Abstract
A new strain QHLA of Lecanicillium uredinophilum was isolated from a Chinese caterpillar fungus complex and its optimum growth temperature and fermentation conditions were studied. Its insecticidal activity was tested against larvae of seven different insect pests, including Henosepilachna vigintioctopunctata, Spodoptera exigua, Plutella xylostella, Spodoptera frugiperda, Sitobion avenae, Hyalopterus perikonus, and Aphis citricola. The optimum growth temperature was 21–24 °C. The highest spore production of the strain QHLA was 4.08 × 106 spore/mL on solid medium with a nitrogen source of NH4Cl. However, the highest mycelial growth rate of the strain QHLA was on solid medium with a nitrogen source from yeast extract (4.63 ± 0.03 mm/d). When the liquid medium contained peptone, yeast extract, and glucose, the water content of the mycelia was the lowest, while the spore production was the highest until day 12. When the liquid medium contained glucose, tussah pupa powder, KH2PO4, and MgSO4, the mycelia production was highest until day 8. The bioassay for insecticidal activity indicated that the LC50 values of QHLA were 6.32 × 103 spore/mL and 6.35 × 103 spore/mL against Sitobion avenae and Aphis citricola, respectively, while the LC90 values of the strain QHLA against Aphis citricola and Sitobion avenae were 2.11 × 107 spore/mL and 2.36 × 108 spore/mL, respectively. Our results demonstrated that the strain QHLA was a high virulence pathogenic fungus against insect pests, with the potential to be developed as a microbial pesticide.
1. Introduction
The Lecanicillium sp. is an important entomopathogenic fungus, and it is widely used in biological control in the field and plays a major role in the control of pests and nematodes [1]. It has been derived from number of sources, including soil, plants, decaying wood, and muscardine cadavers [2]. Previous studies have reported the separation and identification of Lecanicillium sp., including L. muscarium, L. psalliotae, L. lecanii, L. gracile, L. coprophilum, L. rubum, and L. testudineum [3,4,5,6].
Some of the fungi in the genus Lecanicillium are known to be effective against aphids, for example, L. longisporum is used for the control of Myzus persicae [7]. The secondary metabolites of L. attenuatum were effective against Aedes albopictus and Plutella xylostella, and its culture soup has higher insecticidal activity than mycelia cake [8]. L. antillanum that was isolated from soil had a negative effect on the egg structure of the nematode Meloidogyne incognita by secreting chitinase [9]. L. lecanii was isolated from within asymptomatic leaves of cotton, and insect bioassays demonstrated that it significantly reduced the rate and period of reproduction of A. gosspy [10]. L. lecanii also has the potential to control B. tabaci significantly [11], and the partially purified protein of the L. lecanii enhances the plant resistance to B. tabaci by regulating the content of secondary metabolites in the jasmonic acid (JA) and salicylic acid (SA) metabolic pathways of cotton plants [12]. L. muscarium is known to control the adult and larval stages of houseflies [13], Aleurodicus rugioperculatus, Encarsia guadeloupae [14], B. tabaci, Trialeurodes vaporariorum [6], and the sweetpotato whitefly [15]. The L. uredinophilum of Lecanicillium sp. was isolated from rusts and chitinous bodies, but its culture condition and insecticidal activity have not been reported [16,17].
The main objective of this study was to identify the strain QHLA isolated from the fresh samples of Chinese caterpillar fungus complex and to clarify the biological characteristics of the strain QHLA, especially for its optimal growth temperature and fermentation conditions. In addition, the potential pesticide application of QHLA was evaluated by using Henosepilachna vigintioctopunctata (Coleoptera Coccinellidae), Spodoptera exigua (Lepidoptera Noctuidae), Plutella xylostella (Lepidoptera Plutellidae), Spodoptera frugiperda (Lepidoptera Noctuidae), Sitobion avenae (Hemiptera Aphididae), Hayloperus perikonus (Hemiptera Aphididae), and Aphis citricola (Hemiptera Aphididae) [18,19,20,21,22]. This research will provide a better understanding of the optimal growth temperature, carbon and nitrogen sources, the composition of the fermentation broth, and the effective pesticidal range of the QHLA and provide a theoretical basis for its production and application in the field.
2. Materials and Methods
2.1. Specimens Collection and Isolation
Samples were collected from the Tibetan Autonomous Prefecture of Guoluo, Qinghai Province in May 2019. The specimens were isolated by 1/4 SDAY medium containing antibiotics (peptone 2.5 g/L, D-glucose anhydrous 10 g/L, yeast extract 2.5 g/L, streptomycin 0.6 g/L, kanamycin 0.6 g/L, agar 15 g, 1000 mL of water), then cultured in an artificial incubator at 15 °C for 15 d. The edges of the colonies were selected and purified in the new 1/4 SDAY medium.
2.1.1. Morphological Observation of Strain
The purified strain was cultured in 1/4 SDAY medium at 15 °C and shaded for 30 d. The growth characteristics of the strain were observed, including colony morphology, mycelial color, exudate color, and growth rate. The morphological characteristics of strain were observed by microscope (S/N:C 1709110263, SOP TOP; Ningbo Sunny Instruments Co., Ltd., Zhejiang, China), mainly including mycelial characteristics, sporulation structure characteristics, spore morphology, size, and the isolated strain was identified.
2.1.2. Extraction and Amplification of Genomic DNA
The mycelia for DNA extraction were harvested from plated colonies using a sterile needle and placed in a 1.5 mL centrifuge tube. The genomic DNA was isolated using the method described by Aljanabi [23].
The purified DNA was used as a template to amplify the target genes using polymerase chain reaction (PCR). The PCR amplification was performed in 50 μL volumes containing 25 μL PCR mixture (GenStar, Beijing, China), 21 μL of twice-sterilized water, 1 μL of each primer, and 2 μL DNA template. The internal transcribed spacer (ITS), large subunit ribosomal RNA (nrLSU), small subunit ribosomal RNA (nrSSU), RNA polymerase II largest subunit (RPB1), and RNA polymerase II second largest subunit (RPB2) [24,25,26,27,28] were amplified with the primers and procedures mentioned in Supplementary Material Table S1. The PCR products were sequenced by Sangon Biotech (Shanghai, China).
2.1.3. Phylogenetic Analysis
The sequences were submitted to GenBank (accession numbers were listed in Supplementary material Table S2) and compared with available data from the GenBank database (closest identified relatives based on a BLAST search and accessed on 23 June 2022, National Centre for Biotechnology Information website; https://www.ncbi.nlm.nih.gov/) [29]. The closely related and the other entomogenous fungus sequences obtained from GenBank were used for phylogenetic analysis to identify the fungi. Each sequence was an independent file, and MEGA X was used to align each sequence. Then, a cladogram was used for maximum likelihood (ML) analyses in PhyloSuite v1.2.1 [30,31,32], employing a BIC model for all partitions, and nodal support was assessed with nonparametric bootstrap using 1000 pseudo-replicates. ITS, RPB1, and RPB2 were used for Pairwise sequence (p-)distance analysis. (P-)distance analysis was performed with MEGA X using the compute pairwise distances model inferred from distance test and variance estimation method with the bootstrap method.
2.2. Biological Characteristics of the QHLA
2.2.1. Determination of Optimized Culture Temperature for the Fungus
The isolated strain was cultured until the colony was a regular circle with a diameter of about 60.0 mm. Then, the 7.0 mm punch was used to drill holes along the edge of the colony. The discs were inoculated on 1/4 SDAY in 90.0 mm Petri dishes in a chamber (SPX-420B, Shanghai Nanrong laboratory equipment Co., Ltd., Shanghai, China) for 14 d at 15 °C, 18 °C, 21 °C, 24 °C, and 27 °C. Every sample was processed in triplicate.
2.2.2. Determination of Optimized Nitrogen Sources for the Fungus
According to the complete randomized design, based on Czapek–Dox medium (sodium nitrate 3.0 g/L, potassium dihydrogen phosphate 1.0 g/L, magnesium sulfate heptahydrate 0.5 g/L, potassium chloride 0.5 g/L, ferrous sulfate 0.01 g/L, sucrose 30.0 g/L, agar 20.0 g/L), the sodium nitrate was replaced with different nitrogen sources; the specific amount was shown below: soybean power (9.5 g/L), peptone (4.0 g/L), silkworm pupa powder (7.3 g/L), ammonium nitrate (1.4 g/L), ammonium chloride (1.9 g/L), yeast extract (3.9 g/L). No nitrogen source was added to the control group. Then, the 7.0 mm punch was used to drill holes along the edge of the colony. The discs were inoculated on different nitrogen sources medium. They were then grown in an incubator at 24 °C, out of the light. Their diameters were measured after 14 d and their growth rates were calculated. Every sample was processed in triplicate.
