“Force-From-Lipids” Dependence of the MscCG Mechanosensitive Channel Gating on Anionic Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain, Construct, and Cell Culture
2.2. Protein Expression and Purification
2.3. Proteoliposome Preparations Containing Reconstituted Purified Protein
2.4. Liposome Preparations Fused with Corynebacterial Membrane Vesicle or Reconstituted with Membrane Protein Extract
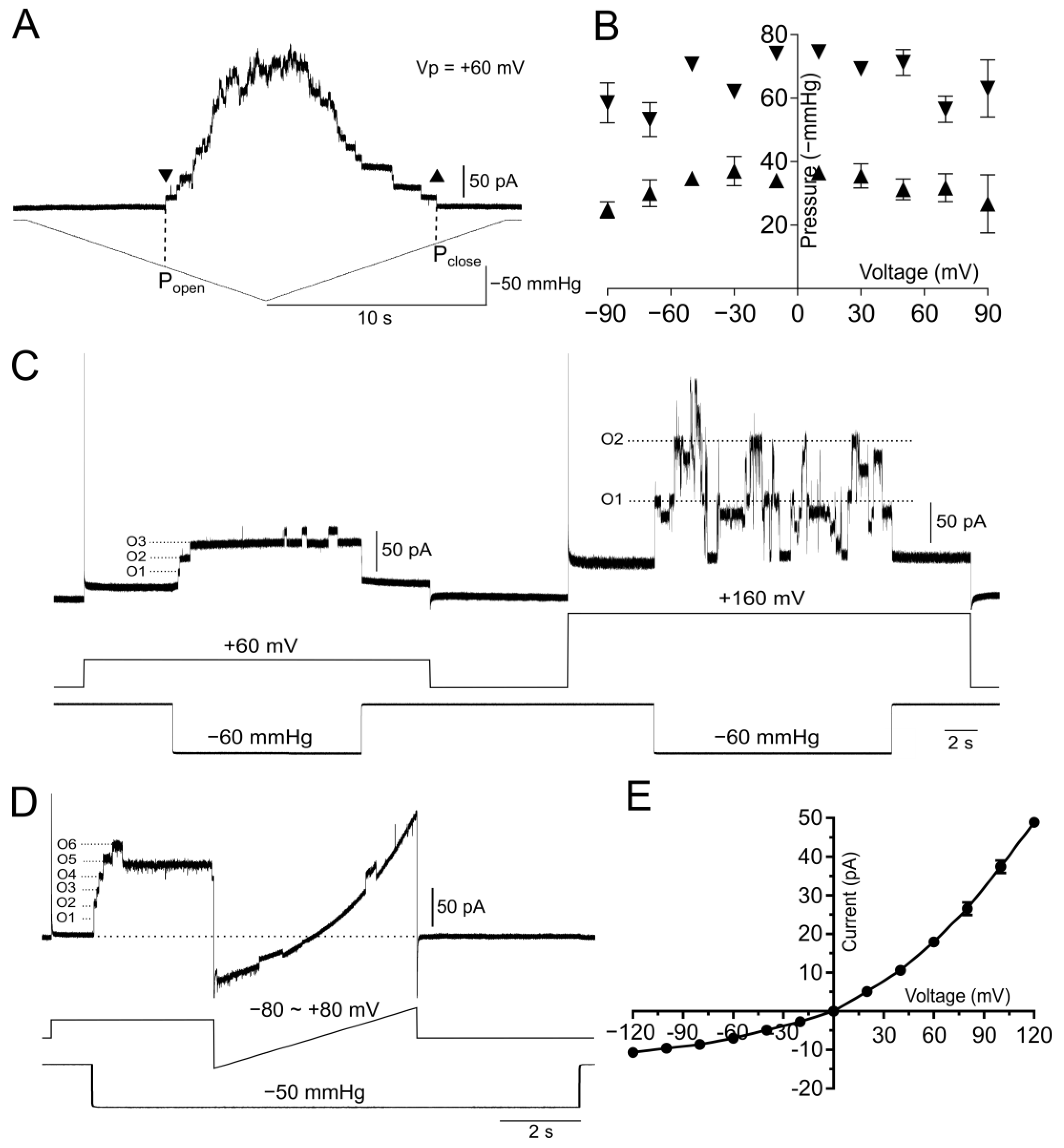
2.5. Patch Clamp Electrophysiology
2.6. Micropipette Aspiration Combined with Patch Fluorometry
3. Results
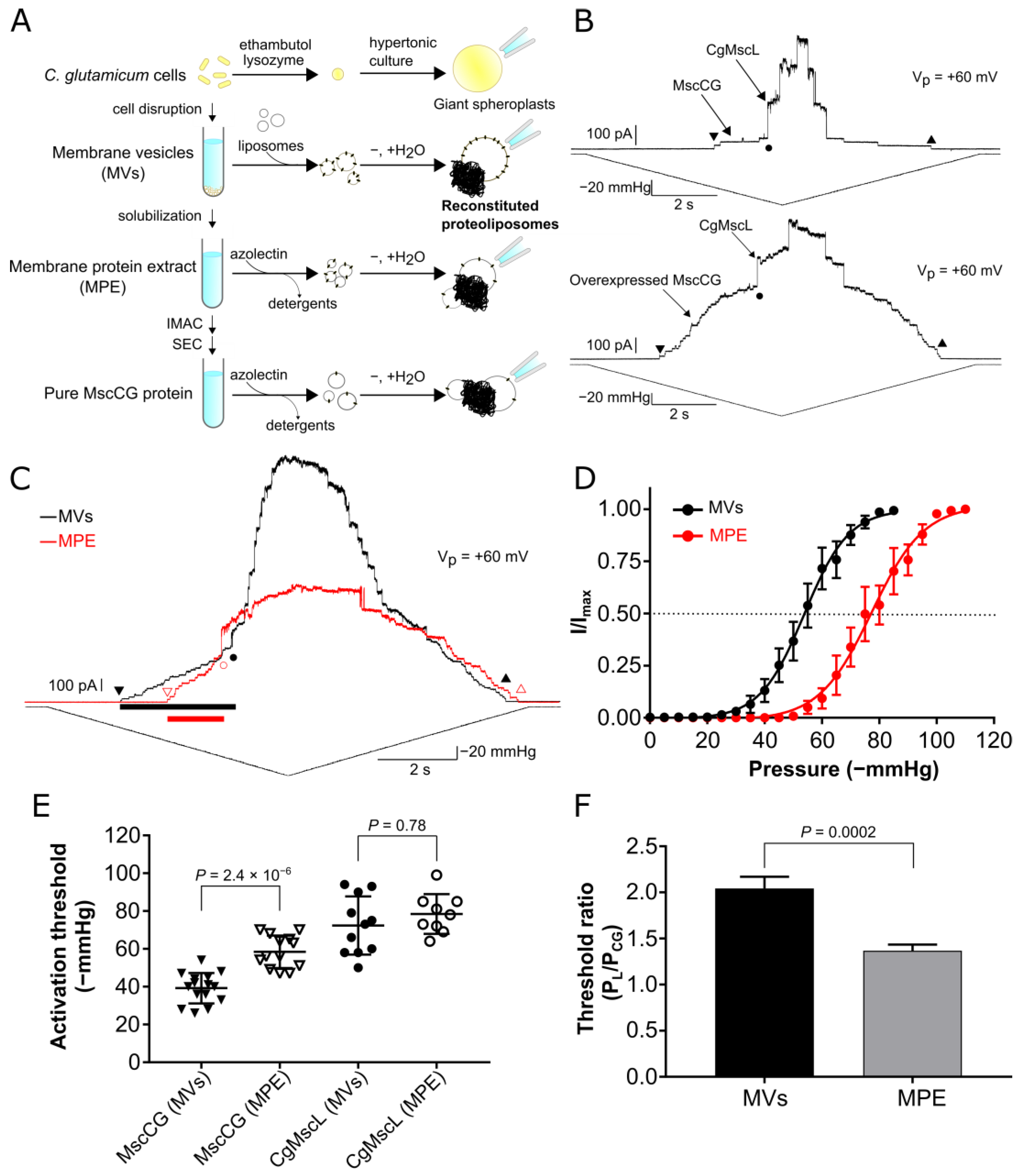
3.1. Mechanosensitivity Difference between the MscCG Channels Reconstituted in Liposomes Fused with Native Membrane Vesicles and Liposomes Containing Membrane Protein Extract
3.2. Protein Purification and Liposomal Reconstitution of the MscCG Channel
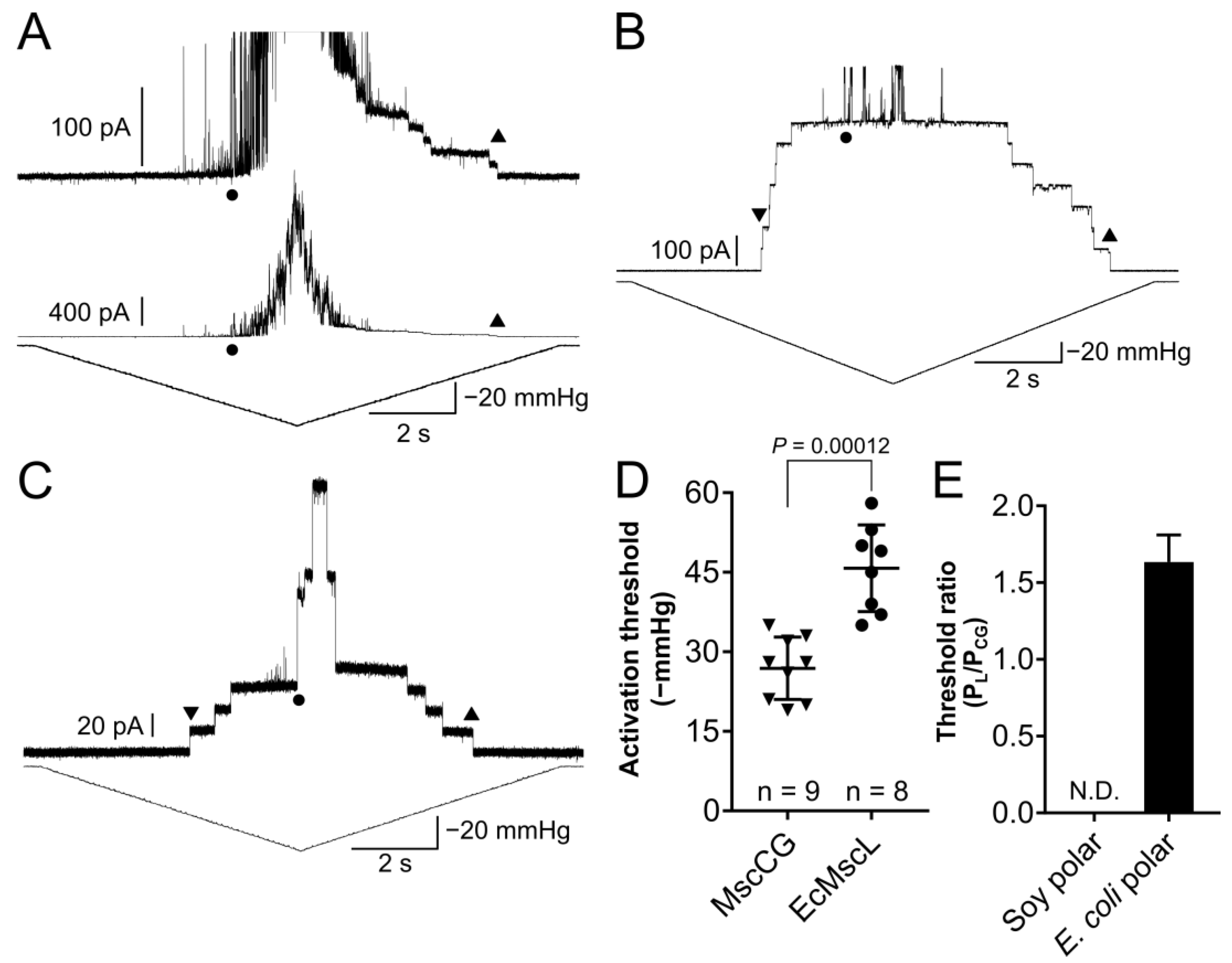
3.3. Comparison between MscCG Mechanosensitivity in Liposomes Made of Soy and E. coli Polar Extract Lipids
3.4. Phosphatidylglycerol Is the Main Charged Lipid for Native MscCG Mechanosensitivity
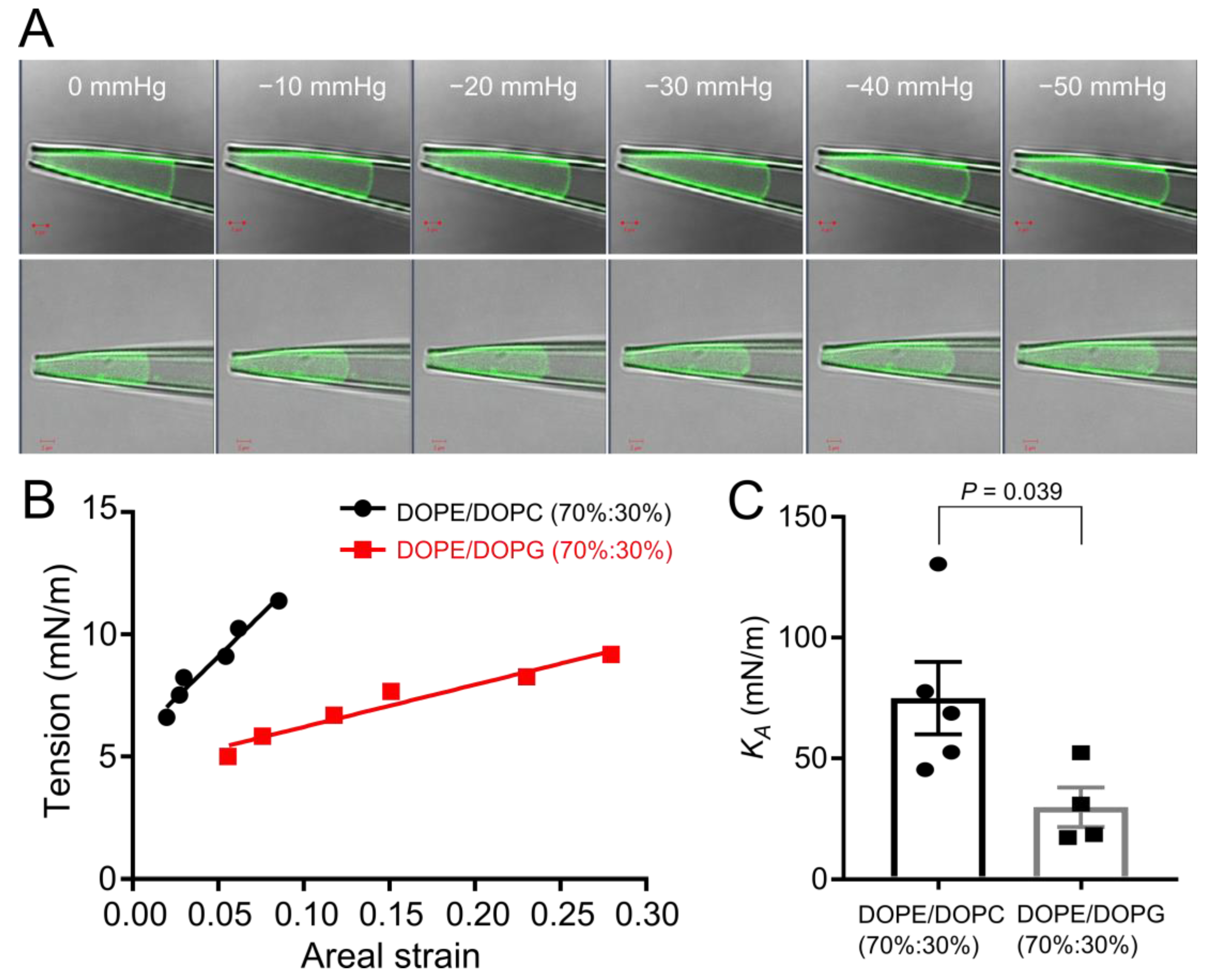
3.5. Anionic Membranes Composed of Phosphatidylglycerol Are Softer than Neutral Membranes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kung, C. A possible unifying principle for mechanosensation. Nature 2005, 436, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Martinac, B.; Cox, C.D. Mechanosensory transduction: Focus on ion channels. In Reference Module in Life Sciences; Edition: Comprehensive biophysics; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Guichard, M.; Thomine, S.; Frachisse, J.M. Mechanotransduction in the spotlight of mechano-sensitive channels. Curr. Opin. Plant Biol. 2022, 68, 102252. [Google Scholar] [CrossRef] [PubMed]
- Levina, N.; Tötemeyer, S.; Stokes, N.R.; Louis, P.; Jones, M.A.; Booth, I.R. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: Identification of genes required for MscS activity. EMBO J. 1999, 18, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Cetiner, U.; Arroyo, D.; Fonbuena, J.; Tiwari, M.; Barrera, P.; Lander, N.; Anishkin, A.; Sukharev, S.; Jimenez, V. A novel mechanosensitive channel controls osmoregulation, differentiation, and infectivity in Trypanosoma cruzi. ELife 2021, 10, e67449. [Google Scholar] [CrossRef] [PubMed]
- Bremer, E.; Krämer, R. Responses of Microorganisms to Osmotic Stress. Annu Rev Microbiol. 2019, 73, 313–334. [Google Scholar] [CrossRef]
