Gram-Positive Bacteria Cell Wall Peptidoglycan Polymers Activate Human Dendritic Cells to Produce IL-23 and IL-1β and Promote TH17 Cell Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacillus anthracis and Staphylococcus aureus peptidoglycan (PGN) and Heat-Killed Bacteria (HKB)
2.2. Generation of Monocyte-Derived Dendritic Cells
2.3. Assessment of DC Activation
2.4. mAbs and Flow Cytometry
2.5. Allogeneic T Cell Assays
2.6. Cytokine Assays
2.7. Statistics
3. Results
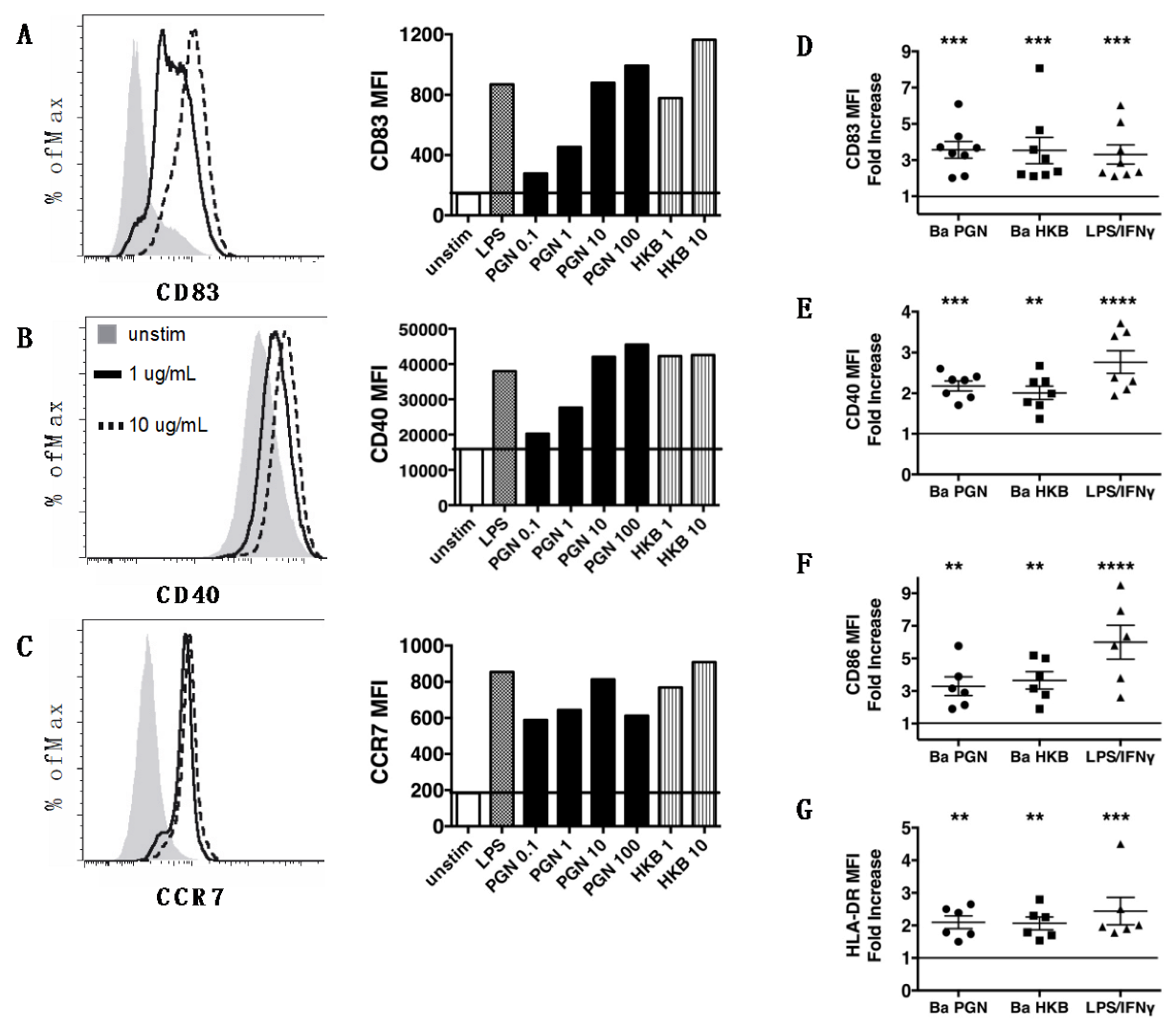
3.1. Peptidoglycan Polymers Isolated from Gram-Positive B. anthracis Activate Human Dendritic Cells
3.2. B. anthracis PGN-Activated DCs Produce IL-23 and IL-1β but Not IL-12p70
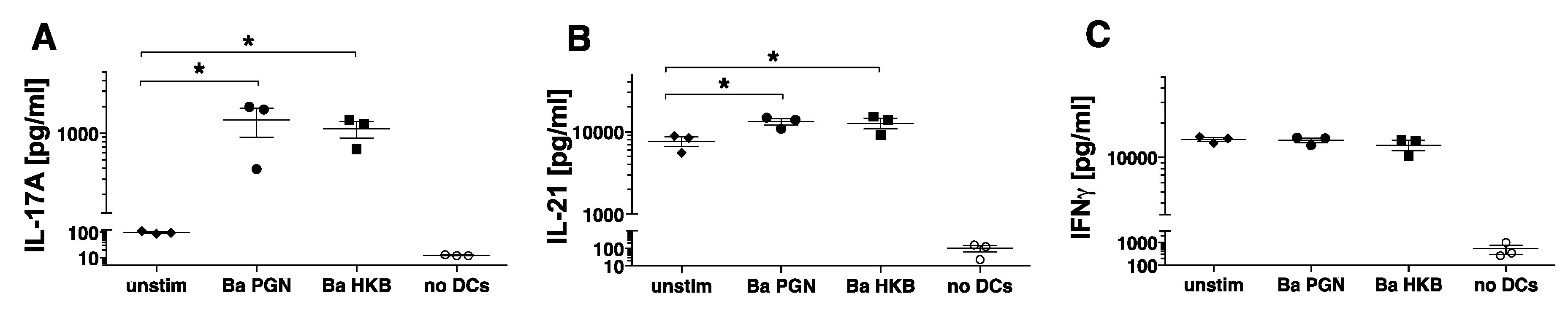
3.3. B. anthracis PGN-Activated DCs Induce Naïve Allogeneic CD4+ T Cells to Produce IL-17
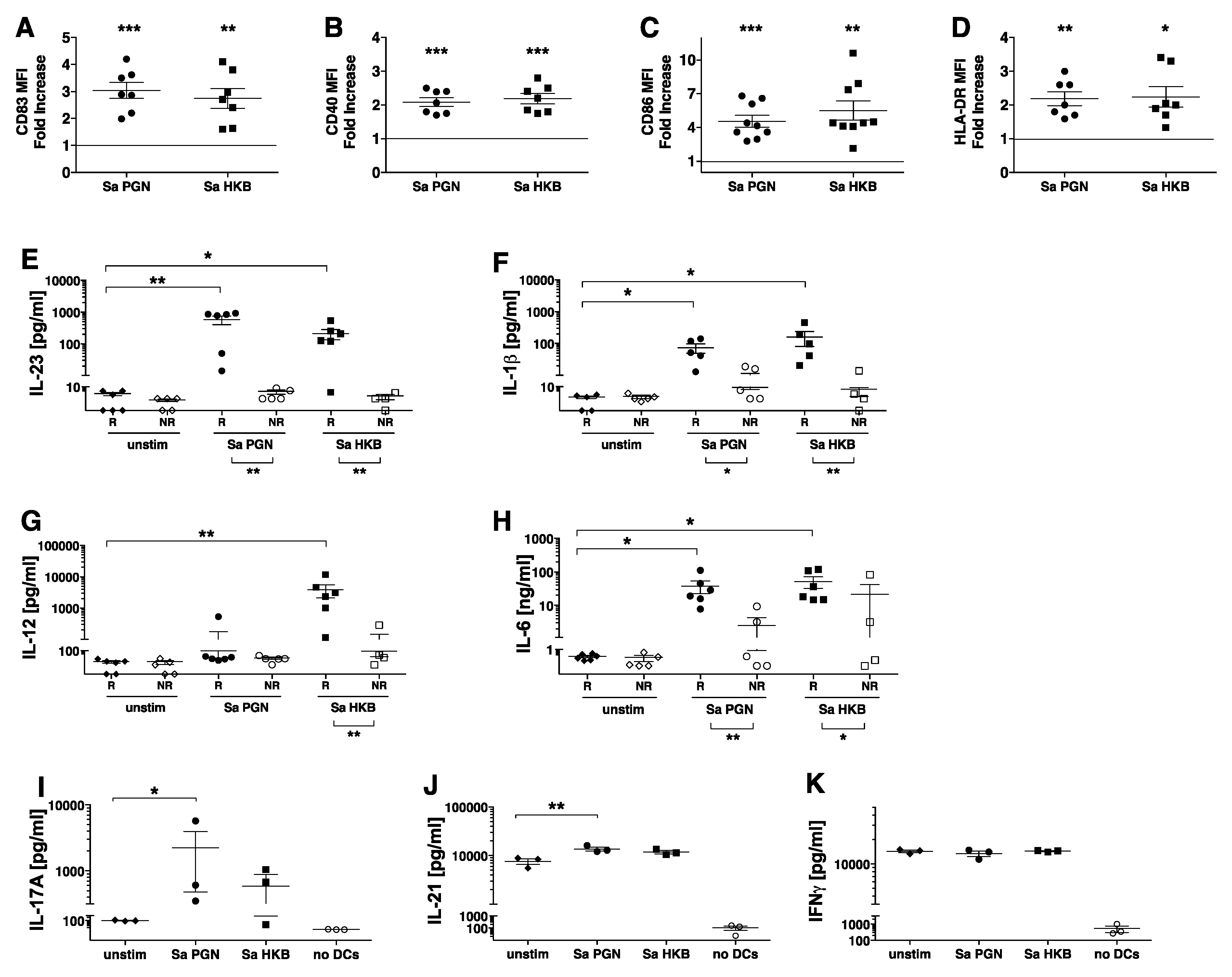
3.4. S. aureus PGN Stimulates Human DC Production of IL-23 and IL-1β, Resulting in their Ability to Promote TH17 Differentiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Miller, L.S.; Cho, J.S. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 2011, 11, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [Green Version]
- Coggeshall, K.M.; Lupu, F.; Ballard, J.; Metcalf, J.P.; James, J.A.; Farris, D.; Kurosawa, S. The sepsis model: An emerging hypothesis for the lethality of inhalation anthrax. J. Cell Mol. Med. 2013, 17, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Doganay, M.; Metan, G.; Alp, E. A review of cutaneous anthrax and its outcome. J. Infect. Public Health 2010, 3, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, M.; Malykhin, A.; Maeda, K.; Chakrabarty, K.; Williamson, K.S.; Feasley, C.L.; West, C.M.; Metcalf, J.P.; Coggeshall, K.M. Bacillus anthracis peptidoglycan stimulates an inflammatory response in monocytes through the p38 mitogen-activated protein kinase pathway. PLoS ONE 2018, 3, e3706. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.K.; Khurana, T.; Langer, M.; West, C.M.; Ballard, J.D.; Metcalf, J.P.; Merkel, T.J.; Coggeshall, K.M. Inflammatory cytokine response to Bacillus anthracis peptidoglycan requires phagocytosis and lysosomal trafficking. Infect. Immun. 2010, 78, 2418–2428. [Google Scholar] [CrossRef] [Green Version]