2.2.3. Determination of Optimized Carbon Sources for the Fungus
According to the complete randomized design, based on the Czapek–Dox medium, the sucrose was replaced with different carbon sources; the specific amount was shown below: glucose (31.6 g/L), maltose (30.0 g/L), lactose (30.0 g/L), D-mannitol (31.6 g/L), fructose (31.6 g/L), D-sorbitol (31.6 g/L). Then the 7.0 mm punch was used to drill holes along the edge of the colony. The discs were inoculated on different carbon sources medium. In addition, no carbon source was added to the control group. They were then grown in an incubator at 24 °C, out of the light. Their diameters were measured after 14 d and their growth rates were calculated. Every sample was processed in triplicate.
2.2.4. Determination of Optimized Liquid Medium for the Fungus
The strains were cultured in 1/4 SDAY liquid medium at 145 rpm/min at 24 °C until the concentration of spores in the medium was 1 × 107 spore/mL. Then, the prepared spore suspension was inoculated in five different media with a volume of 150 mL and at a 5% dose (7.5 mL). The medium used in this study was: A (potato 20.0 g/L, glucose 20.0 g/L), B (D-mannose 22.0 g/L, yeast extract 2.0 g/L, KH2PO4 1.0 g/L, MgSO4 0.5 g/L), C (glucose 22.0 g/L, yeast extract 2.0 g/L, KH2PO4 1.0 g/L, MgSO4 0.5 g/L), D (glucose 20.0 g/L, tussah pupa powder 10.0 g/L, KH2PO4 1.5 g/L, MgSO4 1.2 g/L), E (peptone 10.0 g/L, yeast extract 10.0 g/L, glucose 20.0 g/L) [33]. Each medium was carried out in triplicate. Every 72 h, 4 mL culture medium was taken, 3 mL of which was centrifuged at 10,000 rpm for 10 min. Precipitation was collected and dried with absorbent paper, and the weight of precipitation was measured (m1). After lyophilization, the weight of fermentation was measured again (m2). The water content of metabolism was calculated by the formula: water content (W%) = [1 − (m1 − m2)/m1] × 100. Then we counted the number of spores in the culture medium using a hemocytometer.
2.3. Bioassay for Insecticidal Activity
The fungus was cultured at 24 °C on 1/4 SDAY for 14 d to obtain mycelium. After that, an appropriate amount of mycelium was taken with a sterile needle and inoculated in the broth of D in a shaker cultured for 14 d to obtain conidium. The conidium suspension was harvested from the cultured broth by centrifugation (10,000 rpm, 5 min). The centrifuged spores were suspended with sterile water containing 0.5% glycerin and 0.01% Tween-20 into spore suspension, which have been diluted into the concentrations of 1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107 spore/mL and 50 mL for each concentration. The control group was given sterile water only containing 0.5% glycerin and 0.01% Tween-20.
