A 63-kDa Periplasmic Protein of the Endonuclear Symbiotic Bacterium Holospora obtusa Secreted to the Outside of the Bacterium during the Early Infection Process Binds Weakly to the Macronuclear DNA of the Host Paramecium caudatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Cultures
2.2. Isolation of IFs and RFs of H. obtusa
2.3. Indirect Immunofluorescence Microscopy
2.4. DNA Extraction and Preparation of a DNA Affinity Column
2.5. SDS-PAGE, SDS-DNA PAGE and Immunoblotting
2.6. DNA Affinity Chromatography
3. Results
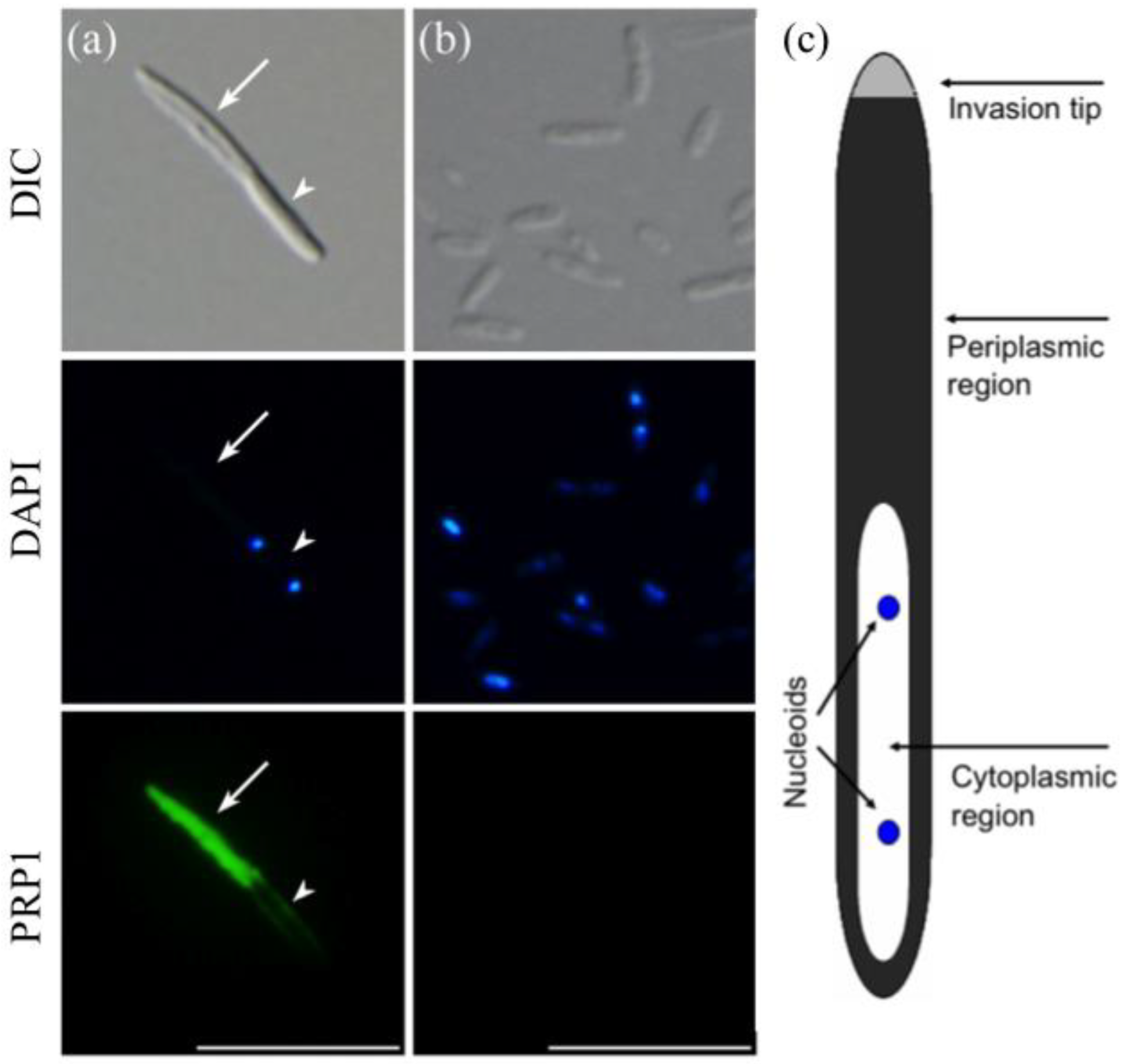
3.1. Indirect Immunofluorescence Microscopy Using a Monoclonal Antibody against the 63-kDa Periplasmic Protein PRP1 of the Infectious form of H. obtusa
3.2. DNA-Binding Ability of the PRP1
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Görtz, H.D.; Wiemann, M. Route of infection of bacteria Holospora elegans and Holospora obtusa into the nuclei of Paramecium caudatum. Eur. J. Protistol. 1989, 24, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Fokin, S.I.; Brigge, T.; Brenner, J.; Görtz, H.D. Holospora species infecting the nuclei of Paramecium appear to belong into two groups of bacteria. Eur. J. Protistol. 1996, 32 (Suppl. 1), 19–24. [Google Scholar] [CrossRef]
- Fokin, S.I.; Görtz, H.D. Diversity of Holospora bacteria in Paramecium and their characterization. In Endosymbionts in Paramecium. Microbiology Monographs; Fujishima, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 12, pp. 161–199. [Google Scholar] [CrossRef]
- Fujishima, M. Infection and maintenance of Holospora species in Paramecium caudatum. In Endosymbionts in Paramecium. Microbiology Monographs; Fujishima, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 12, pp. 201–225. [Google Scholar] [CrossRef]
- Garushyants, S.K.; Beliavskaia, A.Y.; Malko, D.B.; Logacheva, M.D.; Rautian, M.S.; Gelfand, M.S. Comparative genomic analysis of Holospora spp., Intranuclear symbionts of paramecia. Front. Microbiol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokin, S.I.; Serra, V. Bacterial symbiosis in ciliate (Alveolata, Ciliophora): Roads traveled and those still to be taken. J. Eukaryot. Microbiol. 2022, 69, e12886. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Kodama, Y. Mechanisms for establishing primary and secondary endosymbiosis in Paramecium. J. Eukaryot. Microbiol. 2022, 69, e12901. [Google Scholar] [CrossRef]
- Fujishima, M.; Nagahara, K.; Kojima, Y. Change in morphology, buoyant density and protein composition in differentiation from the reproductive short form to the infectious long form of Holospora obtusa, a macronucleus-specific symbiont of the ciliate Paramecium caudatum. Zool. Sci. 1990, 7, 849–860. [Google Scholar]
- Fujishima, M.; Sawabe, H.; Iwatsuki, K. Scanning electron microscopic observations of differentiation from the reproductive short form to the infectious long form of Holospora obtusa. J. Protozool. 1990, 37, 123–128. [Google Scholar] [CrossRef]