- Poddar, A.; Hsu, Y.; Zhang, F.; Shamma, A.; Kreais, Z.; Muller, C.; Malla, M.; Ray, A.; Liu, A.P.; Chen, Q. Membrane stretching activates calcium permeability of a putative channel Pkd2 during fission yeast cytokinesis. Mol. Biol. Cell 2022, 33, ar134. [Google Scholar] [CrossRef]
- Morris, Z.; Sinha, D.; Poddar, A.; Morris, B.; Chen, Q. Fission yeast TRP channel Pkd2p localizes to the cleavage furrow and regulates cell separation during cytokinesis. Mol. Biol. Cell 2019, 30, 1791–1804. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef]
- Takahashi, K.; Takahashi, H.; Furuichi, T.; Toyota, M.; Furutani-Seiki, M.; Kobayashi, T.; Watanabe-Takano, H.; Shinohara, M.; Numaga-Tomita, T.; Sakaue-Sawano, A.; et al. Gravity sensing in plant and animal cells. npj Microgravity 2021, 7, 2. [Google Scholar] [CrossRef]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauve, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Holt, J.R. The Mechanosensory Transduction Machinery in Inner Ear Hair Cells. Annu. Rev. Biophys. 2021, 50, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, Y.; Kusakizako, T.; Wang, Y.; Pan, C.; Zhang, Y.; Nureki, O.; Hattori, M.; Yan, Z. TMC1 and TMC2 Proteins Are Pore-Forming Subunits of Mechanosensitive Ion Channels. Neuron 2020, 105, 310–321.e3. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y. Corynebacterium glutamicum mechanosensing: From osmoregulation to L-Glutamate secretion for the avian microbiota-gut-brain axis. Microorganisms 2021, 9, 201. [Google Scholar] [CrossRef]
- Nakayama, Y.; Komazawa, K.; Bavi, N.; Hashimoto, K.; Kawasaki, H.; Martinac, B. Evolutionary specialization of MscCG, an MscS-like mechanosensitive channel, in amino acid transport in Corynebacterium glutamicum. Sci. Rep. 2018, 8, 12893. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G.; Xu, D.; Fan, L.; Wu, X.; Ni, X.; Zhao, S.; Zheng, P.; Sun, J.; Ma, J. A novel Corynebacterium glutamicum L-glutamate exporter. Appl. Environ. Microbiol. 2018, 84, e02691-17. [Google Scholar] [CrossRef]
- Nakamura, J.; Hirano, S.; Ito, H.; Wachi, M. Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce L-glutamic acid production. Appl. Environ. Microbiol. 2007, 73, 4491–4498. [Google Scholar] [CrossRef]
- Nakayama, Y.; Hashimoto, K.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium glutamicum mechanosensitive channels: Towards unpuzzling “glutamate efflux” for amino acid production. Biophys. Rev. 2018, 10, 1359–1369. [Google Scholar] [CrossRef]