The insects tested in this study were Henosepilachna vigintioctopunctata, Spodoptera exigua, Plutella xylostella, Spodoptera frugiperda, Sitobion avenae, Hyalopterus perikonus, and Aphis citricola. The third instar larva of similar size was selected for each species in 18 groups of 15. The insects were fed in an 11.0 × 8.0 × 5.4 (cm, length × width × height) box. The prepared spore suspension was sprayed into the box evenly, and every box received 1 mL. Some food was placed in each box, and insect deaths were counted every three days.
2.4. Statistical Analysis
All the bioassay experiments were performed independently in triplicates. The lethal concentrations (LC50, LC90) were determined using PoloPlus 2.0 [34]. Our data were analyzed using SPSS, and our presented mean was ±1 standard error of the mean. p < 0.05 was considered statistically significant.
3. Results
3.1. Isolation and Identification of Strain
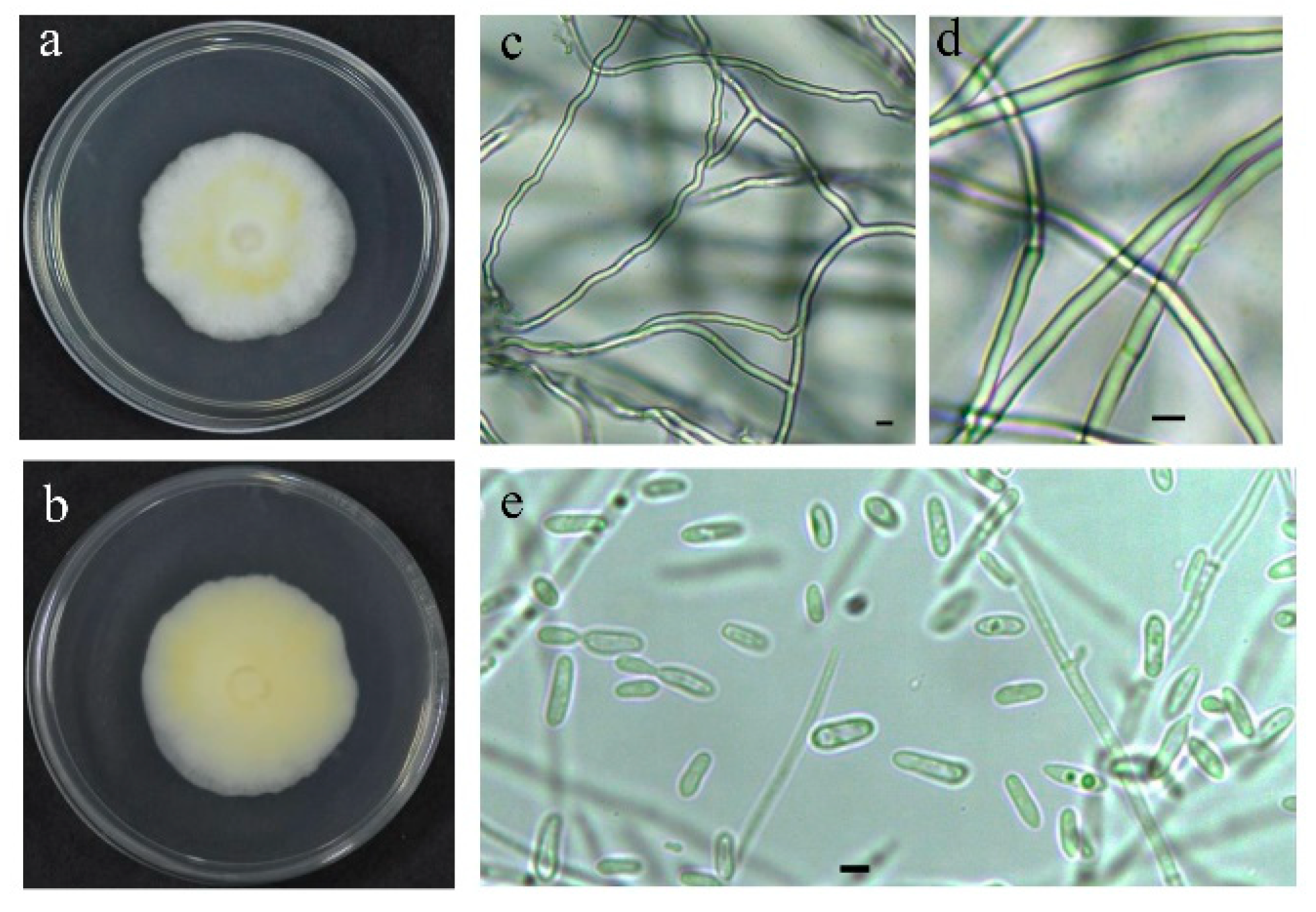
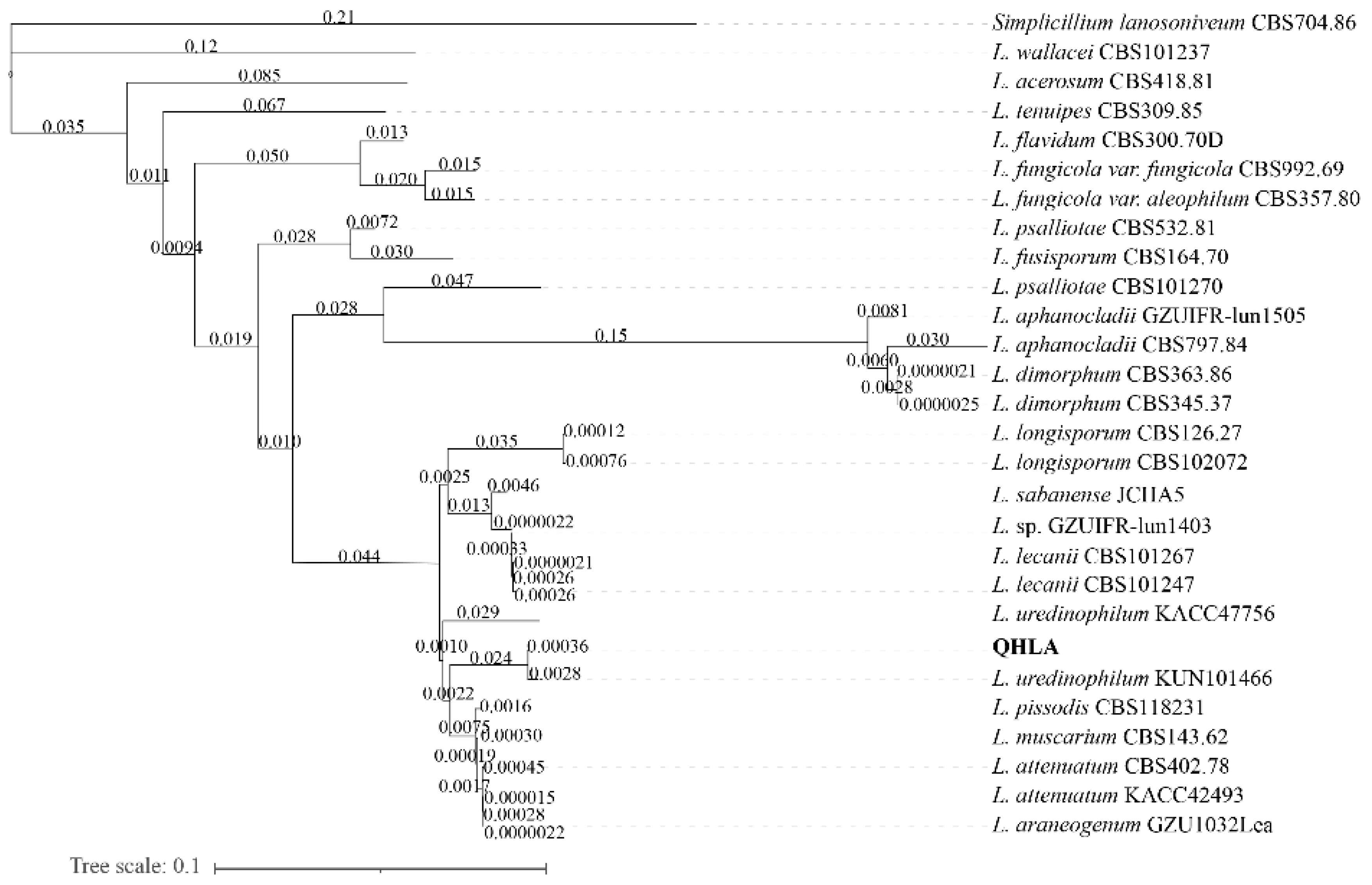
The strain QHLA was isolated from the Chinese caterpillar fungus complex (Cordyceps sinensis) samples collected in Guoluo, Qinghai Province. The strain QHLA was cultured for 30 d on 1/4 SDAY at 15 °C. The colony diameter was 4.5 cm and the middle of the obverse of the colony was light yellow and the surrounding area was white. The mycelia were dense and villous. The reverse of the colony was yellow and the edge was white (Figure 1a,b). Mycelia were colorless, linear, or spiral, without septum, branching, and 0.5–2.0 μm in diameter. The conidia were elliptic to short clubbed, (2.5–5.0) × (1.0–1.5) μm (Figure 1c–e). The phylogenetic tree was established with Simplicillium lanosoniveum CBS704.86 as the outgroup (Figure 2). The strain QHLA and L. uredinophilum KUN101466 were clustered in the same branch, and the results of the pairwise sequence (p-)distance analysis were shown in the Supplementary Material Tables S3–S5. After morphological and molecular identification, QHLA was L. uredinophilum.
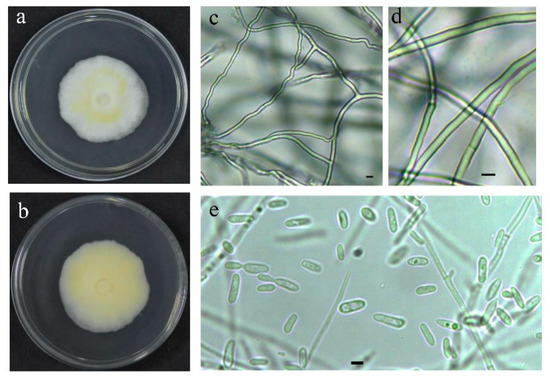
Figure 1.
The characteristic features of the QHLA in a petri dish: (a,b). The microscopic features of the QHLA: (c–e). Bars = 2.5 μm.
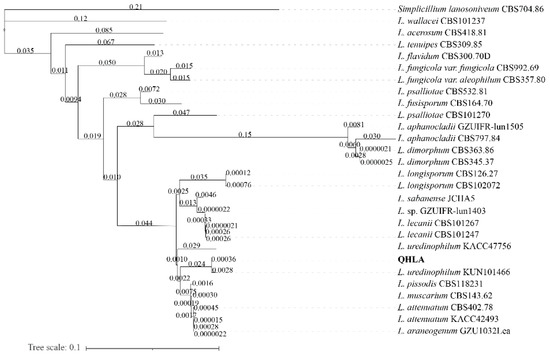
Figure 2.
Phylogenetic tree based on ITS nrSSU nrLSU RPB1 and RPB2 sequences by using PhyloSuite v1.2.1.
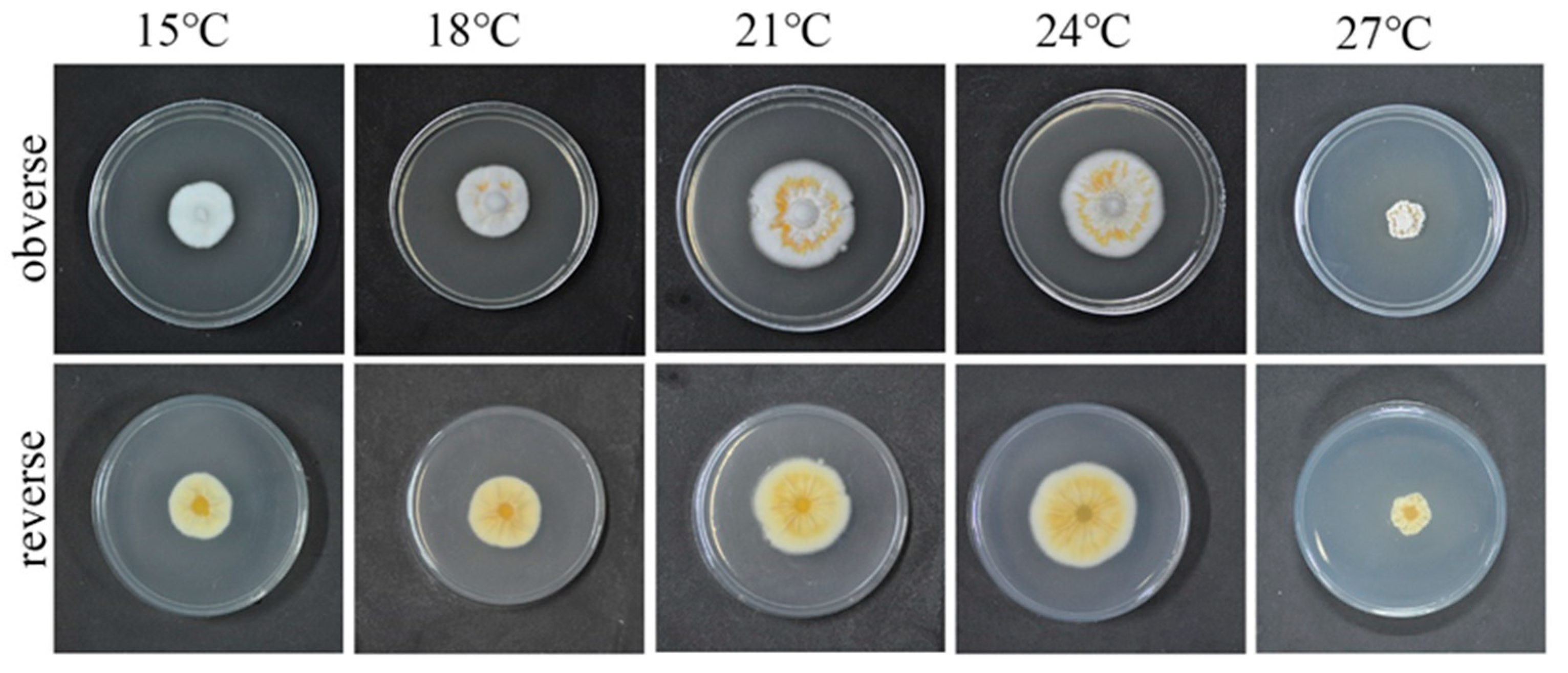
3.2. Optimum Culture Temperature for the QHLA
The growth rate of the strain QHLA growing at 15–24 °C was increased in a temperature-dependent manner, while significant inhibition was observed when the temperature reached 27 °C. The relationship between mycelium growth rate and the temperature was shown in Figure 3. When the temperature was 15 °C, the obverse side of the colony was white and the reverse side was milky-white. At 18 °C, the obverse side of the colony was white, with some yellow aerial mycelia in the middle, and the reverse side was light yellow. The obverse side of the strain was white, the middle circle was orange, and the reverse side was yellow, when the temperature was 21 °C and 24 °C. The mycelia shriveled and grew into the culture medium; it was brown along with the plate and yellow on the reverse side when the temperature was 27 °C.
