Impact of COVID-19 on Uro-Oncological Patients: A Comprehensive Review of the Literature
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
- Bladder cancer (BC);
- Prostate cancer (PCa);
- Renal cell carcinoma (RCC);
- Upper tract urothelial carcinoma (UTUC);
- Penile cancer and Testicular cancer.
2.2. Exclusion Criteria
2.3. Data Extraction and Synthesis
3. Results
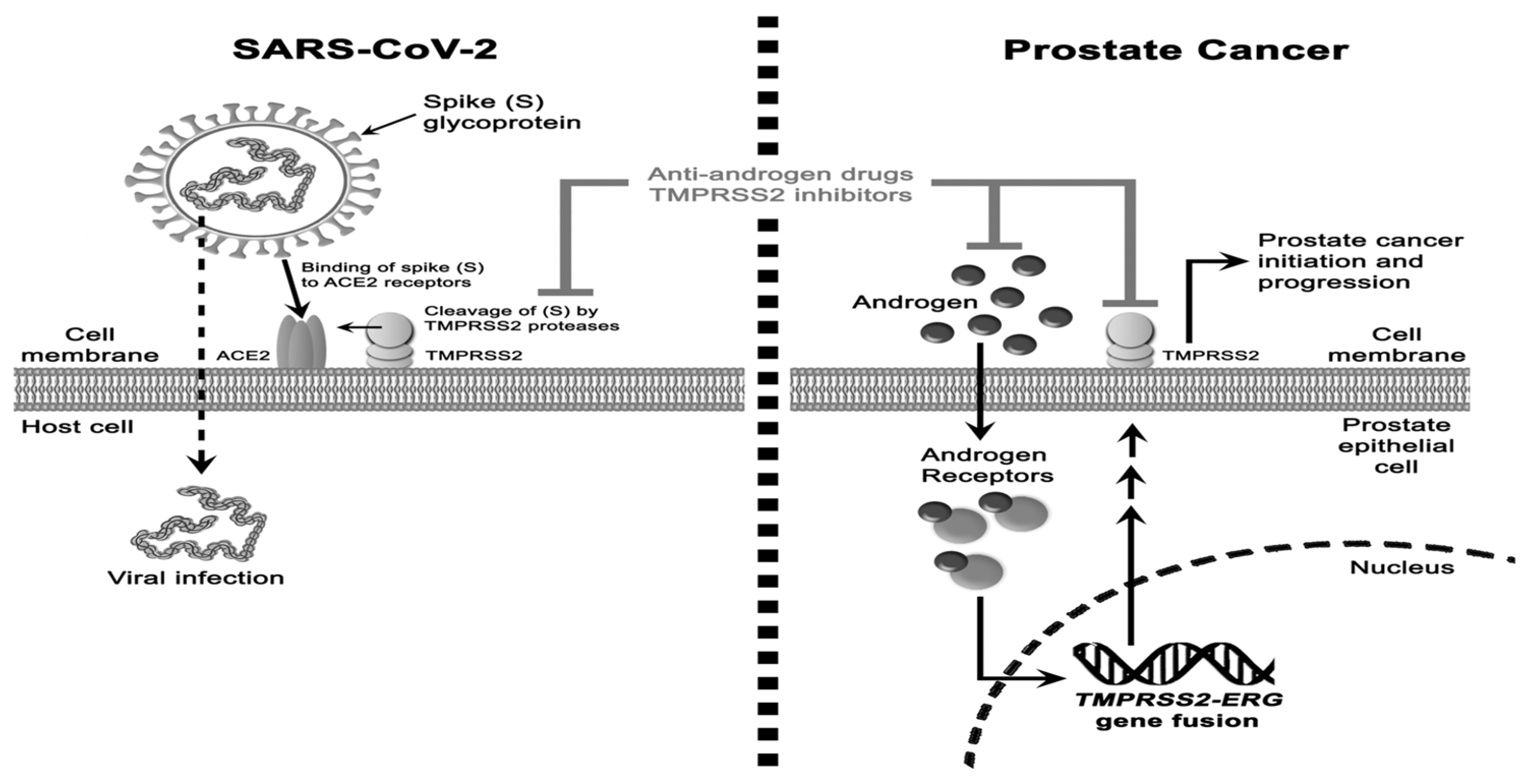
3.1. Prostate Cancer
3.2. Bladder and Upper Tract Urothelial Cancer
3.3. Kidney Cancer
3.4. Penile Cancer
3.5. Testicular Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johns Hopkins University. Coronavirus Resource Center. Available online: https://www.jhu.edu/ (accessed on 1 December 2022).