- Iyer, J.K.; Coggeshall, K.M. Cutting edge: Primary innate immune cells respond efficiently to polymeric peptidoglycan, but not to peptidoglycan monomers. J. Immunol. 2011, 186, 3841–3845. [Google Scholar] [CrossRef] [Green Version]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Müller-Anstett, M.A.; Müller, P.; Albrecht, T.; Nega, M.; Wagener, J.; Gao, Q.; Kaesler, S.; Schaller, M.; Biedermann, T.; Götz, F. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS ONE 2010, 5, e13153. [Google Scholar] [CrossRef] [Green Version]
- Schwandner, R.; Dziarski, R.; Wesche, H.; Rothe, M.; Kirschning, C.J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 1999, 274, 17406–17409. [Google Scholar] [CrossRef]
- Travassos, L.H.; Girardin, S.E.; Philpott, D.J.; Blanot, D.; Nahori, M.A.; Werts, C.; Boneca, I.G. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 2004, 5, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Volz, T.; Nega, M.; Buschmann, J.; Kaesler, S.; Guenova, E.; Peschel, A.; Röcken, M.; Götz, F.; Biedermann, T. Natural Staphylococcus aureus-derived peptidoglycan fragments activate NOD2 and act as potent costimulators of the innate immune system exclusively in the presence of TLR signals. FASEB J. 2010, 24, 4089–4102. [Google Scholar] [CrossRef]
- de Jonge, B.L.; Chang, Y.S.; Gage, D.; Tomasz, A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J. Biol. Chem. 1992, 267, 11248–11254. [Google Scholar] [CrossRef]
- Langer, M.; Girton, A.W.; Popescu, N.I.; Burgett, T.; Metcalf, J.P.; Coggeshall, K.M. Neither Lys- and DAP-type peptidoglycans stimulate mouse or human innate immune cells via Toll-like receptor 2. PLoS ONE 2018, 13, e0193207. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Raisley, B.; Langer, M.; Iyer, J.K.; Vedham, V.; Ballard, J.L.; James, J.A.; Metcalf, J.; Coggeshall, K.M. Anti-peptidoglycan antibodies and Fcγ receptors are the key mediators of inflammation in Gram-positive sepsis. J. Immunol. 2012, 189, 2423–2431. [Google Scholar] [CrossRef] [Green Version]