- Martinac, B.; Adler, J.; Kung, C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 1990, 348, 261–263. [Google Scholar] [CrossRef]
- Teng, J.; Loukin, S.; Anishkin, A.; Kung, C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflug. Arch. 2015, 467, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Martinac, B.; Kung, C. The force-from-lipid principle and its origin, a ‘what is true for E. coli is true for the elephant’ refrain. J. Neurogenet. 2022, 36, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Börngen, K.; Battle, A.R.; Möker, N.; Morbach, S.; Marin, K.; Martinac, B.; Krämer, R. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim. Biophys. Acta 2010, 1798, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Börngen, K.; Nomura, T.; Battle, A.R.; Marin, K.; Martinac, B.; Krämer, R. Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim. Biophys. Acta 2013, 1828, 1230–1240. [Google Scholar] [CrossRef]
- Hashimoto, K.; Murata, J.; Konishi, T.; Yabe, I.; Nakamatsu, T.; Kawasaki, H. Glutamate is excreted across the cytoplasmic membrane through the NCgl1221 channel of Corynebacterium glutamicum by passive diffusion. Biosci. Biotechnol. Biochem. 2012, 76, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, C.; Hashimoto, K.; Kumagai, K.; Maeda, T.; Takada, A.; Yabe, I.; Kawasaki, H.; Wachi, M. L-glutamate secretion by the N-terminal domain of the Corynebacterium glutamicum NCgl1221 mechanosensitive channel. Biosci. Biotechnol. Biochem. 2013, 77, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yoshimura, K.; Iida, H. Electrophysiological characterization of the mechanosensitive channel MscCG in Corynebacterium glutamicum. Biophys. J. 2013, 105, 1366–1375. [Google Scholar] [CrossRef]
- Nomura, T.; Cranfield, C.G.; Deplazes, E.; Owen, D.M.; Macmillan, A.; Battle, A.R.; Constantine, M.; Sokabe, M.; Martinac, B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc. Natl. Acad. Sci. USA 2012, 109, 8770–8775. [Google Scholar] [CrossRef]
- Martinac, B.; Nikolaev, Y.A.; Silvani, G.; Bavi, N.; Romanov, V.; Nakayama, Y.; Martinac, A.D.; Rohde, P.; Bavi, O.; Cox, C.D. Cell membrane mechanics and mechanosensory transduction. Curr. Top. Membr. 2020, 86, 83–141. [Google Scholar] [CrossRef]
- Reddy, B.; Bavi, N.; Lu, A.; Park, Y.; Perozo, E. Molecular basis of force-from-lipids gating in the mechanosensitive channel MscS. eLife 2019, 8, e50486. [Google Scholar] [CrossRef]