- He, F.; Deng, Y.; Li, W. Coronavirus Disease 2019: What We Know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, F.; Wang, R.; Guan, K.; Jiang, T.; Xu, G.; Sun, J.; Chang, C. The Deadly Coronaviruses: The 2003 SARS Pandemic and the 2020 Novel Coronavirus Epidemic in China. J. Autoimmun. 2020, 109, 102434. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, I.; Santoro, P.E.; Fiorilli, C.; Angelini, G.; Buonomo, I.; Benevene, P.; Romano, L.; Gualano, M.R.; Amantea, C.; Moscato, U. A New Tool to Evaluate Burnout: The Italian Version of the BAT for Italian Healthcare Workers. BMC Public Health 2022, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Gualano, M.R.; Rossi, M.F.; Borrelli, I.; Santoro, P.E.; Amantea, C.; Daniele, A.; Tumminello, A.; Moscato, U. Returning to Work and the Impact of Post COVID-19 Condition: A Systematic Review. Work 2022, 73, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology for Public Health, Istituto Superiore di Sanità EpiCentro. COVID-19: Gestione Dello Stress Tra Gli Operatori Sanitari. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-gestione-stress (accessed on 1 January 2023).
- Burnout: Sintomi e Intervento. Available online: https://www.giuntipsy.it/informazioni/notizie/burnout-sintomi-e-intervento (accessed on 1 January 2023).
- Franc-Guimond, J.; Mcneil, B.; Schlossberg, S.M.; North, A.C.; Sener, A. Urologist Burnout: Frequency, Causes, and Potential Solutions to an Unspoken Entity. Can. Urol. Assoc. J. 2018, 12, 137–142. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for Potential Pathogenesis of SARS-CoV-2 Infection—A Review of Immune Changes in Patients with Viral Pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.-Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical Characteristics of COVID-19-Infected Cancer Patients: A Retrospective Case Study in Three Hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative; GlobalSurg Collaborative. Timing of Surgery Following SARS-CoV-2 Infection: An International Prospective Cohort Study. Anaesthesia 2021, 76, 748–758. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer Patients in SARS-CoV-2 Infection: A Nationwide Analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Foschi, N.; Santoro, P.E.; Borrelli, I.; Gavi, F.; Amantea, C.; Russo, P.; Moscato, U. Urological Safety and COVID-19 Vaccinations. Vaccines 2022, 10, 1887. [Google Scholar] [CrossRef]
- Beccia, F.; Rossi, M.F.; Amantea, C.; Villani, L.; Daniele, A.; Tumminello, A.; Aristei, L.; Santoro, P.E.; Borrelli, I.; Ricciardi, W.; et al. COVID-19 Vaccination and Medical Liability: An International Perspective in 18 Countries. Vaccines 2022, 10, 1275. [Google Scholar] [CrossRef]
- OncologyPRO. Available online: https://oncologypro.esmo.org/ (accessed on 1 December 2022).
- COVID-19 Coronavirus|Fight Bladder Cancer. Available online: https://www.fightbladdercancer.co.uk/get-help/covid-19-coronavirus (accessed on 1 December 2022).
- COVID-19 Vaccines in People with Cancer. Available online: https://www.cancer.org/treatment/coronavirus-covid-19-and-cancer/covid-19-vaccines-in-people-with-cancer.html (accessed on 1 December 2022).