- Dohra, H.; Fujishima, M. Effects of antibiotics on early infection process of a macronuclear endosymbiotic bacterium Holospora obtusa of Paramecium caudatum. FEMS Microbiol. Lett. 1999, 179, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, M.; Hoshide, K. Light and electron microscopic observations of Holospora obtusa: A macronucleus-specific bacterium of the ciliate Paramecium caudatum. Zool. Sci. 1988, 5, 791–799. [Google Scholar]
- Dohra, H.; Fujishima, M. Cell structure of the infectious form of Holospora, an endonuclear symbiotic bacterium of the ciliate Paramecium. Zool. Sci. 1999, 16, 93–98. [Google Scholar] [CrossRef]
- Iwatani, K.; Dohra, H.; Lang, B.F.; Burger, G.; Hori, M.; Fujishima, M. Translocation of an 89-kDa periplasmic protein is associated with Holospora obtusa. Biochem. Biophys. Res. Commun. 2005, 337, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Fujishima, M. Paramecium as a model organism for studies on primary and secondary endosymbioses. In Biocommunication of Ciliates; Witzany, G., Nowacki, M., Eds.; Springer International Publishing: Switzerland, 2016; pp. 227–304. ISBN 978-3-319-32209-4. [Google Scholar]
- Hori, M.; Fujishima, M. The endosymbiotic bacterium Holospora obtusa enhances heat-shock gene expression of the host Paramecium caudatum. J. Eukaryot. Microbiol. 2003, 50, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Kawai, M.; Yamamoto, R. Paramecium caudatum acquires heat-shock resistance in ciliary movement by infection with the endonuclear symbiotic bacterium Holospora obtusa. FEMS Microbiol. Lett. 2005, 243, 101–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, M.; Fujii, K.; Fujishima, M. Micronucleus-specific bacterium Holospora elegans irreversibly enhances stress gene expression of the host Paramecium caudatum. J. Eukaryot. Microbiol. 2008, 55, 515–521. [Google Scholar] [CrossRef]
- Nakamura, Y.; Aki, M.; Aikawa, T.; Hori, M.; Fujishima, M. Differences in gene expression of the ciliate Paramecium caudatum caused by endonuclear symbiosis with Holospora obtusa, revealed using differential display reverse transcribed PCR. FEMS Microbiol. Lett. 2004, 240, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Smurov, A.O.; Fokin, S.I. Resistance of Paramecium caudatum infected with endonuclear bacteria Holospora against salinity impact. Proc. Zool. Inst. RAS. 1988, 276, 175–178. [Google Scholar]
- Duncan, A.B.; Fellous, S.; Accot, R.; Alart, M.; Sobandi, K.C.; Cosiaux, A.; Kaltz, O. Parasite-mediated protection against osmotic stress for Paramecium caudatum infected by Holospora undulata is host genotype specific. FEMS Microbiol. Ecol. 2010, 74, 353–360. [Google Scholar] [CrossRef]
- Fujishima, M.; Nakata, K.; Kodama, Y. Paramecium acquires resistance for high concentrations of various metal chlorides by infection of endonuclear symbiotic bacterium Holospora. Zool. Sci. 2006, 23, 1161. [Google Scholar]
- Preer, J.R.; Preer, L.B.; Jurand, A. Kappa and other endosymbionts in Paramecium aurelia. Bacterial Rev. 1974, 38, 113–163. [Google Scholar] [CrossRef]