- Zhang, Y.; Daday, C.; Gu, R.; Cox, C.D.; Martinac, B.; de Groot, B.L.; Walz, T. Visualization of the mechanosensitive ion channel MscS under membrane tension. Nature 2021, 590, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.D.; Zhang, Y.; Zhou, Z.; Walz, T.; Martinac, B. Cyclodextrins increase membrane tension and are universal activators of mechanosensitive channels. Proc. Natl. Acad. Sci. USA 2021, 118, e2104820118. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Maksaev, G.; Schlegel, A.M.; Zhang, J.; Rau, M.; Fitzpatrick, J.A.J.; Haswell, E.S.; Yuan, P. Structural mechanism for gating of a eukaryotic mechanosensitive channel of small conductance. Nat. Commun. 2020, 11, 3690. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Wang, J.; Liu, X.; Zhang, W.; Sun, L. Structural Insights into a Plant Mechanosensitive Ion Channel MSL1. Cell Rep. 2020, 30, 4518–4527. [Google Scholar] [CrossRef] [PubMed]
- Flegler, V.J.; Rasmussen, A.; Rao, S.; Wu, N.; Zenobi, R.; Sansom, M.S.P.; Hedrich, R.; Rasmussen, T.; Böttcher, B. The MscS-like channel YnaI has a gating mechanism based on flexible pore helices. Proc. Natl. Acad. Sci. USA 2020, 117, 28754–28762. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Zheng, H. Mechanosensitive channel YnaI has lipid-bound extended sensor paddles. Commun. Biol. 2021, 4, 602. [Google Scholar] [CrossRef]
- Mount, J.; Maksaev, G.; Summers, B.T.; Fitzpatrick, J.A.J.; Yuan, P. Structural basis for mechanotransduction in a potassium-dependent mechanosensitive ion channel. Nat. Commun. 2022, 13, 6904. [Google Scholar] [CrossRef]
- Yang, X.; Lin, C.; Chen, X.; Li, S.; Li, X.; Xiao, B. Structure deformation and curvature sensing of PIEZO1 in lipid membranes. Nature 2022, 604, 377–383. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2015, 40, 133–159. [Google Scholar] [CrossRef]
- López-Lara, I.M.; Geiger, O. Bacterial lipid diversity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1287–1299. [Google Scholar] [CrossRef]
- Wilson, M.E.; Maksaev, G.; Haswell, E.S. MscS-like mechanosensitive channels in plants and microbes. Biochemistry 2013, 52, 5708–5722. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.D.; Nakayama, Y.; Nomura, T.; Martinac, B. The evolutionary ‘tinkering’ of MscS-like channels: Generation of structural and functional diversity. Pflug. Arch. 2015, 467, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, H.R.; Maurer, J.A. The Mechanosensitive Channel of Small Conductance (MscS) Superfamily: Not Just Mechanosensitive Channels Anymore. Chembiochem 2012, 13, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Krämer, R. MscCG from Corynebacterium glutamicum: Functional significance of the C-terminal domain. Eur. Biophys. J. 2015, 44, 577–588. [Google Scholar] [CrossRef]