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative Therapeutic Efficacy of Remdesivir and Combination Lopinavir, Ritonavir, and Interferon Beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef]
- Poggio, F.; Tagliamento, M.; Di Maio, M.; Martelli, V.; De Maria, A.; Barisione, E.; Grosso, M.; Boccardo, F.; Pronzato, P.; Del Mastro, L.; et al. Assessing the Impact of the COVID-19 Outbreak on the Attitudes and Practice of Italian Oncologists Toward Breast Cancer Care and Related Research Activities. JCO Oncol. Pract. 2020, 16, e1304–e1314. [Google Scholar] [CrossRef]
- Gualano, M.R.; Santoro, P.E.; Borrelli, I.; Rossi, M.F.; Amantea, C.; Tumminello, A.; Daniele, A.; Beccia, F.; Moscato, U. Employee Participation in Workplace Vaccination Campaigns: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1898. [Google Scholar] [CrossRef]
- Englum, B.R.; Prasad, N.K.; Lake, R.E.; Mayorga-Carlin, M.; Turner, D.J.; Siddiqui, T.; Sorkin, J.D.; Lal, B.K. Impact of the COVID-19 Pandemic on Diagnosis of New Cancers: A National Multicenter Study of the Veterans Affairs Healthcare System. Cancer 2022, 128, 1048–1056. [Google Scholar] [CrossRef]
- Marino, F.; Totaro, A.; Gandi, C.; Bientinesi, R.; Moretto, S.; Gavi, F.; Pierconti, F.; Iacovelli, R.; Bassi, P.; Sacco, E. Germline Mutations in Prostate Cancer: A Systematic Review of the Evidence for Personalized Medicine. Prostate Cancer Prostatic Dis. 2022. [Google Scholar] [CrossRef]
- Greiner, B.; Tipton, S.; Nelson, B.; Hartwell, M. Cancer Screenings during the COVID-19 Pandemic: An Analysis of Public Interest Trends. Curr. Probl. Cancer 2022, 46, 100766. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Haynes, K.; Du, S.; Barron, J.; Katz, A.J. Association of Cancer Screening Deficit in the United States With the COVID-19 Pandemic. JAMA Oncol. 2021, 7, 878. [Google Scholar] [CrossRef] [PubMed]
- Castellani, D.; Ragonese, M.; Di Rosa, M.; Marzio, V.; Di Gianfrancesco, L.; Bassi, P.; De Dominicis, M.; Dellabella, M.; Antonucci, M. An Italian Multicenter Analysis of Emergency Admissions and Treatment of Upper Tract Urolithiasis during the Lockdown and Reopening Phases of the COVID-19 Pandemic: Are We Ready for a Second Wave of the Outbreak? Int. J. Urol. 2021, 28, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Puliatti, S.; Eissa, A.; Eissa, R.; Amato, M.; Mazzone, E.; Dell’Oglio, P.; Sighinolfi, M.C.; Zoeir, A.; Micali, S.; Bianchi, G.; et al. COVID-19 and Urology: A Comprehensive Review of the Literature: COVID-19 and Urology. BJU Int. 2020, 125, E7–E14. [Google Scholar] [CrossRef] [PubMed]
- Ribal, M.J.; Cornford, P.; Briganti, A.; Knoll, T.; Gravas, S.; Babjuk, M.; Harding, C.; Breda, A.; Bex, A.; Rassweiler, J.J.; et al. European Association of Urology Guidelines Office Rapid Reaction Group: An Organisation-Wide Collaborative Effort to Adapt the European Association of Urology Guidelines Recommendations to the Coronavirus Disease 2019 Era. Eur. Urol. 2020, 78, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Stensland, K.D.; Morgan, T.M.; Moinzadeh, A.; Lee, C.T.; Briganti, A.; Catto, J.W.F.; Canes, D. Considerations in the Triage of Urologic Surgeries During the COVID-19 Pandemic. Eur. Urol. 2020, 77, 663–666. [Google Scholar] [CrossRef]
- Diamand, R.; Ploussard, G.; Roumiguié, M.; Oderda, M.; Benamran, D.; Fiard, G.; Peltier, A.; Simone, G.; Van Damme, J.; Malavaud, B.; et al. Timing and Delay of Radical Prostatectomy Do Not Lead to Adverse Oncologic Outcomes: Results from a Large European Cohort at the Times of COVID-19 Pandemic. World J. Urol. 2021, 39, 1789–1796. [Google Scholar] [CrossRef]