- Popescu, N.I.; Cochran, J.; Duggan, E.; Kluza, J.; Silasi, R.; Coggeshall, K.M. Internalization of Polymeric Bacterial Peptidoglycan Occurs through Either Actin or Dynamin Dependent Pathways. Microorganisms 2022, 10, 552. [Google Scholar] [CrossRef]
- Girardin, S.E.; Travassos, L.H.; Hervé, M.; Blanot, D.; Boneca, I.G.; Philpott, D.J.; Sansonetti, P.J.; Mengin-Lecreulx, D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 2003, 278, 41702–41708. [Google Scholar] [CrossRef] [Green Version]
- Wolf, A.J.; Reyes, C.N.; Liang, W.; Becker, C.; Shimada, K.; Wheeler, M.L.; Cho, H.C.; Popescu, N.I.; Coggeshall, K.M.; Arditi, M.; et al. Hexokinase Is an Innate Immune Receptor for the Detection of Bacterial Peptidoglycan. Cell 2016, 166, 624–636. [Google Scholar] [CrossRef] [Green Version]
- Irazoki, O.; Hernandez, S.B.; Cava, F. Peptidoglycan Muropeptides: Release, Perception, and Functions as Signaling Molecules. Front. Microbiol. 2019, 10, 500. [Google Scholar] [CrossRef] [Green Version]
- Geginat, J.; Nizzoli, G.; Paroni, M.; Maglie, S.; Larghi, P.; Pascolo, S.; Abrignani, S. Immunity to Pathogens Taught by Specialized Human Dendritic Cell Subsets. Front. Immunol. 2015, 6, 527. [Google Scholar] [CrossRef]
- van Beelen, A.J.; Zelinkova, Z.; Taanman-Kueter, E.W.; Muller, F.J.; Hommes, D.W.; Zaat, S.A.; Kapsenberg, M.L.; de Jong, E.C. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 2007, 27, 660–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balraadjsing, P.P.; Lund, L.D.; Souwer, Y.; Zaat, S.A.J.; Frøkiær, H.; de Jong, E.C. The Nature of Antibacterial Adaptive Immune Responses against Staphylococcus aureus Is Dependent on the Growth Phase and Extracellular Peptidoglycan. Infect. Immun. 2019, 88, e00733-19. [Google Scholar] [CrossRef]
- Gramlich, R.; Aliahmadi, E.; Peiser, M. In Vitro Induction of T Helper 17 Cells by Synergistic Activation of Human Monocyte-Derived Langerhans Cell-Like Cells with Bacterial Agonists. Int. J. Mol. Sci. 2019, 20, E1367. [Google Scholar] [CrossRef] [Green Version]
- Zipperle, G.F.; Ezzell, J.W.; Doyle, R.J. Glucosamine substitution and muramidase susceptibility in Bacillus anthracis. Can. J. Microbiol. 1984, 30, 553–559. [Google Scholar] [CrossRef]
