HIV and Drug-Resistant Subtypes
Abstract
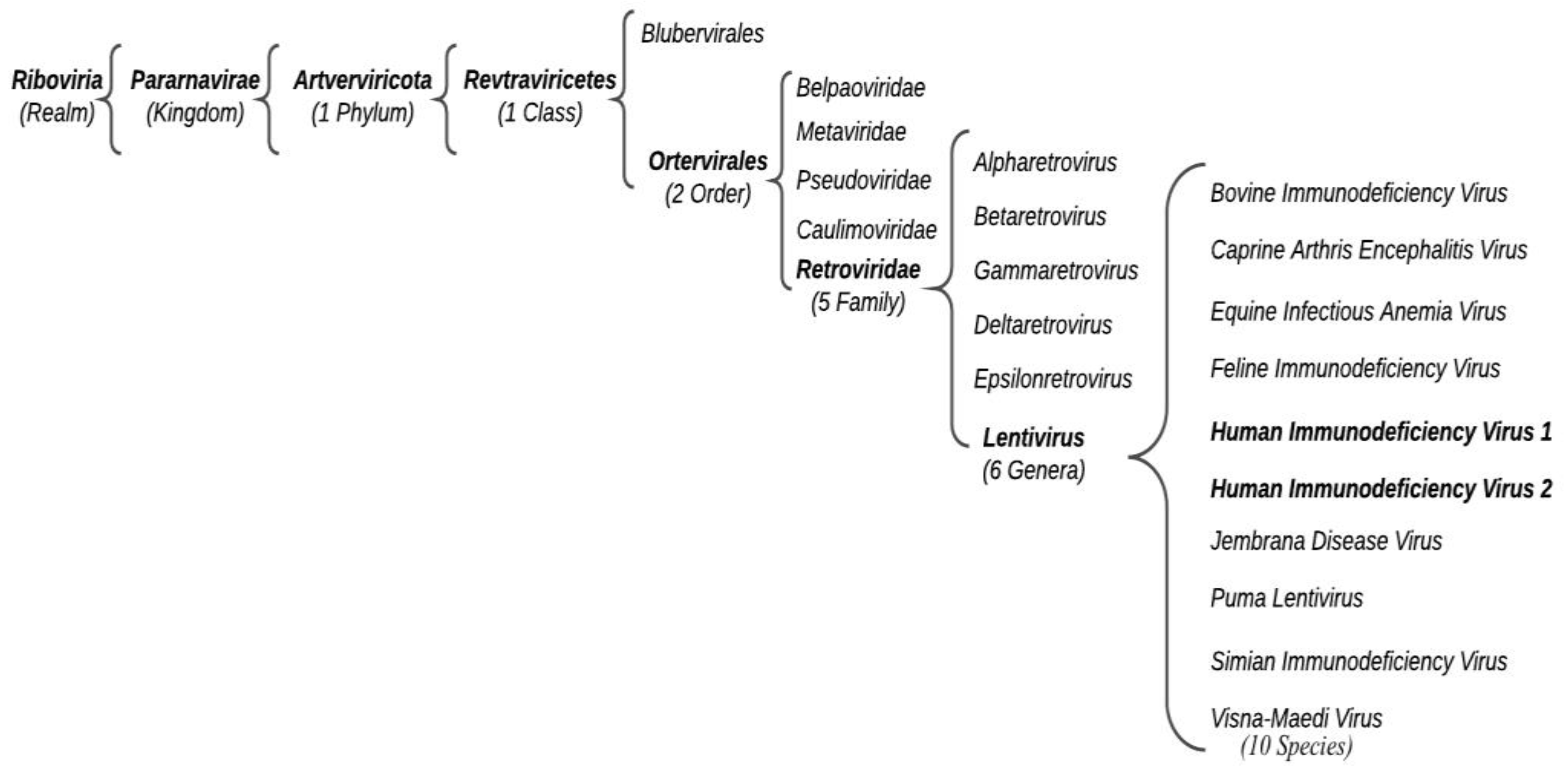
:1. Introduction: HIV and Its Distribution
2. HIV Diagnosis and Clinical Course
3. Treatment
4. Drug Resistance Mechanism
5. NRTI Drug Resistance
6. NNRTIs Drugs Resistance
7. PIs Drugs Resistance
8. INSTIs Drugs Resistance
9. Entry Inhibitor Drugs Resistance
9.1. Chemokine Receptor 5 (CCR5) Antagonists
9.2. Entry Inhibitor of gp41
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bbosa, N.; Kaleebu, P.; Ssemwanga, D. HIV Subtype Diversity Worldwide. Curr. Opin. HIV AIDS 2019, 14, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C.; Montagnier, L. The Discovery of HIV as the Cause of AIDS. N. Engl. J. Med. 2003, 349, 2283–2285. [Google Scholar] [CrossRef] [PubMed]
- Montagnier, L. 25 Years after HIV Discovery: Prospects for Cure and Vaccine. Virology 2010, 397, 248–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahlne, A. A Historical Reflection on the Discovery of Human Retroviruses. Retrovirology 2009, 6, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemelaar, J.; Gouws, E.; Ghys, P.D.; Osmanov, S. Global and Regional Distribution of HIV-1 Genetic Subtypes and Recombinants in 2004. AIDS 2006, 20, W13–W23. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, N.; Peeters, M.; Mulanga-Kabeya, C.; Nzilambi, N.; Robertson, D.; Ilunga, W.; Sema, H.; Tshimanga, K.; Bongo, B.; Delaporte, E. Unprecedented Degree of Human Immunodeficiency Virus Type 1 (HIV-1) Group M Genetic Diversity in the Democratic Republic of Congo Suggests That the HIV-1 Pandemic Originated in Central Africa. J. Virol. 2000, 74, 10498–10507. [Google Scholar] [CrossRef] [Green Version]
- Casado, G.; Thomson, M.M.; Sierra, M.; Nájera, R. Identification of a Novel HIV-1 Circulating ADG Intersubtype Recombinant Form (CRF19_cpx) in Cuba. J. Acquir. Immune Defic. Syndr. 2005, 40, 532–537. [Google Scholar] [CrossRef]
- Ng, K.T.; Ong, L.Y.; Takebe, Y.; Kamarulzaman, A.; Tee, K.K. Genome Sequence of a Novel HIV-1 Circulating Recombinant Form 54_01B from Malaysia. J. Virol. 2012, 86, 11405–11406. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Giorgi, E.E.; Ganusov, V.V.; Cai, F.; Athreya, G.; Yoon, H.; Carja, O.; Hora, B.; Hraber, P.; Romero-Severson, E.; et al. Tracking HIV-1 Recombination to Resolve Its Contribution to HIV-1 Evolution in Natural Infection. Nat. Commun. 2018, 9, 1928. [Google Scholar] [CrossRef]
