K.; Busscher, H.J.; van der Mei, H.C.; Krom, B.P. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl. Environ. Microbiol. 2010, 76, 3405–3408. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Kutty, S.K.; Kumar, N.; Manefield, M. Pyocyanin facilitates extracellular DNA binding to Pseudomonas aeruginosa influencing cell surface properties and aggregation. PLoS ONE 2013, 8, e58299. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Keren-Paz, A.; Maan, H.; Karunker, I.; Olender, T.; Kapishnikov, S.; Dersch, S.; Kartvelishvily, E.; Wolf, S.G.; Gal, A.; Graumann, P.L.; et al. The roles of intracellular and extracellular calcium in Bacillus subtilis biofilms. iScience 2022, 25, 104308. [Google Scholar] [CrossRef]
- Keren-Paz, A.; Brumfeld, V.; Oppenheimer-Shaanan, Y.; Kolodkin-Gal, I. Micro-CT X-ray imaging exposes structured diffusion barriers within biofilms. NPJ Biofilms Microbiomes 2018, 4, 8. [Google Scholar] [CrossRef]
- Oppenheimer-Shaanan, Y.; Sibony-Nevo, O.; Bloom-Ackermann, Z.; Suissa, R.; Steinberg, N.; Kartvelishvily, E.; Brumfeld, V.; Kolodkin-Gal, I. Spatio-temporal assembly of functional mineral scaffolds within microbial biofilms. NPJ Biofilms Microbiomes 2016, 2, 15031. [Google Scholar] [CrossRef]
- Keren-Paz, A.; Kolodkin-Gal, I. A brick in the wall: Discovering a novel mineral component of the biofilm extracellular matrix. New Biotechnol. 2020, 56, 9–15. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Koop, L.; Wong, Y.K.; Ahmed, S.; Siddiqui, K.S.; Manefield, M. Influence of calcium in extracellular DNA mediated bacterial aggregation and biofilm formation. PLoS ONE 2014, 9, e91935. [Google Scholar] [CrossRef] [PubMed]
- Astasov-Frauenhoffer, M.; Varenganayil, M.M.; Decho, A.W.; Waltimo, T.; Braissant, O. Exopolysaccharides regulate calcium flow in cariogenic biofilms. PLoS ONE 2017, 12, e0186256. [Google Scholar] [CrossRef] [PubMed]
- Safari, A.; Habimana, O.; Allen, A.; Casey, E. The significance of calcium ions on Pseudomonas fluorescens biofilms—A structural and mechanical study. Biofouling 2014, 30, 859–869. [Google Scholar] [CrossRef]
- Wilson, R.F.; Ashley, F.P. The relationship between the biochemical composition of dental plaque from both approximal and free smooth surfaces of teeth and subsequent 3-year caries increment in adolescents. Arch. Oral Biol. 1990, 35, 933–937. [Google Scholar] [CrossRef]
- Leitao, T.J.; Cury, J.A.; Tenuta, L.M.A. Kinetics of calcium binding to dental biofilm bacteria. PLoS ONE 2018, 13, e0191284. [Google Scholar] [CrossRef] [PubMed]
- Margolis, H.C.; Moreno, E.C. Composition of pooled plaque fluid from caries-free and caries-positive individuals following sucrose exposure. J. Dent. Res. 1992, 71, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pinder, K.L. Effects of calcium on development of anaerobic acidogenic biofilms. Biotechnol. Bioeng. 1995, 45, 212–218. [Google Scholar] [CrossRef]
- Rose, R.K.; Dibdin, G.H.; Shellis, R.P. A quantitative study of calcium binding and aggregation in selected oral bacteria. J. Dent. Res. 1993, 72, 78–84. [Google Scholar] [CrossRef]
- Rose, R.K.; Hogg, S.D.; Shellis, R.P. A quantitative study of calcium binding by isolated streptococcal cell walls and lipoteichoic acid: Comparison with whole cells. J. Dent. Res. 1994, 73, 1742–1747. [Google Scholar] [CrossRef]
- Wang, T.; Flint, S.; Palmer, J. Magnesium and calcium ions: Roles in bacterial cell attachment and biofilm structure maturation. Biofouling 2019, 35, 959–974. [Google Scholar] [CrossRef]
- Turakhia, M.H.; Characklis, W.G. Activity of Pseudomonas aeruginosa in biofilms: Effect of calcium. Biotechnol. Bioeng. 1989, 33, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Steiger, E.L.; Muelli, J.R.; Braissant, O.; Waltimo, T.; Astasov-Frauenhoffer, M. Effect of divalent ions on cariogenic biofilm formation. BMC Microbiol. 2020, 20, 287. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Kobayashi, K. Calcium Prevents Biofilm Dispersion in Bacillus subtilis. J. Bacteriol. 2021, 203, e0011421. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.J.; Hsu, R.B.; Shun, C.T.; Hsu, C.C.; Chia, J.S. AtlA Mediates Extracellular DNA Release, Which Contributes to Streptococcus mutans Biofilm Formation in an Experimental Rat Model of Infective Endocarditis. Infect. Immun. 2017, 85, e00252-17. [Google Scholar] [CrossRef] [PubMed]