- Yim, S.S.; An, S.J.; Kang, M.; Lee, J.; Jeong, K.J. Isolation of fully synthetic promoters for high-level gene expression in Corynebacterium glutamicum. Biotechnol. Bioeng. 2013, 110, 2959–2969. [Google Scholar] [CrossRef]
- Häse, C.C.; Le Dain, A.C.; Martinac, B. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J. Biol. Chem. 1995, 270, 18329–18334. [Google Scholar] [CrossRef]
- Bavi, N.; Nakayama, Y.; Bavi, O.; Cox, C.D.; Qin, Q.; Martinac, B. Biophysical implications of lipid bilayer rheometry for mechanosensitive channels. Proc. Natl. Acad. Sci. USA 2014, 111, 13864–13869. [Google Scholar] [CrossRef]
- Grage, S.L.; Keleshian, A.M.; Turdzeladze, T.; Battle, A.R.; Tay, W.C.; May, R.P.; Holt, S.A.; Contera, S.A.; Haertlein, M.; Moulin, M.; et al. Bilayer-mediated clustering and functional interaction of MscL channels. Biophys. J. 2011, 100, 1252–1260. [Google Scholar] [CrossRef]
- Cox, C.D.; Nomura, T.; Ziegler, C.S.; Campbell, A.K.; Wann, K.T.; Martinac, B. Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat. Commun. 2013, 4, 2137. [Google Scholar] [CrossRef]
- Nagakubo, T.; Tahara, Y.O.; Miyata, M.; Nomura, N.; Toyofuku, M. Mycolic acid-containing bacteria trigger distinct types of membrane vesicles through different routes. iScience 2021, 24, 102015. [Google Scholar] [CrossRef]
- Pluhackova, K.; Horner, A. Native-like membrane models of E. coli polar lipid extract shed light on the importance of lipid composition complexity. BMC Biol. 2021, 19, 4. [Google Scholar] [CrossRef]
- Nakayama, Y.; Fujiu, K.; Sokabe, M.; Yoshimura, K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc. Natl. Acad. Sci. USA 2007, 104, 5883–5888. [Google Scholar] [CrossRef]
- Haswell, E.S.; Meyerowitz, E.M. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr. Biol. 2006, 16, 1–11. [Google Scholar] [CrossRef]