- Osmanov, S.; Pattou, C.; Walker, N.; Schwardländer, B.; Esparza, J. WHO-UNAIDS Network for HIV Isolation and Characterization Estimated Global Distribution and Regional Spread of HIV-1 Genetic Subtypes in the Year 2000. J. Acquir. Immune Defic. Syndr. 2002, 29, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Balaguera, H.U.; Freedberg, K.A.; Werner, B.G.; Steger Craven, K.A.; Craven, D.E.; D’Aquila, R.T. Drug-Selected Resistance Mutations and Non-B Subtypes in Antiretroviral-Naive Adults with Established Human Immunodeficiency Virus Infection. J. Infect. Dis. 2003, 188, 986–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, S.-Y.; Shafer, R.W. Geographically-Stratified HIV-1 Group M Pol Subtype and Circulating Recombinant Form Sequences. Sci. Data 2018, 5, 180148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartner, M.J.; Roche, M.; Churchill, M.J.; Gorry, P.R.; Flynn, J.K. Understanding the Mechanisms Driving the Spread of Subtype C HIV-1. EBioMedicine 2020, 53, 102682. [Google Scholar] [CrossRef]
- Khan, S.; Zahid, M.; Qureshi, M.A.; Mughal, M.N.; Ujjan, I.D. HIV-1 Genetic Diversity, Geographical Linkages and Antiretroviral Drug Resistance among Individuals from Pakistan. Arch. Virol. 2018, 163, 33–40. [Google Scholar] [CrossRef]
- Korber, B.; Gaschen, B.; Yusim, K.; Thakallapally, R.; Kesmir, C.; Detours, V. Evolutionary and Immunological Implications of Contemporary HIV-1 Variation. Br. Med. Bull. 2001, 58, 19–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menéndez-Arias, L.; Alvarez, M. Antiretroviral Therapy and Drug Resistance in Human Immunodeficiency Virus Type 2 Infection. Antivir. Res. 2014, 102, 70–86. [Google Scholar] [CrossRef]
- Lu, H.; Tang, Y.-W. Myths in the Laboratory Diagnosis of HIV Infection. Emerg. Microbes Infect. 2019, 8, 1240–1242. [Google Scholar] [CrossRef] [Green Version]
- Hurt, C.B.; Nelson, J.A.E.; Hightow-Weidman, L.B.; Miller, W.C. Selecting an HIV Test: A Narrative Review for Clinicians and Researchers. Sex. Transm. Dis. 2017, 44, 739–746. [Google Scholar] [CrossRef]
- Alexander, T.S. Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution. Clin. Vaccine Immunol. 2016, 23, 249–253. [Google Scholar] [CrossRef]
- Pitasi, M.A.; Patel, S.N.; Wesolowski, L.G.; Masciotra, S.; Luo, W.; Owen, S.M.; Delaney, K.P. Performance of an Alternative Laboratory-Based HIV Diagnostic Testing Algorithm Using HIV-1 RNA Viral Load. Sex. Transm. Dis. 2020, 47, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Braunstein, S.L.; Hoover, D.R.; Li, S.; Nash, D. Timeliness of Human Immunodeficiency Virus Diagnosis and