- Popescu, N.I.; Girton, A.; Burgett, T.; Lovelady, K.; Coggeshall, K.M. Monocyte procoagulant responses to anthrax peptidoglycan are reinforced by proinflammatory cytokine signaling. Blood Adv. 2019, 3, 2436–2447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munroe, M.E.; Vista, E.S.; Guthridge, J.M.; Thompson, L.F.; Merrill, J.T.; James, J.A. Proinflammatory adaptive cytokine and shed tumor necrosis factor receptor levels are elevated preceding systemic lupus erythematosus disease flare. Arthritis Rheumatol. 2014, 66, 1888–1899. [Google Scholar] [CrossRef] [Green Version]
- Sharif, S.; Singh, M.; Kim, S.J.; Schaefer, J. Staphylococcus aureus peptidoglycan tertiary structure from carbon-13 spin diffusion. J. Am. Chem. Soc. 2009, 131, 7023–7030. [Google Scholar] [CrossRef] [Green Version]
- Dixon, T.C.; Meselson, M.; Guillemin, J.; Hanna, P.C. Anthrax. N. Engl. J. Med. 1999, 341, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.C.; Lyons, C.R.; Lipscomb, M.F. Effect of Bacillus anthracis virulence factors on human dendritic cell activation. Hum. Immunol. 2008, 69, 552–561. [Google Scholar] [CrossRef] [Green Version]
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.P.; Liu, S. Anthrax Pathogenesis. Annu. Rev. Microbiol. 2015, 69, 185–208. [Google Scholar] [CrossRef]
- Stearns-Kurosawa, D.J.; Lupu, F.; Taylor, F.B.J.; Kinasewitz, G.; Kurosawa, S. Sepsis and pathophysiology of anthrax in a nonhuman primate model. Am. J. Pathol. 2006, 169, 433–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, N.I.; Silasi, R.; Keshari, R.S.; Girton, A.; Burgett, T.; Zeerleder, S.S.; Gailani, D.; Gruber, A.; Lupu, F.; Coggeshall, K.M. Peptidoglycan induces disseminated intravascular coagulation in baboons through activation of both coagulation pathways. Blood 2018, 132, 849–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Popescu, N.I.; Raisley, B.; Keshari, R.S.; Dale, G.L.; Lupu, F.; Coggeshall, K.M. Bacillus anthracis peptidoglycan activates human platelets through FcγRII and complement. Blood 2013, 122, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Gerosa, F.; Baldani-Guerra, B.; Lyakh, L.A.; Batoni, G.; Esin, S.; Winkler-Pickett, R.T.; Consolaro, M.R.; De Marchi, M.; Giachino, D.; Robbiano, A.; et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 2008, 205, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Aiba, S.; Shibata, K.; Ohteki, T.; Takada, H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect. Immun. 2005, 73, 7967–7976. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Zhao, Q.; Zheng, H.; Li, X.; Zhang, T.; Ma, X. A novel crosstalk between TLR4- and NOD2-mediated signaling in the regulation of intestinal inflammation. Sci. Rep. 2015, 5, 12018. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Vidyarthi, A.; Pahari, S.; Negi, S.; Aqdas, M.; Nadeem, S.; Agnihotri, T.; Agrewala, J.N. Signaling through NOD-2 and TLR-4 Bolsters the T cell Priming Capability of Dendritic cells by Inducing Autophagy. Sci. Rep. 2016, 6, 19084. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.M.; Ramachandran, G.; Basu, S.; Rollins, S.; Mann, D.; Cross, A.S. The IL-23/Th17 axis is involved in the adaptive immune response to Bacillus anthracis in humans. Eur. J. Immunol. 2014, 44, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Shieh, W.J.; Guarner, J.; Paddock, C.; Greer, P.; Tatti, K.; Fischer, M.; Layton, M.; Philips, M.; Bresnitz, E.; Quinn, C.P.; et al. The critical role of pathology in the investigation of bioterrorism-related cutaneous anthrax. Am. J. Pathol. 2003, 163, 1901–1910. [Google Scholar] [CrossRef] [Green Version]
- Grinberg, L.M.; Abramova, F.A.; Yampolskaya, O.V.; Walker, D.H.; Smith, J.H. Quantitative pathology of inhalational anthrax I: Quantitative microscopic findings. Mod. Pathol. 2001, 14, 482–495. [Google Scholar] [CrossRef]
- Ingram, R.J.; Ascough, S.; Reynolds, C.J.; Metan, G.; Doganay, M.; Baillie, L.; Williamson, D.E.; Robinson, J.H.; Maillere, B.; Boyton, R.J.; et al. Natural cutaneous anthrax infection, but not vaccination, induces a CD4(+) T cell response involving diverse cytokines. Cell Biosci. 