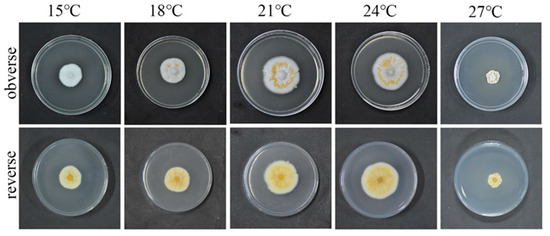
Figure 3.
QHLA growth at different temperatures.
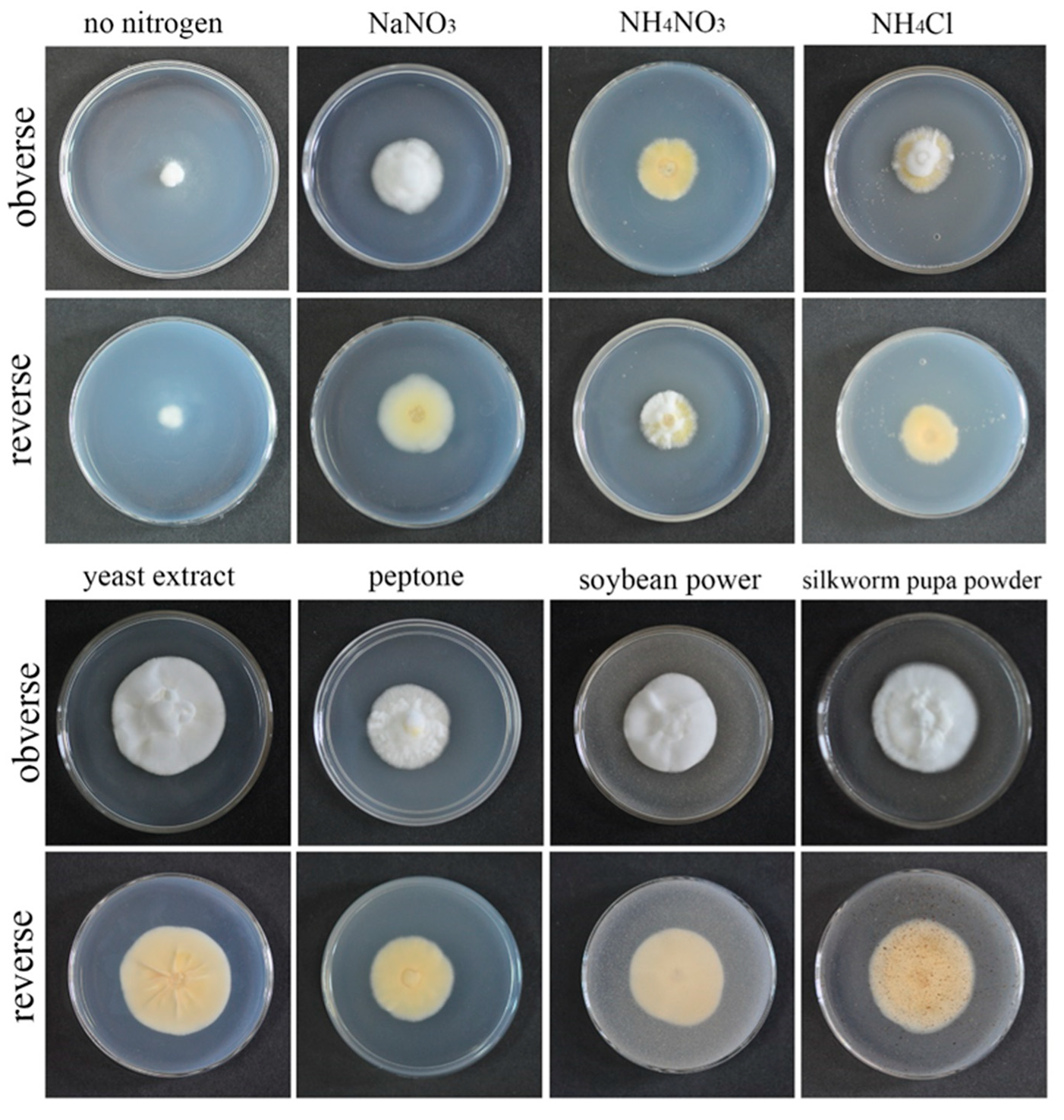
3.3. Effects of Different Nitrogen Sources on Growth Rate and Sporulation Quantity of the Strain QHLA
QHLA grew normally on eight different kinds of media. The growth rate of the QHLA was yeast extract > silkworm pupa powder > soybean power > peptone > NaNO3 > NH4NO3 > NH4Cl > no nitrogen. The highest mycelial growth rate of QHLA was observed on yeast extract and except for the silkworm pupa powder (there was no significant difference between them), the growth rate was significantly difference from that of other media. The spore production of the QHLA was the highest in the medium with NH4Cl as a nitrogen source, which was significantly different from other media. No spores were produced in a medium without any nitrogen source (Table 1, Figure 4).

Table 1.
Colony growth rate, spore production, and colony morphology of the QHLA on different nitrogen sources.
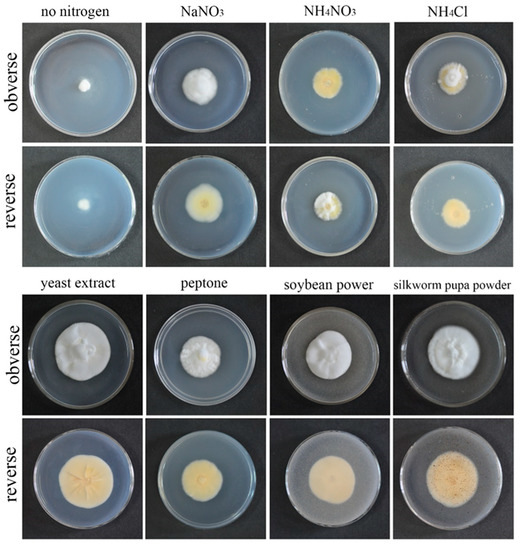
Figure 4.
Growth of the QHLA on different media with different nitrogen sources.