- Gupta, N.; Bivalacqua, T.J.; Han, M.; Gorin, M.A.; Challacombe, B.J.; Partin, A.W.; Mamawala, M.K. Evaluating the Impact of Length of Time from Diagnosis to Surgery in Patients with Unfavourable Intermediate-Risk to Very-High-Risk Clinically Localised Prostate Cancer. BJU Int. 2019, 124, 268–274. [Google Scholar] [CrossRef]
- Gurel, A.; Baylan, B.; Keles, I.; Demirbas, A.; Karalar, M.; Eren, B.; Ozen, A.; Oztekin, U.; Benlioglu, C.; Dogan, A.E.; et al. Effect of the COVID-19 Pandemic on Radical Prostatectomy: A Turkish Multicenter Study. Turk. J. Urol. 2022, 48, 339–345. [Google Scholar] [CrossRef]
- Ward, Z.J.; Walbaum, M.; Walbaum, B.; Guzman, M.J.; de la Jara, J.J.; Nervi, B.; Atun, R. Estimating the Impact of the COVID-19 Pandemic on Diagnosis and Survival of Five Cancers in Chile from 2020 to 2030: A Simulation-Based Analysis. Lancet Oncol. 2021, 22, 1427–1437. [Google Scholar] [CrossRef]
- Jiménez-Alcaide, E.; García-Fuentes, C.; Hernández, V.; De la Peña, E.; Pérez-Fernández, E.; Castro, A.; Caballero-Perea, B.; Guijarro, A.; Llorente, C. Influence of Androgen Deprivation Therapy on the Severity of COVID-19 in Prostate Cancer Patients. Prostate 2021, 81, 1349–1354. [Google Scholar] [CrossRef]
- Montopoli, M.; Zumerle, S.; Vettor, R.; Rugge, M.; Zorzi, M.; Catapano, C.V.; Carbone, G.M.; Cavalli, A.; Pagano, F.; Ragazzi, E.; et al. Androgen-Deprivation Therapies for Prostate Cancer and Risk of Infection by SARS-CoV-2: A Population-Based Study (N = 4532). Ann. Oncol. 2020, 31, 1040–1045. [Google Scholar] [CrossRef]
- Wambier, C.G.; Goren, A.; Vaño-Galván, S.; Ramos, P.M.; Ossimetha, A.; Nau, G.; Herrera, S.; McCoy, J. Androgen Sensitivity Gateway to COVID -19 Disease Severity. Drug Dev. Res. 2020, 81, 771–776. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Abou-Kheir, W. Crosstalk between COVID-19 and Prostate Cancer. Prostate Cancer Prostatic Dis. 2020, 23, 561–563. [Google Scholar] [CrossRef]
- Caffo, O.; Messina, M.; Veccia, A.; Kinspergher, S.; Maines, F.; Messina, C. Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Patients with Prostate Cancer: A Critical Review. Crit. Rev. Oncol. Hematol. 2021, 167, 103491. [Google Scholar] [CrossRef]
- Bernstein, A.N.; Talwar, R.; Handorf, E.; Syed, K.; Danella, J.; Ginzburg, S.; Belkoff, L.; Reese, A.C.; Tomaszewski, J.; Trabulsi, E.; et al. Assessment of Prostate Cancer Treatment Among Black and White Patients During the COVID-19 Pandemic. JAMA Oncol. 2021, 7, 1467–1473. [Google Scholar] [CrossRef]
- Thomas, F.; Noon, A.P.; Rubin, N.; Goepel, J.R.; Catto, J.W.F. Comparative Outcomes of Primary, Recurrent, and Progressive High-Risk Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2013, 63, 145–154. [Google Scholar] [CrossRef]
- Khadhouri, S.; Gallagher, K.M.; MacKenzie, K.R.; Shah, T.T.; Gao, C.; Moore, S.; Zimmermann, E.F.; Edison, E.; Jefferies, M.; Nambiar, A.; et al. Developing a Diagnostic Multivariable Prediction Model for Urinary Tract Cancer in Patients Referred with Haematuria: Results from the IDENTIFY Collaborative Study. Eur. Urol. Focus 2022, 8, 1673–1682. [Google Scholar] [CrossRef]
- Travassos, T.C.; De Oliveira JM, I.; Selegatto, I.B.; Reis, L.O. COVID-19 Impact on Bladder Cancer-Orientations for Diagnosing, Decision Making, and Treatment. Am. J. Clin. Exp. Urol. 2021, 9, 132. [Google Scholar]
- Zhou, J.; Kelsey, K.T.; Smith, S.; Giovannucci, E.; Michaud, D.S. Lower Urinary Tract Symptoms and Risk of Bladder Cancer in Men: Results From the Health Professionals Follow-up Study. Urology 2015, 85, 1312–1318. [Google Scholar] [CrossRef]
- Bientinesi, R.; Gavi, F.; Coluzzi, S.; Nociti, V.; Marturano, M.; Sacco, E. Neurologic Urinary Incontinence, Lower Urinary Tract Symptoms and Sexual Dysfunctions in Multiple Sclerosis: Expert Opinions Based on the Review of Current Evidences. J. Clin. Med. 2022, 11, 6572. [Google Scholar] [CrossRef] [PubMed]