- Lee, C.P.; Maksaev, G.; Jensen, G.S.; Murcha, M.W.; Wilson, M.E.; Fricker, M.; Hell, R.; Haswell, E.S.; Millar, A.H.; Sweetlove, L.J. MSL1 is a mechanosensitive ion channel that dissipates mitochondrial membrane potential and maintains redox homeostasis in mitochondria during abiotic stress. Plant J. 2016, 88, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yoshimura, K.; Iida, H. Organellar mechanosensitive channels in fission yeast regulate the hypo-osmotic shock response. Nat. Commun. 2012, 3, 1020. [Google Scholar] [CrossRef]
- Dionysopoulou, M.; Yan, N.; Wang, B.; Pliotas, C.; Diallinas, G. Genetic and cellular characterization of MscS-like putative channels in the filamentous fungus Aspergillus nidulans. Channels 2022, 16, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H.; Martinac, B.; Adler, J.; Kung, C. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys. J. 1989, 56, 631–636. [Google Scholar] [CrossRef]
- Battle, A.R.; Ridone, P.; Bavi, N.; Nakayama, Y.; Nikolaev, Y.A.; Martinac, B. Lipid-protein interactions: Lessons learned from stress. Biochim. Biophys. Acta 2015, 1848, 1744–1756. [Google Scholar] [CrossRef]
- Yoshimura, K.; Iida, K.; Iida, H. MCAs in Arabidopsis are Ca2+-permeable mechanosensitive channels inherently sensitive to membrane tension. Nat. Commun. 2021, 12, 6074. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.G.; Su, Z.; MacKinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. USA 2014, 111, 3614–3619. [Google Scholar] [CrossRef]
- Iwamoto, M.; Oiki, S. Hysteresis of a Tension-Sensitive K+ Channel Revealed by Time-Lapse Tension Measurements. JACS Au 2021, 1, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Cox, C.D.; Bavi, N.; Rohde, P.R.; Nakayama, Y.; Martinac, B. Membrane stiffness is one of the key determinants of E. coli MscS channel mechanosensitivity. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183203. [Google Scholar] [CrossRef] [PubMed]
- Ridone, P.; Grage, S.L.; Patkunarajah, A.; Battle, A.R.; Ulrich, A.S.; Martinac, B. “Force-from-lipids” gating of mechanosensitive channels modulated by PUFAs. J. Mech. Behav. Biomed. Mater. 2018, 79, 158–167. [Google Scholar] [CrossRef]
- Martinac, B.; Bavi, N.; Ridone, P.; Nikolaev, Y.A.; Martinac, A.D.; Nakayama, Y.; Rohde, P.R.; Bavi, O. Tuning ion channel mechanosensitivity by asymmetry of the transbilayer pressure profile. Biophys. Rev. 2018, 10, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Blount, P. Phosphatidylinositol is crucial for the mechanosensitivity of Mycobacterium tuberculosis MscL. Biochemistry 2013, 52, 5415–5420. [Google Scholar] [CrossRef]