3.4. Effects of Different Carbon Sources on Growth Rate and Sporulation Quantity of the Strain QHLA
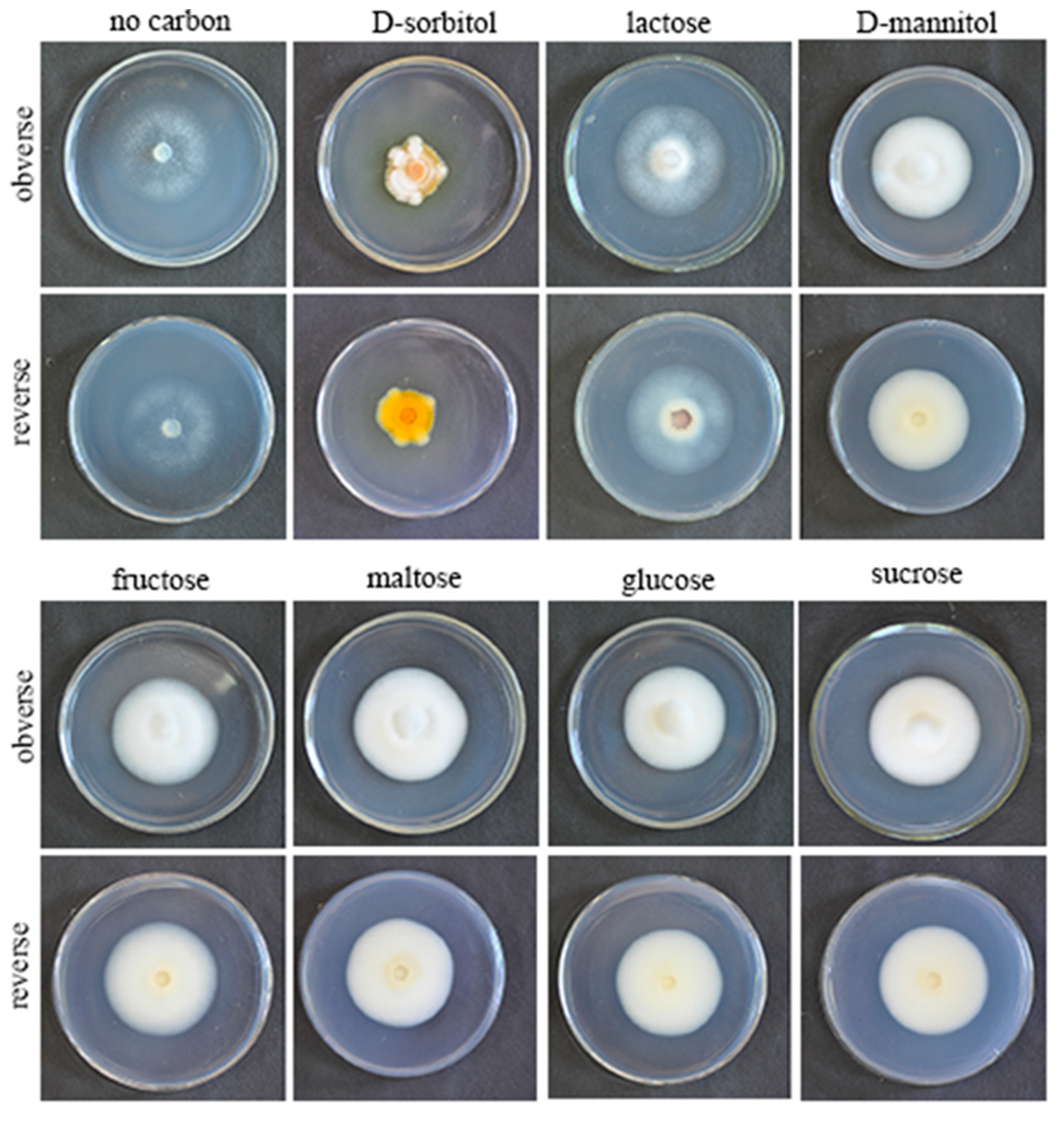
QHLA grew normally on eight different kinds of media. The growth rate of QHLA was maltose > fructose > D-mannitol > lactose > glucose > sucrose > D-sorbitol > no carbon (Figure 2). The highest mycelial growth rate of QHLA was observed on maltose; but there was no significant difference between maltose, fructose, D-mannitol, lactose, and glucose. The spore production of the QHLA was the highest in the medium with D-sorbitol as a nitrogen source, which was significantly different from other media. No spores were produced in a medium without any carbon source (Table 2, Figure 5).

Table 2.
Colony growth rate, spore production, and colony morphology of QHLA on different carbon sources.
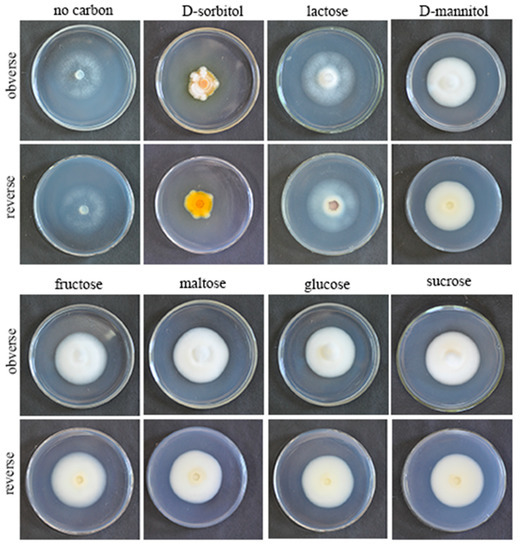
Figure 5.
Growth of the QHLA on different media with different carbon sources.
3.5. Optimum Liquid Medium for the QHLA
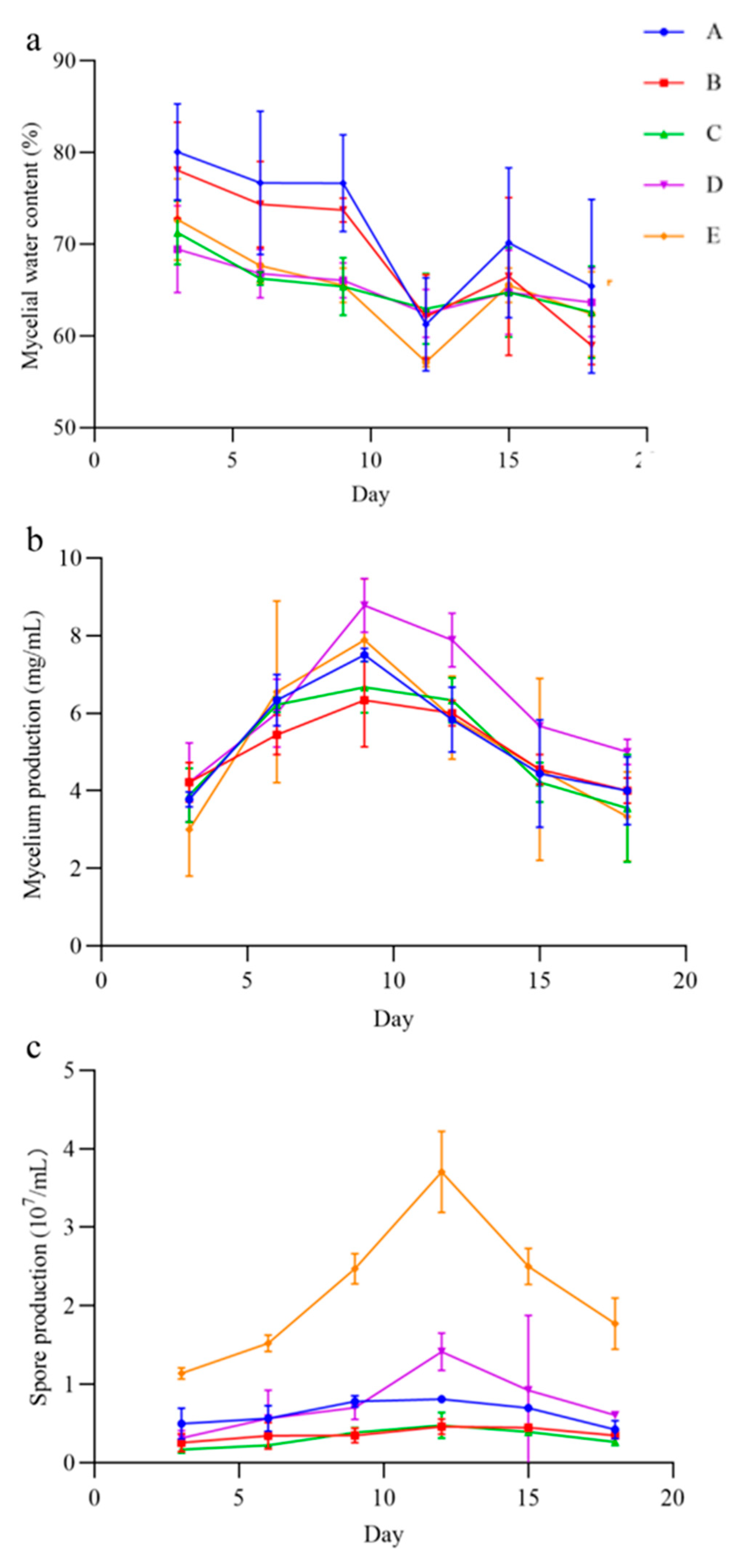
In the five liquid culture, the QHLA grew mycelia and produced spores, but the yield of mycelia and spores varied with the different media (Figure 6). The mycelia water content of the QHLA was the lowest in medium A, at 57.15 ± 0.54%, and was the highest in medium E. The mycelium production was the highest in medium D, which was 8.78 ± 0.69 mg/mL and the lowest in medium E. The spore production was significantly higher in medium E than in other media, at 3.71 ± 0.52 mg/mL, and the lowest in medium B.