- Bientinesi, R.; Coluzzi, S.; Gavi, F.; Nociti, V.; Gandi, C.; Marino, F.; Moretto, S.; Mirabella, M.; Bassi, P.; Sacco, E. The Impact of Neurogenic Lower Urinary Tract Symptoms and Erectile Dysfunctions on Marital Relationship in Men with Multiple Sclerosis: A Single Cohort Study. J. Clin. Med. 2022, 11, 5639. [Google Scholar] [CrossRef] [PubMed]
- Batura, D.; Hashemzehi, T.; Gayed, W. Should Contrast CT Urography Replace Non-Contrast CT as an Investigation for Ureteric Colic in the Emergency Department in Those Aged 65 and Over? Emerg. Radiol. 2018, 25, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Goldman, H.B.; Haber, G.P. Recommendations for Tiered Stratification of Urological Surgery Urgency in the COVID-19 Era. J. Urol. 2020, 204, 11–13. [Google Scholar] [CrossRef]
- Matulay, J.T.; Soloway, M.; Witjes, J.A.; Buckley, R.; Persad, R.; Lamm, D.L.; Boehle, A.; Palou, J.; Colombel, M.; Brausi, M.; et al. Risk-adapted Management of Low-grade Bladder Tumours: Recommendations from the International Bladder Cancer Group (IBCG). BJU Int. 2020, 125, 497–505. [Google Scholar] [CrossRef]
- Russell, B.; Liedberg, F.; Khan, M.S.; Nair, R.; Thurairaja, R.; Malde, S.; Kumar, P.; Bryan, R.T.; Van Hemelrijck, M. A Systematic Review and Meta-Analysis of Delay in Radical Cystectomy and the Effect on Survival in Bladder Cancer Patients. Eur. Urol. Oncol. 2020, 3, 239–249. [Google Scholar] [CrossRef]
- Waldert, M.; Karakiewicz, P.I.; Raman, J.D.; Remzi, M.; Isbarn, H.; Lotan, Y.; Capitanio, U.; Bensalah, K.; Marberger, M.J.; Shariat, S.F. A Delay in Radical Nephroureterectomy Can Lead to Upstaging. BJU Int. 2010, 105, 812–817. [Google Scholar] [CrossRef]
- Lee, J.N.; Kwon, S.Y.; Choi, G.-S.; Kim, H.T.; Kim, T.-H.; Kwon, T.G.; Kim, B.W. Impact of Surgical Wait Time on Oncologic Outcomes in Upper Urinary Tract Urothelial Carcinoma: Surgical Timing of Upper Urinary Tract Urothelial Carcinoma. J. Surg. Oncol. 2014, 110, 468–475. [Google Scholar] [CrossRef]
- Nison, L.; Rouprêt, M.; Bozzini, G.; Ouzzane, A.; Audenet, F.; Pignot, G.; Ruffion, A.; Cornu, J.-N.; Hurel, S.; Valeri, A.; et al. The Oncologic Impact of a Delay between Diagnosis and Radical Nephroureterectomy Due to Diagnostic Ureteroscopy in Upper Urinary Tract Urothelial Carcinomas: Results from a Large Collaborative Database. World J. Urol. 2013, 31, 69–76. [Google Scholar] [CrossRef]
- Moon, Y.J.; Cho, K.S.; Jeong, J.Y.; Chung, D.Y.; Kang, D.H.; Jung, H.D.; Lee, J.Y. Effects of Intravesical BCG Maintenance Therapy Duration on Recurrence Rate in High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC): Systematic Review and Network Meta-Analysis According to EAU COVID-19 Recommendations. PLoS ONE 2022, 17, e0273733. [Google Scholar] [CrossRef]
- Tulchiner, G.; Staudacher, N.; Fritz, J.; Radmayr, C.; Culig, Z.; Horninger, W.; Pichler, R. The “COVID-19 Pandemic Gap” and Its Influence on Oncologic Outcomes of Bladder Cancer. Cancers 2021, 13, 1754. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Huillard, O.; Dabi, Y.; Anract, J.; Sibony, M.; Zerbib, M.; Xylinas, E. Neoadjuvant Chemotherapy in Patients With Muscle-Invasive Bladder Cancer and Its Impact on Surgical Morbidity and Oncological Outcomes: A Real-World Experience. Front. Surg. 2018, 5, 58. [Google Scholar] [CrossRef]
- Boeri, L.; Soligo, M.; Frank, I.; Boorjian, S.A.; Thompson, R.H.; Tollefson, M.; Quevedo, F.J.; Cheville, J.C.; Karnes, R.J. Delaying Radical Cystectomy After Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer Is Associated with Adverse Survival Outcomes. Eur. Urol. Oncol. 2019, 2, 390–396. [Google Scholar] [CrossRef]