- Laganowsky, A.; Reading, E.; Allison, T.M.; Ulmschneider, M.B.; Degiacomi, M.T.; Baldwin, A.J.; Robinson, C.V. Membrane proteins bind lipids selectively to modulate their structure and function. Nature 2014, 510, 172–175. [Google Scholar] [CrossRef]
- Iwamoto, M.; Oiki, S. Amphipathic antenna of an inward rectifier K+ channel responds to changes in the inner membrane leaflet. Proc. Natl. Acad. Sci. USA 2013, 110, 749–754. [Google Scholar] [CrossRef]
- Shi, T.; Fan, X.; Wu, Y.; Ma, Q.; Xu, Q.; Xie, X.; Chen, N. Mutation of genes for cell membrane synthesis in Corynebacterium glutamicum causes temperature-sensitive trait and promotes L-glutamate excretion. Biotechnol. Biotechnol. Equip. 2020, 34, 38–47. [Google Scholar] [CrossRef]
- Nampoothiri, K.; Hoischen, C.; Bathe, B.; Möckel, B.; Pfefferle, W.; Krumbach, K.; Sahm, H.; Eggeling, L. Expression of genes of lipid synthesis and altered lipid composition modulates L-glutamate efflux of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2002, 58, 89–96. [Google Scholar] [CrossRef]
- Perozo, E.; Kloda, A.; Cortes, D.M.; Martinac, B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002, 9, 696–703. [Google Scholar] [CrossRef]
- Bavi, O.; Cox, C.D.; Vossoughi, M.; Naghdabadi, R.; Jamali, Y.; Martinac, B. Influence of Global and Local Membrane Curvature on Mechanosensitive Ion Channels: A Finite Element Approach. Membranes 2016, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Bavi, N.; Cortes, D.M.; Cox, C.D.; Rohde, P.R.; Liu, W.; Deitmer, J.W.; Bavi, O.; Strop, P.; Hill, A.P. The role of MscL amphipathic N terminus indicates a blueprint for bilayer-mediated gating of mechanosensitive channels. Nat. Commun. 2016, 7, 11984. [Google Scholar] [CrossRef] [PubMed]
- Pliotas, C.; Dahl, A.C.; Rasmussen, T.; Mahendran, K.R.; Smith, T.K.; Marius, P.; Gault, J.; Banda, T.; Rasmussen, A.; Miller, S.; et al. The role of lipids in mechanosensation. Nat. Struct. Mol. Biol. 2015, 22, 991–998. [Google Scholar] [CrossRef]
- Flegler, V.J.; Rasmussen, T.; Böttcher, B. How Functional Lipids Affect the Structure and Gating of Mechanosensitive MscS-like Channels. Int. J. Mol. Sci. 2022, 23, 15071. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Goda, Y.; Sakai-Kato, K. Atomic Force Microscopy Study on the Stiffness of Nanosized Liposomes Containing Charged Lipids. Langmuir 2018, 34, 7805–7812. [Google Scholar] [CrossRef]
- Saha, S.K.; Alam Shibly, S.U.; Yamazaki, M. Membrane Tension in Negatively Charged Lipid Bilayers in a Buffer under Osmotic Pressure. J. Phys. Chem. B 2020, 124, 5588–5599. [Google Scholar] [CrossRef] [PubMed]