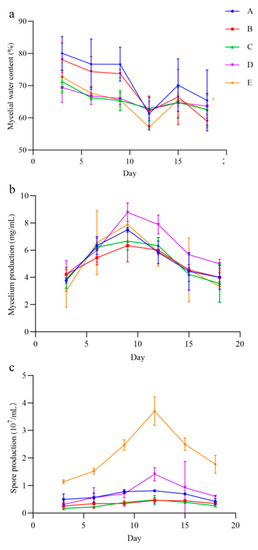
Figure 6.
The mycelia water content, mycelium production, and spore production in different media of the QHLA. A (potato, glucose), B (D-mannose, yeast extract, KH2PO4, MgSO4), C (glucose, yeast extract, KH2PO4, MgSO4), D (glucose, tussah pupa powder, KH2PO4, MgSO4), E (peptone, yeast extract, glucose). Note: (a): mycelial water content of the QHLA; (b): mycelium production of the QHLA; (c): spore production of the QHLA.
With the increase in incubation time, the mycelial water content; mycelium production; and spore production of the QHLA also changed. The water content of mycelia was the highest at the initial stage of culture, gradually decreasing with the advance of culture time, and it reached its lowest point at the 12th d of culture. After that, the water content of mycelia increased again. Both mycelium production and spore production had a trend of first increasing and then decreasing during the incubation time. Mycelium production reached the highest value on the 9th d, while spore production reached the highest value on the 12th d.
3.6. Bioassay
The QHLA had the best biocontrol effect on S. avenae and A. citricola, with LC50 of 6.32 × 103 spore/mL and 6.35 × 103 spore/mL, respectively. However, as the concentration of spores increased, the QHLA had different control effects on the two aphids. LC90 of the QHLA on A. citricola was 2.11 × 107 spore/mL and 2.36 × 108 spore/mL on S. avenae (Table 3).

Table 3.
The LC50 and LC90 of the QHLA.
4. Discussion
The Chinese caterpillar fungus (C. sinensis), a well-known entomopathogenic fungus in traditional Chinese medicine, is a complex formed by Ophiocordycepes sinensis parasitic larvae of the ghost moth (Hepialus armoricanus Obertheir, Lepidoptera) [35]. Previous experiments have also isolated a variety of other fungi, such as Isaria feline, Samsoniella hepialid, I. farinose, I. fumosorosea [36,37]. In addition, the fungi of the Lecanicillium sp. come from a wide range of sources and are widely distributed. For example, L. cauligalbarum emerges from the corpse of a stemborer (Lepidoptera) [38], and L. primulinum was isolated from soil in Okinawa’s main island and the Bonin Islands [39]. L. subprimulium originates from decaying wood in Baoshan City, Yunnan Province, China [1].
In this study, the strain QHLA was isolated from C. sinensis. After phylogenetic analysis of a combined data set comprising ITS, nrSSU, nrLSU, RPB1, and RPB2, sequence data supported that QHLA was L. uredinophilum. At the same time, the morphological result of QHLA was consistent with the results described by Zare and Gams for the Lecanicillium sp. [40]. The L. uredinophilum was first reported in Korea and was isolated from a rust fungus [16]. In China, L. uredinophilum was first obtained from an infected insect collected in Yunnan Province [17].
Environmental factors, especially temperature and humidity, affect the growth rate of fungi [41,42]. The optimum culture temperature for Lecanicillium sp. is 24 °C, and for the strain, QHLA is between 21 °C to 24 °C [43]. In addition to temperature and humidity, nutrition, mineral elements, vitamins, and other cultural conditions also have a great influence on the growth rate and sporulation of fungi, and the Lecanicillium sp. fungi are cultured in a variety of media. For example, L. lecanii CA-A-G is favorable for sporulation at 29 °C, a pH of 4, and under full light. However, when the temperature is 23 °C, the pH drops to 3, and photology is 12L:12D, the environment is more conducive to the accumulation of mycelia and other biomass of the strain [44]. L. lecanii SN21 is cultured in a variety of media, and a medium with a high C/N ratio is favorable for producing spores [45].
Lecanicillium sp. are entomopathogenic fungi that invade host insects by secreting cell wall-degrading enzymes, such as chitinase and lipase, and then its host insects are effectively killed [46,47]. L. attenuatum was reported to be effective in controlling cypress aphids (Cinara cupressi) with LC50 3 × 105 spore/mL [48], and L. attenuatum also has a control effect on cotton aphid (A. gossypii) [49]. The LT50 for L. attenuatum and L. longisporum to M. persicae, Macrosiphum euphorbiae, and Aulacorthum solani are between 2 d to 4 d, indicating a potential for biological control [50].
L. muscarium works with A. nigripes to enhance virulence against Potato Aphid [51], and strains of L. muscarium are also used for the control of B. tabaci, T. vaporariorum, A. craccivora, and Pristiphora abietina. Moreover, the activity of L. muscarium to Aphis craccivora is higher than that of M. pingshaense and B. Bassiana [6,52,53]. The V-3 strain of L. lecanii has a high control effect on whitefly (B. tabaci), with a maximum mortality rate of 90.6%. In addition, the filtrate of the V-3 strain also controls B. tabaci [11], and the temperature at which L. lecanii is most active against B. tabaci is 24 °C [54].
L. lecanii SN21 is also used to control Frankliniella occidentalis (Thysanoptera: Thripidae) [39]. However, different L. lecanii and L. muscarium have varied virulence to T. castaneum, and LC50, which ranged from 2.83 × 105 to 6.133 × 1022 spore/mL [55]. According to current research results, Lecanicillium sp. has prospective application potential in biological control, especially for Hemiptera control. In addition, the virulence of Lecanicillium sp. against various biological stages of T. vaporariorum was enhanced after protoplasmic fusion [56].
There are many species of entomogenous fungi that are used for aphid biological control, and our study focused on the insecticidal range of L. uredinophilum. We found that the strain of L. uredinophilum QHLA has the best control effect on aphids, especially on S. avenae and A. citricola. The LT50 of Pandora neoaphidis and Entomophthora planchoniana for S. avenae was about 5 d, which merits further study of the QHLA [57].
5. Conclusions
A new strain QHLA of L. uredinophilumwas was isolated from the Chinese caterpillar fungus. The QHLA was highly pathogenic against S. avenae and A. citricola with LC50 of 6.32 × 103 spore/mL and 6.35 × 103 spore/mL, respectively. For optimized culture conditions, the best sporulation was produced with NH4Cl as the nitrogen source and D-sorbitol as the carbon source, while the best mycelial growth rate was obtained with the yeast extract as the nitrogen source and maltose as the carbon source. For the liquid culture of the strain QHLA, peptone; yeast extract; and glucose were necessary for the best spore production with a fermentation period of 12 days, while glucose, tussah pupa powder, KH2PO4, and MgSO4 were necessary for the highest mycelia production with a fermentation period of 8 days.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10091832/s1, Table S1: Gene and primers used in the phylogenetic analyses; Table S2. Specimen information and GenBank accession number for strains used in this study. Tables S3–S5: the results of the pairwise sequence (p-)distance analysis.