- Claps, F.; van de Kamp, M.W.; Mayr, R.; Bostrom, P.J.; Boormans, J.L.; Eckstein, M.; Mertens, L.S.; Boevé, E.R.; Neuzillet, Y.; Burger, M.; et al. Risk Factors Associated with Positive Surgical Margins’ Location at Radical Cystectomy and Their Impact on Bladder Cancer Survival. World J. Urol. 2021, 39, 4363–4371. [Google Scholar] [CrossRef]
- Afferi, L.; Lonati, C.; Montorsi, F.; Briganti, A.; Necchi, A.; Mari, A.; Minervini, A.; Tellini, R.; Campi, R.; Schulz, G.B.; et al. Selecting the Best Candidates for Cisplatin-Based Adjuvant Chemotherapy After Radical Cystectomy Among Patients with PN+ Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 722–725. [Google Scholar] [CrossRef]
- Sarkis, J.; Samaha, R.; Kattan, J.; Sarkis, P. Bladder Cancer during the COVID-19 Pandemic: The Calm before the Storm? Future Sci. OA 2020, 6, FSO615. [Google Scholar] [CrossRef]
- Deng, Y.-Y.; Zheng, Y.; Cai, G.-Y.; Chen, X.-M.; Hong, Q. Single-Cell RNA Sequencing Data Suggest a Role for Angiotensin-Converting Enzyme 2 in Kidney Impairment in Patients Infected with 2019-Novel Coronavirus. Chin. Med. J. 2020, 133, 1129–1131. [Google Scholar] [CrossRef]
- Ye, M.; Wysocki, J.; William, J.; Soler, M.J.; Cokic, I.; Batlle, D. Glomerular Localization and Expression of Angiotensin-Converting Enzyme 2 and Angiotensin-Converting Enzyme: Implications for Albuminuria in Diabetes. J. Am. Soc. Nephrol. 2006, 17, 3067–3075. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical Characteristics of 113 Deceased Patients with Coronavirus Disease 2019: Retrospective Study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, H.; Yang, Y. Challenges and Countermeasures of Integrative Cancer Therapy in the Epidemic of COVID-19. Integr. Cancer Ther. 2020, 19, 153473542091281. [Google Scholar] [CrossRef]
- Staehler, M.; Battle, D.; Pal, S.K.; Bergerot, C.D. Counterbalancing COVID-19 with Cancer Surveillance and Therapy: A Survey of Patients with Renal Cell Carcinoma. Eur. Urol. Focus 2021, 7, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Dabestani, S.; Beisland, C.; Stewart, G.D.; Bensalah, K.; Gudmundsson, E.; Lam, T.B.; Gietzmann, W.; Zakikhani, P.; Marconi, L.; Fernandéz-Pello, S.; et al. Long-Term Outcomes of Follow-up for Initially Localised Clear Cell Renal Cell Carcinoma: RECUR Database Analysis. Eur. Urol. Focus 2019, 5, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Reuther, J.; Scheel, C.H.; Becker, J.C. On the Use of Immune Checkpoint Inhibitors in Patients with Viral Infections Including COVID-19. J. Immunother. Cancer 2020, 8, e001145. [Google Scholar] [CrossRef]
- Vardhana, S.A.; Wolchok, J.D. The Many Faces of the Anti-COVID Immune Response. J. Exp. Med. 2020, 217, e20200678. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa, C.J.; Glickman, M.S.; et al. Determinants of COVID-19 Disease Severity in Patients with Cancer. Nat. Med. 2020, 26, 1218–1223. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Switzer, B.; Haanen, J.; Lorigan, P.C.; Puzanov, I.; Turajlic, S. Clinical and Immunologic Implications of COVID-19 in Patients with Melanoma and Renal Cell Carcinoma Receiving Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e002835. [Google Scholar] [CrossRef]
- Kuusk, T.; Cullen, D.; Neves, J.B.; Campain, N.; Barod, R.; Boleti, E.; El-Sheihk, S.; Grant, L.; Kelly, J.; Marchetti, M.; et al. Impact of the First Surge of the COVID-19 Pandemic on a Tertiary Referral Centre for Kidney Cancer. BJU Int. 2021, 128, 752–758. [Google Scholar] [CrossRef]
- Srivastava, A.; Patel, H.V.; Kim, S.; Shinder, B.; Sterling, J.; Tabakin, A.L.; Polotti, C.F.; Saraiya, B.; Mayer, T.; Kim, I.Y.; et al. Delaying Surgery for Clinical T1b-T2bN0M0 Renal Cell Carcinoma: Oncologic Implications in the COVID-19 Era and Beyond. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 247–257. [Google Scholar] [CrossRef]