- Karal, M.A.; Alam, J.M.; Takahashi, T.; Levadny, V.; Yamazaki, M. Stretch-Activated Pore of Antimicrobial Peptide Magainin 2. Langmuir 2015, 31, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Komatsuzawa, H.; Fujiwara, T.; McCallum, N.; Sugai, M. Reduced Content of Lysyl-Phosphatidylglycerol in the Cytoplasmic Membrane Affects Susceptibility to Moenomycin, as Well as Vancomycin, Gentamicin, and Antimicrobial Peptides, in Staphylococcus aureus. Antimicrob. Agents Chemother. 2004, 48, 4800–4807. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Role of adhesion forces in mechanosensitive channel gating in Staphylococcus aureus adhering to surfaces. NJP Biofilms Microbiomes. 2020, 6, 31. [Google Scholar] [CrossRef]
- Sidarta, M.; Baruah, L.; Wenzel, M. Roles of Bacterial Mechanosensitive Channels in Infection and Antibiotic Susceptibility. Pharmaceuticals 2022, 15, 770. [Google Scholar] [CrossRef]


| Soy Polar Lipid Extract | E. coli Polar Lipid Extract | C. glutamicum Cell Membranes Ref. [51] | |
|---|---|---|---|
| Component | wt/wt% | wt/wt% | Relative Abundance (%) |
| Phosphatidylcholine (PC) | 45.7 | - | - |
| Phosphatidylethanolamine (PE) | 22.1 | 67.0 | - |
| Phosphatidylinositol (PI) | 18.4 | - | 6.7 |
| Phosphatidic acid (PA) | 6.9 | - | - |
| Phosphatidylglycerol (PG) | - | 23.2 | 72.4 |
| Cardiolipin (CA) | - | 9.8 | 6.5 |
| Diacylglycerol (DAG) | - | 14.4 | |
| Unknown | 6.9 | 0.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, Y.; Rohde, P.R.; Martinac, B. “Force-From-Lipids” Dependence of the MscCG Mechanosensitive Channel Gating on Anionic Membranes. Microorganisms 2023, 11, 194. https://doi.org/10.3390/microorganisms11010194
Nakayama Y, Rohde PR, Martinac B. “Force-From-Lipids” Dependence of the MscCG Mechanosensitive Channel Gating on Anionic Membranes. Microorganisms. 2023; 11(1):194. https://doi.org/10.3390/microorganisms11010194
Chicago/Turabian StyleNakayama, Yoshitaka, Paul R. Rohde, and Boris Martinac. 2023. "“Force-From-Lipids” Dependence of the MscCG Mechanosensitive Channel Gating on Anionic Membranes" Microorganisms 11, no. 1: 194. https://doi.org/10.3390/microorganisms11010194
APA StyleNakayama, Y., Rohde, P. R., & Martinac, B. (2023). “Force-From-Lipids” Dependence of the MscCG Mechanosensitive Channel Gating on Anionic Membranes. Microorganisms, 11(1), 194. https://doi.org/10.3390/microorganisms11010194