Author Contributions
The study protocol was designed by Y.M. and D.W. The samples were collected for this study by Y.M. and D.W. The experimental work was done by Y.M. and P.I.D.W.H.D. The draft writing was done by Y.M. and P.I.D.W.H.D. Analysis and interpretation of the results were done by Y.M. and D.W. All authors contributed to editing and approving the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Shaanxi Key Project of Science and Technology (2020zdzx03-03-02) and the National key research and development project (2018YFD0600202-03).
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
The sequences of QHLA (Accession numbers are listed in Table S2) are available in the Genack datable.
Acknowledgments
We thank Ken Smith from University of Arizona for the language editing for this manuscript.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Huang, S.K.; Maharachchikumbura, S.; Jeewon, R.; Bhat, D.J.; Al-Sadi, A.M. Lecanicillium subprimulinum (Cordycipitaceae, Hypocreales), a novel species from Baoshan, Yunnan. Phytotaxa 2018, 348, 99–108. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Q.; Wang, D.; Zou, W.Q.; Tang, D.X.; Hongthong, P.; Yu, H. Species diversity and virulence potential of the Beauveria bassiana complex and Beauveria scarabaeidicola complex. Front. Microbiol. 2022, 13, 841604. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, X.; Tian, B.; Wang, M.; Niu, Q.; Zhang, K. Isolation and characterization of a serine protease from the nematophagous fungus, Lecanicillium psalliotae, displaying nematicidal activity. Biotechnol. Lett. 2005, 27, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhu, H.; Guo, Y.; Du, X.; Guo, J.; Zhang, L.; Qin, C. Lecanicillium coprophilum (Cordycipitaceae, Hypocreales), a new species of fungus from the feces of Marmota monax in China. Phytotaxa 2019, 387, 55–62. [Google Scholar] [CrossRef]
- Ponizovskaya, V.B.; GrumGrzhimaylo, A.A.; Georgieva, M.L.; Kokaeva, L.T.; Bilanenko, E.N. Lecanicillium gracile (Cordycipitaceae), a new species isolated from mineral building materials. Phytotaxa 2020, 443, 265–278. [Google Scholar] [CrossRef]
- Moyo, D.; Ishikura, S.; Rakotondrafara, A.; Clayton, M.; Kinoshita, R.; Tani, M.; Koike, M.; Aiuchi, D. Behavioral change of Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) infected by Lecanicillium muscarium (Hypocreales: Cordycipitaceae). Appl. Entomol. Zool. 2021, 56, 327–336. [Google Scholar] [CrossRef]
- Mitina, G.V.; Stepanycheva, E.A.; Choglokova, A.A.; Cherepanova, M.A. Features of behavioral reactions of the peach aphid Myzus persicae (Sulzer, 1776) (Hemiptera, Aphididae) to volatile organic compounds of entomopathogenic fungi of the genus Lecanicillium. Entomol. Rev. 2022, 101, 1015–1023. [Google Scholar] [CrossRef]
- Woo, R.M.; Park, M.G.; Choi, J.Y.; Park, D.H.; Kim, J.Y.; Wang, M.; Kim, H.J.; Woo, S.D.; Kim, J.S.; Je, Y.H. Insecticidal and insect growth regulatory activities of secondary metabolites from entomopathogenic fungi, Lecanicillium attenuatum. J. Appl. Entomol. 2020, 144, 655–663. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Wang, S.L.; Nguyen, A.D.; Doan, M.D.; Tran, D.M.; Nguyen, T.H.; Ngo, V.A.; Doan, C.T.; Tran, T.N.; Do, V.C.; et al. Potential application of rhizobacteria isolated from the Central Highland of Vietnam as an effective biocontrol agent of Robusta Coffee nematodes and as a bio-fertilizer. Agronomy 2021, 11, 1887. [Google Scholar] [CrossRef]
- Gonalves, M.F.M.; Abreu, A.C.; Sandra, H.; Alves, A. Diversity of marine fungi associated with wood baits in the estuary Ria de Aveiro, with descriptions of Paralulworthia halima, comb. nov. Remispora submersa, sp. nov. and Zalerion pseudomaritima, sp. Nov. Mycologia 2021, 113, 664–683. [Google Scholar] [CrossRef]
- Abdulle, A.Y.; Nazir, T.; Keerio, A.U.; Ali, H.; Zaman, S.; Anwar, T.; Nam, T.D.; Qiu, D. In vitro virulence of three Lecanicillium lecanii strains against the whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae). Egypt. J. Biol. Pest Control 2020, 30, 129. [Google Scholar] [CrossRef]
- Abdulle, A.Y.; Nazir, T.; Sayed, S.; Mahmoud, F.S.; Majeed, Z.M.; Aslam, U.M.H.; Iqbal, Z.; Nisar, S.M.; Keerio, U.A.; Ali, H.; et al. Sub-Lethal Effects of Lecanicillium lecanii (Zimmermann)-derived partially purified protein and its potential implication in Cotton (Gossypium hirsutum L.) defense against Bemisia tabaci Gennadius (Aleyrodidae: Hemiptera). Agriculture 2021, 11, 778. [Google Scholar] [CrossRef]
- Mochi, D.A.; Monteiro, A.C.; Simi, L.D.; Sampaio, M.A.A. Susceptibility of adult and larval stages of the horn fly, Haematobia irritans, to the entomopathogenic fungus Metarhizium anisopliae under field conditions. Vet. Parasitol. 2009, 166, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Sumalatha, B.V.; Selvaraj, K.; Poomesha, B.; Ramanujam, B. Pathogenicity of entomopathogenic fungus Isaria fumosorosea on rugose spiralling whitefly Aleurodicus rugioperculatus and its effect on parasitoid Encarsia guadeloupae. Biocontrol Sci. Technol. 2020, 30, 1150–1161. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Mathers, J.J.; Northing, P. The integrated use of chemical insecticides and the entomopathogenic nematode, Steinernema carpocapsae (Nematoda: Steinernematidae), for the control of sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Sci. 2008, 15, 447–453. [Google Scholar] [CrossRef]
- Park, M.J.; Hong, S.B.; Shin, H.D. Lecanicillium uredinophilum sp. nov. associated with rust fungi from Korea. Mycotaxon 2015, 12, 997–1005. [Google Scholar] [CrossRef]
- Wei, D.P.; Wanasinghe, D.N.; Chaiwat, T.A.; Hyde, K.D. Lecanicillium uredinophilum known from rusts, also occurs on animal hosts with chitinous bodies. Asian J. Mycol. 2018, 1, 63–73. [Google Scholar] [CrossRef]
- He, W.; Zhao, X.; Ali, A.; Ge, S.; Zhang, H.; He, L.; Wu, K. Population dynamics and reproductive developmental analysis of Helicoverpa armigera (Lepidoptera: Noctuidae) trapped using food attractants in the field. J. Econ. Entomol. 2021, 114, 1533–1541. [Google Scholar] [CrossRef]
- Garlet, C.G.; Moreira, R.P.; Gubiani, P.D.S.; Palharini, R.B.; Farias, J.R.; Bernardi, O. Fitness cost of chlorpyrifos resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) on different host plants. Environ. Entomol. 2021, 50, 898–908. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Wu, X.; Deng, Q.; Zhu, Z.; Ren, M.; Ye, M.; Zeng, R. Seed priming with calcium chloride enhances wheat resistance against wheat aphid Schizaphis graminum Rondani. Pest Manag. Sci. 2021, 77, 4709–4718. [Google Scholar] [CrossRef]
- Rimantas, R.; Jekaterina, H.; Audrius, Z. Mitochondrial COI and morphological specificity of the mealy aphids (Hyalopterus ssp.) collected from different hosts in Europe (Hemiptera, Aphididae). ZooKeys 2013, 319, 255–267. [Google Scholar] [CrossRef]
- Li, S.; Lv, M.; Li, T.; Hao, M.; Xu, H. Spirodiclofen ether derivatives: Semisynthesis, structural elucidation, and pesticidal activities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot and Mythimna separata Walker. Pest Manag. Sci. 2021, 77, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Sun, B.L. Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 4599–4603. [Google Scholar] [CrossRef] [PubMed]