- Görtz, H.D.; Fokin, S.I. Diversity of endosymbiotic bacteria in Paramecium. In Endosymbionts in Paramecium. Microbiology Monographs; Fujishima, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 12, pp. 132–160. [Google Scholar] [CrossRef]
- Dohra, H.; Tanaka, K.; Suzuki, T.; Fujishima, M.; Suzuki, H. Draft genome sequences of three Holospora species (Holospora obtusa, Holospora undulata, and Holospora elegans), endonuclear symbiotic bacteria of the ciliate Paramecium caudatum. FEMS Microbiol. Lett. 2014, 359, 16–18. [Google Scholar] [CrossRef] [Green Version]
- Abamo, F.; Dohra, H.; Fujishima, M. Fate of 63-kDa periplasmic protein of the infectious form of the endonuclear symbiotic bacterium Holospora obtusa during the infection process. FEMS Microbiol. Lett. 2008, 280, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dryl, S. Antigenic transformation in Paramecium aurelia after homologous antiserum treatment during autogamy and conjugation. J. Protozool. 1959, 6, 25. [Google Scholar]
- Hiwatashi, K. Determination and inheritance of mating type in Paramecium caudatum. Genetics 1968, 58, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Görtz, H.D. Infection of macronuclear anlagen of Paramecium caudatum with the macronucleus-specific symbiont Holospora obtusa. J. Cell Sci. 1983, 64, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Tonooka, Y.; Mizukami, Y.; Fujishima, M. One-base excess adaptor ligation method for walking uncloned genomic DNA. Appl. Microbiol. Biotechnol. 2008, 78, 173–180. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 121, 680–685. [Google Scholar] [CrossRef]
- Rosenthal, A.L.; Lacks, S.A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal. Biochem. 1977, 80, 76–90. [Google Scholar] [CrossRef]
- Miyakawa, I.; Kitamura, Y.; Jyozaki, T.; Sato, H.; Umezaki, T. Simple detection of a yeast mitochondiral DNA-binding protein, Abf2p, on SDS-DNA gels. J. Gen. Appl. Microbiol. 2000, 46, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Dohra, H.; Fujishima, M.; Hoshide, K. Monoclonal; antibodies specific for periplasmic materials of the macronuclear specific bacterium Holospora obtusa of the ciliate Paramecium caudatum. Eur. J. Protistol. 1994, 30, 288–294. [Google Scholar] [CrossRef]
- Dohra, H.; Yamamoto, K.; Fujishima, M.; Ishikawa, H. Cloning and sequencing of gene coding for a periplasmic 5.4 kDa peptide of the macronucley-specific symbiont Holospora obtusa of the ciliate Paramecium caudatum. Zool. Sci. 1997, 14, 69–75. [Google Scholar] [CrossRef]
- Tseng, T.T.; Tyler, B.M.; Setubal, J.C. Protein secretion systems in bacterial-host associations, and their description in the gene ontology. BMC Microbiol. 2009, 9 (Suppl. 1), S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbibe, L.; Kim, D.W.; Batsche, E.; Pedron, T.; Mateescu, B.; Muchardt, C.; Parsot, C.; Sansonetti, P.J. An injected bacterial effector targets chromatin access for transcription factor NF-κB to alter transcription of host genes involved in immune responses. Nat. Immunol. 2007, 8, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, K.J.; Choi, K.S.; Grab, D.J.; Dumler, J.S. Anaplasma phagocytophilum AnkA binds to granulocyte DNA and nuclear proteins. Cell. Microbiol. 2004, 6, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, J.C.; Rennoll-Banket, K.E.; Pelly, S.; Milstone, A.M.; Dumler, J.S. Silencing of host cell CYBB gene expression by the nuclear effector AnkA of the intracellular pathogen Anaplasma phagocytophilum. Infect. Immun. 2009, 77, 2385–2391. [Google Scholar] [CrossRef] [Green Version]