2015, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedl, M.; Abraham, C. IRF5 risk polymorphisms contribute to interindividual variance in pattern recognition receptor-mediated cytokine secretion in human monocyte-derived cells. J. Immunol. 2012, 188, 5348–5356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.N.; Ye, C.; Villani, A.C.; Raj, T.; Li, W.; Eisenhaure, T.M.; Imboywa, S.H.; Chipendo, P.I.; Ran, F.A.; Slowikowski, K.; et al. Common genetic variants modulate pathogen-sensing responses in human dendritic cells. Science 2014, 343, 1246980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairfax, B.P.; Humburg, P.; Makino, S.; Naranbhai, V.; Wong, D.; Lau, E.; Jostins, L.; Plant, K.; Andrews, R.; McGee, C.; et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science 2014, 343, 1246949. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, S.; Raisley, B.; Roach, K.; Bajaña, S.; Munroe, M.E.; James, J.A.; Coggeshall, K.M.; Kovats, S. Gram-Positive Bacteria Cell Wall Peptidoglycan Polymers Activate Human Dendritic Cells to Produce IL-23 and IL-1β and Promote TH17 Cell Differentiation. Microorganisms 2023, 11, 173. https://doi.org/10.3390/microorganisms11010173
Turner S, Raisley B, Roach K, Bajaña S, Munroe ME, James JA, Coggeshall KM, Kovats S. Gram-Positive Bacteria Cell Wall Peptidoglycan Polymers Activate Human Dendritic Cells to Produce IL-23 and IL-1β and Promote TH17 Cell Differentiation. Microorganisms. 2023; 11(1):173. https://doi.org/10.3390/microorganisms11010173
Chicago/Turabian StyleTurner, Sean, Brent Raisley, Kimberly Roach, Sandra Bajaña, Melissa E. Munroe, Judith A. James, K. Mark Coggeshall, and Susan Kovats. 2023. "Gram-Positive Bacteria Cell Wall Peptidoglycan Polymers Activate Human Dendritic Cells to Produce IL-23 and IL-1β and Promote TH17 Cell Differentiation" Microorganisms 11, no. 1: 173. https://doi.org/10.3390/microorganisms11010173
APA StyleTurner, S., Raisley, B., Roach, K., Bajaña, S., Munroe, M. E., James, J. A., Coggeshall, K. M., & Kovats, S. (2023). Gram-Positive Bacteria Cell Wall Peptidoglycan Polymers Activate Human Dendritic Cells to Produce IL-23 and IL-1β and Promote TH17 Cell Differentiation. Microorganisms, 11(1), 173. https://doi.org/10.3390/microorganisms11010173