- Guhr, A.; Weig, A.R. Assessment of prokaryote to eukaryote ratios in environmental samples by SSU rDNA length polymorphism. Antonie Leeuwenhoek 2020, 113, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.H.; Hyten, A.S.; Spatafora, J.W. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef]
- Liu, Y.; Whelen, S.; Hall, B. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, X.; Zhi, J.; Xie, J.; Jiang, T. Fast recognition of Lecanicillium spp. and its virulence against Frankliniella occidentalis. Front. Microbiol. 2020, 11, 561381. [Google Scholar] [CrossRef]
- Zhang, D.F.; Gao, I.; Jakovlić, H.; Zou, J.; Zhang, W.; Li, X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, M.Y.; Liu, S.; Liu, M.K.; Ding, C.Y.; Qi, Y.Z.; Yan, X.L.; Qian, Y.B.; Xu, Y.F.; Yan, F.F. Optimization of culture conditions for a highly virulent Lecanicillium psalliotae and its pathogenicity against Geen Peach Aphid. Chin. J. Biol. Control 2021, 37, 349–355. [Google Scholar] [CrossRef]
- Russell, R.M.; Robertson, J.L.; Savin, N.E. POLO: A New Computer Program for Probit Analysis. Bull. Entomol. Soc. Am. 1977, 23, 209–213. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Wang, M.; Zhang, H.; Huang, Z.; Ma, J. Comparative study of the composition of cultivated, naturally grown Cordyceps sinensis, and stiff worms across different sampling years. PLoS ONE 2019, 14, e0225750. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Zhao, L.; Yang, Y.; Yan, L.; Tai, G.; Zhang, H. Cordyceps sinensis-derived fungus Isaria felina ameliorates experimental autoimmune thyroiditis in mice. Biomed. Pharmacother. 2021, 140, 111733. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Shi, J.D.; Wang, D. Three fungal strains isolated from stroma of Ophiocordyceps sinensis and their culture conditions. Mycosystema 2021, 40, 1991–2007. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Zhi, J.R.; Ye, M.; Zhang, Z.Y.; Yue, W.B.; Zou, X. Lecanicillium cauligalbarum sp. nov. (Cordycipitaceae, Hypocreales), a novel fungus isolated from a stemborer in the Yao Ren National Forest Mountain Park, Guizhou. Mycokeys 2018, 43, 59–74. [Google Scholar] [CrossRef]
- Mseddi, J.; FarhatTouzri, D.B.; Azzouz, H. Selection and characterization of thermotolerant Beauveria bassiana isolates and with insecticidal activity against the cotton-melon aphid Aphis gossypii (Glover) (Hemiptera: Aphididae). Pest Manag. Sci. 2022, 78, 2183–2195. [Google Scholar] [CrossRef]
- Kaifuchi, S.; Nonaka, K.; Mori, M.; Shiomi, K.; Satoshi, O.; Masuma, R. Lecanicillium primulinum, a new hyphomycete (Cordycipitaceae) from soils in the Okinawa’s main island and the Bonin Islands, Japan. Mycoscience 2013, 54, 291–296. [Google Scholar] [CrossRef]
- Omuse, E.R.; Niassy, S.; Wagacha, J.M.; Ong’amo, G.O.; Azrag, A.G.A.; Dubois, T. Suitable models to describe the effect of temperature on conidial germination and mycelial growth of Metarhizium anisopliae and Beauveria bassiana. Biocontrol Sci. Technol. 2022, 32, 281–298. [Google Scholar] [CrossRef]
- Tang, H.; Yoshizawa, S. Influences of temporal variations in environmental temperature and humidity on the fungus groethrate. Biocontrol Sci. 2000, 5, 51–55. [Google Scholar] [CrossRef][Green Version]
- Zare, R.; Gams, W. A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycol. Res. 2008, 112, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Gao, L. Optimization of nutritional and environmental conditions for biomass production and sporulation of entomogenous fungus Lecanicillium lecanii CA-1-G using two-stage cultivation system. J. Yeast Fungal Res. 2018, 9, 14–20. [Google Scholar] [CrossRef]
- Wang, H.; Lei, Z.; Reitz, S.; Li, Y.; Xu, X. Production of microsclerotia of the fungal entomopathogen Lecanicillium lecanii (Hypocreales: Cordycipitaceae) as a biological control agent against soil-dwelling stages of Frankliniella occidentalis (Thysanoptera: Thripidae). Biocontrol Sci. Technol. 2013, 23, 234–238. [Google Scholar] [CrossRef]
- Gan, Z.; Yang, J.; Tao, N.; Liang, L.; Mi, Q.; Li, J.; Zhang, K.-Q. Cloning of the gene Lecanicillium psalliotae chitinase Lpchi1 and identification of its potential role in the biocontrol of root-knot nematode Meloidogyne incognita. Appl. Microbiol. Biotechnol. 2007, 76, 1309–1317. [Google Scholar] [CrossRef]
- Radwan, O.; Gunasekera, T.S.; Ruiz, O.N. Draft Genome Sequence of Lecanicillium sp. isolate LEC01, a fungus capable of hydrocarbon degradation. Microbiol. Resour. Announc. 2019, 8, e01744-18. [Google Scholar] [CrossRef]
- Montalva, C.; Valenzuela, E.; Barta, M.; Rojas, E.; Arismendi, N.; Rodrigues, J.; Humber, R.A. Lecanicillium attenuatum isolates affecting the invasive cypress aphid (Cinara cupressi) in Chile. Biocontrol 2017, 62, 625–637. [Google Scholar] [CrossRef]
- Kim, J.J.; Roberts, D.W. The relationship between conidial dose, moulting and insect developmental stage on the susceptibility of cotton aphid, Aphis gossypii, to conidia of Lecanicillium attenuatum, an entomopathogenic fungus. Biocontrol Sci. Technol. 2012, 22, 319–331. [Google Scholar] [CrossRef]
- Kim, J.J.; Goettel, M.S.; Gillespie, D.R. Potential of Lecanicillium species for dual microbial control of aphids and the cucumber powdery mildew fungus, Sphaerotheca fuliginea. Biol. Control 2007, 40, 327–332. [Google Scholar] [CrossRef]
- Askary, H.; Ajam, H.M.; Yarmand, H. Investigation on survival of Aphidius nigripes Ashmead (Hymenoptera: Aphidiidae) reared on infected potato aphid by Lecanicillium muscarium (Deut.: Moniliaceae). Commun. Agric. Appl. Biol. Sci. 2006, 71, 375–385. [Google Scholar]
- Biryol, S.; Araz, N.; Eski, A.; Aktürk, R.; Aksu, Y.; Çelik Göktürk, B.; Bilgin, L.; Demir, İ. Biodiversity and pathogenicity of entomopathogenic fungi associated with the lesser spruce sawfly, Pristiphora abietina. Entomol. Exp. Appl. 2021, 169, 414–423. [Google Scholar] [CrossRef]
- Juliya, R.F. Phylogeny, chitinase activity, and pathogenicity of Beauveria, Metarhizium and Lecanicillium species against cowpea aphid, Aphis craccivora Koch. Int. J. Trop. Insect Sci. 2019, 40, 309–314. [Google Scholar] [CrossRef]
- Keerio, A.U.; Nazir, T.; Abdulle, Y.A.; Jatoi, G.H.; Gadhi, M.A.; Anwar, T.; Sokea, T.; Qiu, D. In vitro pathogenicity of the fungi Beauveria bassiana and Lecanicillium lecanii at different temperatures against the whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae). Egypt. J. Biol. Pest Control 2020, 30, 41. [Google Scholar] [CrossRef]
- Broumandnia, F.; Rajabpour, A. Efficacies of some isolates of Lecanicillium lecanii to control Tribolium castaneum (Col. Tenebrionidae). J. Plant Dis. Prot. 2020, 127, 625–631. [Google Scholar] [CrossRef]
- Aiuchi, D.; Moyo, D.; Ishikura, S.; Tani, M.; Kinoshita, R.; Rakotondrafara, A.M.; Koike, M. Virulence of Lecanicillium spp. (Hypocreales: Cordycipitaceae) hybrid strains against various biological stages of the greenhouse whitefly, Trialeurodes vaporariorum (Hemiptera: Aleyroidae). Biocontrol Sci. Technol. 2020, 30, 1006–1017. [Google Scholar] [CrossRef]
- Ben Fekih, I.; Jensen, A.B.; Boukhris-Bouhachem, S.; Pozsgai, G.; Rezgui, S.; Rensing, C.; Eilenberg, J. Virulence of two entomophthoralean fungi, Pandora neoaphidis and Entomophthora planchoniana, to Their Conspecific (Sitobion avenae) and Heterospecific (Rhopalosiphum padi) Aphid Hosts. Insects 2019, 10, 54. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).