- Sugai, T.; Hiwatashi, K. Cytologic and autoradiographic studies of the micronucleus at meiotic prophase in Tetrahymena pyriformis. J. Protozool. 1974, 21, 542–548. [Google Scholar] [CrossRef]
- Tanaka, T.; Watanabe, T. Spatiotemporal sites of DNA replication in macro- and micronuclei of the ciliate Paramecium caudatum. Chromosome Research 2003, 11, 153–164. [Google Scholar] [CrossRef]
- Mochizuki, K.; Gorovsky, M.A. RNA polymerase II localizes in Tetrahymena thermophila meiotic micronuclei when micronuclear transcription associated with genome rearrangement occurs. Eukaryot. Cell. 2004, 3, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, M.; Watanabe, T. Transplantation of germ nuclei in Paramecium caudatum. III. Role of germinal micronucleus in vegetative growth. Exp. Cell Res. 1981, 132, 47–56. [Google Scholar] [CrossRef]
- Fokin, S.I.; Skovorodkin, I.N. Holospora obtusa–endonucleobiont of the ciliate Paramecium caudatum in search for macronucleus. Cytology 1991, 33, 101–115. [Google Scholar]
- Fokin, S.I.; Skovorodkin, I. Holospora undulata–endonucleobiont of the ciliate Paramecium caudatum in search for micronucleus. Cytology 1991, 3, 64–65. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujishima, M.; Kawano, H.; Miyakawa, I. A 63-kDa Periplasmic Protein of the Endonuclear Symbiotic Bacterium Holospora obtusa Secreted to the Outside of the Bacterium during the Early Infection Process Binds Weakly to the Macronuclear DNA of the Host Paramecium caudatum. Microorganisms 2023, 11, 155. https://doi.org/10.3390/microorganisms11010155
Fujishima M, Kawano H, Miyakawa I. A 63-kDa Periplasmic Protein of the Endonuclear Symbiotic Bacterium Holospora obtusa Secreted to the Outside of the Bacterium during the Early Infection Process Binds Weakly to the Macronuclear DNA of the Host Paramecium caudatum. Microorganisms. 2023; 11(1):155. https://doi.org/10.3390/microorganisms11010155
Chicago/Turabian StyleFujishima, Masahiro, Hideaki Kawano, and Isamu Miyakawa. 2023. "A 63-kDa Periplasmic Protein of the Endonuclear Symbiotic Bacterium Holospora obtusa Secreted to the Outside of the Bacterium during the Early Infection Process Binds Weakly to the Macronuclear DNA of the Host Paramecium caudatum" Microorganisms 11, no. 1: 155. https://doi.org/10.3390/microorganisms11010155
APA StyleFujishima, M., Kawano, H., & Miyakawa, I. (2023). A 63-kDa Periplasmic Protein of the Endonuclear Symbiotic Bacterium Holospora obtusa Secreted to the Outside of the Bacterium during the Early Infection Process Binds Weakly to the Macronuclear DNA of the Host Paramecium caudatum. Microorganisms, 11(1), 155. https://doi.org/10.3390/microorganisms11010155