- Chan, V.W.-S.; Tan, W.S.; Leow, J.J.; Ong, W.L.K.; Chiu, P.K.-F.; Gurung, P.; Pirola, G.M.; Orecchia, L.; Liew, M.P.C.; Lee, H.-Y.; et al. Delayed Surgery for Localised and Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis for the COVID-19 Pandemic. World J. Urol. 2021, 39, 4295–4303. [Google Scholar] [CrossRef]
- Marchioni, M.; Kriegmair, M.; Heck, M.; Amiel, T.; Porpiglia, F.; Ceccucci, E.; Campi, R.; Minervini, A.; Mari, A.; Van Bruwaene, S.; et al. Development of a Novel Risk Score to Select the Optimal Candidate for Cytoreductive Nephrectomy Among Patients with Metastatic Renal Cell Carcinoma. Results from a Multi-Institutional Registry (REMARCC). Eur. Urol. Oncol. 2021, 4, 256–263. [Google Scholar] [CrossRef]
- Oderda, M.; Roupret, M.; Marra, G.; Merseburger, A.S.; Oderda, G.; Falcone, M.; Ceruti, C.; Shariat, S.F.; Gontero, P. The Impact of COVID-19 Outbreak on Uro-Oncological Practice Across Europe: Which Burden of Activity Are We Facing Ahead? Eur. Urol. 2020, 78, 124–126. [Google Scholar] [CrossRef]
- Collins, P.M.; Madden, A.; O’Connell, C.; Omer, S.A.; Inder, M.S.; Casey, R.G.; Flynn, R.J.; Thomas, A.Z.; Smyth, L.G.; Manecksha, R.P. Urological Service Provision during the COVID-19 Period: The Experience from an Irish Tertiary Centre. Ir. J. Med. Sci. (1971-) 2021, 190, 455–460. [Google Scholar] [CrossRef]
- McIntosh, A.G.; Ristau, B.T.; Ruth, K.; Jennings, R.; Ross, E.; Smaldone, M.C.; Chen, D.Y.T.; Viterbo, R.; Greenberg, R.E.; Kutikov, A.; et al. Active Surveillance for Localized Renal Masses: Tumor Growth, Delayed Intervention Rates, and >5-Yr Clinical Outcomes. Eur. Urol. 2018, 74, 157–164. [Google Scholar] [CrossRef]
- Khene, Z.-E.; Guérin, S.; Khene, F.; Pradère, B.; Roumiguié, M.; Mathieu, R.; Pignot, G.; Massard, C.; Neuzillet, Y.; Ploussard, G.; et al. Online Public Interest in Urological Cancers During the COVID-19 Pandemic: What Can “Dr. Google” Teach Us? Eur. Urol. Open Sci. 2022, 37, 73–79. [Google Scholar] [CrossRef]
- Reitblat, C.; Bain, P.A.; Porter, M.E.; Bernstein, D.N.; Feeley, T.W.; Graefen, M.; Iyer, S.; Resnick, M.J.; Stimson, C.J.; Trinh, Q.-D.; et al. Value-Based Healthcare in Urology: A Collaborative Review. Eur. Urol. 2021, 79, 571–585. [Google Scholar] [CrossRef]
- Pecoraro, A.; Roussel, E.; Serni, S.; Campi, R. Re-Envisioning Patient Education and Public Awareness of Urological Cancers at the Time of the COVID-19 Pandemic. Eur. Urol. Open Sci. 2022, 38, 67–68. [Google Scholar] [CrossRef]
- Gao, W.; Song, L.; Yang, J.; Song, N.; Wu, X.; Song, N.; Qiao, D.; Chen, C.; Zhang, J.; Wang, Z. Risk Factors and Negative Consequences of Patient’s Delay for Penile Carcinoma. World J. Surg. Oncol. 2016, 14, 124. [Google Scholar] [CrossRef]
- Cakir, O.O.; Castiglione, F.; Tandogdu, Z.; Collins, J.; Alnajjar, H.M.; Akers, C.; Albersen, M.; Alifrangis, C.; Ayres, B.; Brouwer, O.; et al. Management of Penile Cancer Patients during the COVID-19 Pandemic: An EUROGEN Accelerated Delphi Consensus Study. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 197.e9–197.e17. [Google Scholar] [CrossRef]