- Pink, D.A.; Truelstrup Hansen, L.; Gill, T.A.; Quinn, B.E.; Jericho, M.H.; Beveridge, T.J. Divalent Calcium Ions Inhibit the Penetration of Protamine through the Polysaccharide Brush of the Outer Membrane of Gram-Negative Bacteria. Langmuir 2003, 19, 8852–8858. [Google Scholar] [CrossRef]
- Thomas, K.J., 3rd; Rice, C.V. Revised model of calcium and magnesium binding to the bacterial cell wall. Biometals 2014, 27, 1361–1370. [Google Scholar] [CrossRef]
- Kotra, L.P.; Golemi, D.; Amro, N.A.; Liu, G.-Y.; Mobashery, S. Dynamics of the Lipopolysaccharide Assembly on the Surface of Escherichia coli. J. Am. Chem. Soc. 1999, 121, 8707–8711. [Google Scholar] [CrossRef]
- Smithies, W.R.; Gibbons, N.E. The deoxyribose nucleic acid slime layer of some halophilic bacteria. Can. J. Microbiol. 1955, 1, 614–621. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: Conceptually new antibiotics. J. Bacteriol. 1996, 178, 2767–2774. [Google Scholar] [CrossRef]
- Ibanez de Aldecoa, A.L.; Zafra, O.; Gonzalez-Pastor, J.E. Mechanisms and Regulation of Extracellular DNA Release and Its Biological Roles in Microbial Communities. Front. Microbiol. 2017, 8, 1390. [Google Scholar] [CrossRef] [Green Version]
- Das, T.; Manefield, M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e46718. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.M.; Johnsen, P.J.; Bensasson, D.; Daffonchio, D. Release and persistence of extracellular DNA in the environment. Environ. Biosafety Res. 2007, 6, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Jabbouri, S.; Sadovskaya, I. Extracellular DNA-dependent biofilm formation by Staphylococcus epidermidis RP62A in response to subminimal inhibitory concentrations of antibiotics. Res. Microbiol. 2011, 162, 535–541. [Google Scholar] [CrossRef]
- Wagner, A.O.; Malin, C.; Knapp, B.A.; Illmer, P. Removal of free extracellular DNA from environmental samples by ethidium monoazide and propidium monoazide. Appl. Environ. Microbiol. 2008, 74, 2537–2539. [Google Scholar] [CrossRef]
- Hu, W.; Li, L.; Sharma, S.; Wang, J.; McHardy, I.; Lux, R.; Yang, Z.; He, X.; Gimzewski, J.K.; Li, Y.; et al. DNA builds and strengthens the extracellular matrix in Myxococcus xanthus biofilms by interacting with exopolysaccharides. PLoS ONE 2012, 7, e51905. [Google Scholar] [CrossRef] [PubMed]
- DeFrancesco, A.S.; Masloboeva, N.; Syed, A.K.; DeLoughery, A.; Bradshaw, N.; Li, G.W.; Gilmore, M.S.; Walker, S.; Losick, R. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2017, 114, E5969–E5978. [Google Scholar] [CrossRef]
- Dengler, V.; Foulston, L.; DeFrancesco, A.S.; Losick, R. An Electrostatic Net Model for the Role of Extracellular DNA in Biofilm Formation by Staphylococcus aureus. J. Bacteriol. 2015, 197, 3779–3787. [Google Scholar] [CrossRef]
- Kavanaugh, J.S.; Flack, C.E.; Lister, J.; Ricker, E.B.; Ibberson, C.B.; Jenul, C.; Moormeier, D.E.; Delmain, E.A.; Bayles, K.W.; Horswill, A.R. Identification of Extracellular DNA-Binding Proteins in the Biofilm Matrix. MBio 2019, 10, e01137-19. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokeen, B.; Pham, E.; Esfandi, J.; Kondo, T.; Okawa, H.; Nishimura, I.; Lux, R. Effect of Calcium Ion Supplementation on Oral Microbial Composition and Biofilm Formation In Vitro. Microorganisms 2022, 10, 1780. https://doi.org/10.3390/microorganisms10091780
Shokeen B, Pham E, Esfandi J, Kondo T, Okawa H, Nishimura I, Lux R. Effect of Calcium Ion Supplementation on Oral Microbial Composition and Biofilm Formation In Vitro. Microorganisms. 2022; 10(9):1780. https://doi.org/10.3390/microorganisms10091780
Chicago/Turabian StyleShokeen, Bhumika, Elaine Pham, Julia Esfandi, Takeru Kondo, Hiroko Okawa, Ichiro Nishimura, and Renate Lux. 2022. "Effect of Calcium Ion Supplementation on Oral Microbial Composition and Biofilm Formation In Vitro" Microorganisms 10, no. 9: 1780. https://doi.org/10.3390/microorganisms10091780
APA StyleShokeen, B., Pham, E., Esfandi, J., Kondo, T., Okawa, H., Nishimura, I., & Lux, R. (2022). Effect of Calcium Ion Supplementation on Oral Microbial Composition and Biofilm Formation In Vitro. Microorganisms, 10(9), 1780. https://doi.org/10.3390/microorganisms10091780