- Chipollini, J.; Tang, D.H.; Gilbert, S.M.; Poch, M.A.; Pow-Sang, J.M.; Sexton, W.J.; Spiess, P.E. Delay to Inguinal Lymph Node Dissection Greater than 3 Months Predicts Poorer Recurrence-Free Survival for Patients with Penile Cancer. J. Urol. 2017, 198, 1346–1352. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Abrate, A.; Bartoletti, R.; Crestani, A.; De Nunzio, C.; Giannarini, G.; Gregori, A.; Liguori, G.; Mirone, V.; et al. Urology Practice during the COVID-19 Pandemic. Minerva Urol. Nefrol. 2020, 72, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Manecksha, R.P.; Fitzpatrick, J.M. Epidemiology of Testicular Cancer. BJU Int. 2009, 104, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Chalya, P.L.; Simbila, S.; Rambau, P.F. Ten-Year Experience with Testicular Cancer at a Tertiary Care Hospital in a Resource-Limited Setting: A Single Centre Experience in Tanzania. World J. Surg. Oncol. 2014, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Wishnow, K.I.; Johnson, D.E.; Preston, W.L.; Tenney, D.M.; Brown, B.W. Prompt Orchiectomy Reduces Morbidity and Mortality from Testicular Carcinoma. Br. J. Urol. 1990, 65, 629–633. [Google Scholar] [CrossRef]
- Tachibana, I.; Ferguson, E.L.; Mahenthiran, A.; Natarajan, J.P.; Masterson, T.A.; Bahler, C.D.; Sundaram, C.P. Delaying Cancer Cases in Urology during COVID-19: Review of the Literature. J. Urol. 2020, 204, 926–933. [Google Scholar] [CrossRef]
- Socarrás, M.R.; Loeb, S.; Teoh, J.Y.-C.; Ribal, M.J.; Bloemberg, J.; Catto, J.; N’Dow, J.; Van Poppel, H.; Rivas, J.G. Telemedicine and Smart Working: Recommendations of the European Association of Urology. Eur. Urol. 2020, 78, 812–819. [Google Scholar] [CrossRef]
- Monroy-Iglesias, M.J.; Rai, S.; Mistretta, F.A.; Roberts, G.; Dickinson, H.; Russell, B.; Moss, C.; De Berardinis, R.; Ferro, M.; Musi, G.; et al. Impact of the COVID-19 Pandemic on Urological Cancers: The Surgical Experience of Two Cancer Hubs in London and Milan. BJUI Compass 2022, 3, 277–286. [Google Scholar] [CrossRef]
- Palmieri, C.; Turtle, L.; Docherty, A.; Harrison, E.; Drake, T.; Greenhalf, B.; Openshaw, P.J.; Baillie, J.K.; Semple, M.G. 1670O Prospective Data of First 1797 Hospitalised Patients with Cancer and COVID-19 Derived from the COVID-19 Clinical Information Network and International Severe Acute Respiratory and Emerging Infections Consortium, WHO Coronavirus Clinical Characterisation Consortium. Ann. Oncol. 2020, 31, S992. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical Impact of COVID-19 on Patients with Cancer (CCC19): A Cohort Study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Castelo-Branco, L.; Cervantes, A.; Curigliano, G.; Garassino, M.C.; Giesen, N.; Grivas, P.; Haanen, J.; Jordan, K.; Liebert, U.G.; Lordick, F.; et al. ESMO Statements on Vaccination against COVID-19 in People with Cancer. Available online: https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination (accessed on 7 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavi, F.; Santoro, P.E.; Amantea, C.; Russo, P.; Marino, F.; Borrelli, I.; Moscato, U.; Foschi, N. Impact of COVID-19 on Uro-Oncological Patients: A Comprehensive Review of the Literature. Microorganisms 2023, 11, 176. https://doi.org/10.3390/microorganisms11010176
Gavi F, Santoro PE, Amantea C, Russo P, Marino F, Borrelli I, Moscato U, Foschi N. Impact of COVID-19 on Uro-Oncological Patients: A Comprehensive Review of the Literature. Microorganisms. 2023; 11(1):176. https://doi.org/10.3390/microorganisms11010176
Chicago/Turabian StyleGavi, Filippo, Paolo Emilio Santoro, Carlotta Amantea, Pierluigi Russo, Filippo Marino, Ivan Borrelli, Umberto Moscato, and Nazario Foschi. 2023. "Impact of COVID-19 on Uro-Oncological Patients: A Comprehensive Review of the Literature" Microorganisms 11, no. 1: 176. https://doi.org/10.3390/microorganisms11010176
APA StyleGavi, F., Santoro, P. E., Amantea, C., Russo, P., Marino, F., Borrelli, I., Moscato, U., & Foschi, N. (2023). Impact of COVID-19 on Uro-Oncological Patients: A Comprehensive Review of the Literature. Microorganisms, 11(1), 176. https://doi.org/10.3390/microorganisms11010176