Antiretroviral Treatment Initiation in the Era of Universal Testing and Treatment. J. Infect. Dis. 2019, 220, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Yang, O.O.; Cumberland, W.G.; Escobar, R.; Liao, D.; Chew, K.W. Demographics and Natural History of HIV-1-Infected Spontaneous Controllers of Viremia. AIDS 2017, 31, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Platten, M.; Linnemann, R.; Kümmerle, T.; Jung, N.; Wyen, C.; Ehren, K.; Gravemann, S.; Gillor, D.; Cornely, O.A.; Fischer, J.; et al. Clinical Course and Quality of Care in ART-Naïve Patients Newly Presenting in a HIV Outpatient Clinic. Infection 2014, 42, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Sneller, M.C.; Blazkova, J.; Justement, J.S.; Shi, V.; Kennedy, B.D.; Gittens, K.; Tolstenko, J.; McCormack, G.; Whitehead, E.J.; Schneck, R.F.; et al. Combination Anti-HIV Antibodies Provide Sustained Virological Suppression. Nature 2022, 606, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Merigan, T.C. Treatment of AIDS with Combinations of Antiretroviral Agents. Am. J. Med. 1991, 90, 8S–17S. [Google Scholar] [CrossRef]
- Nastri, B.M.; Zannella, C.; Folliero, V.; Rinaldi, L.; Restivo, L.; Stelitano, D.; Sperlongano, R.; Adinolfi, L.E.; Franci, G. Editorial-Role of Highly Active Antiretroviral Therapy (HAART) for the COVID-19 Treatment. Eur. Rev. Med. Pharm. Sci. 2020, 24, 11982–11984. [Google Scholar] [CrossRef]
- Sanna, G.; Madeddu, S.; Murgia, G.; Serreli, G.; Begala, M.; Caboni, P.; Incani, A.; Franci, G.; Galdiero, M.; Giliberti, G. Potent and Selective Activity against Human Immunodeficiency Virus 1 (HIV-1) of Thymelaea Hirsuta Extracts. Viruses 2020, 12, E664. [Google Scholar] [CrossRef]
- Scott, L.J. Dolutegravir/Lamivudine Single-Tablet Regimen: A Review in HIV-1 Infection. Drugs 2020, 80, 61–72. [Google Scholar] [CrossRef]
- Sarafianos, S.G.; Hughes, S.H.; Arnold, E. Designing Anti-AIDS Drugs Targeting the Major Mechanism of HIV-1 RT Resistance to Nucleoside Analog Drugs. Int. J. Biochem. Cell Biol. 2004, 36, 1706–1715. [Google Scholar] [CrossRef]
- Voshavar, C. Protease Inhibitors for the Treatment of HIV/AIDS: Recent Advances and Future Challenges. Curr. Top. Med. Chem. 2019, 19, 1571–1598. [Google Scholar] [CrossRef] [PubMed]
- Farady, C.J.; Craik, C.S. Mechanisms of Macromolecular Protease Inhibitors. ChemBiochem 2010, 11, 2341–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 5172–5208. [Google Scholar] [CrossRef] [Green Version]
- Brik, A.; Wong, C.-H. HIV-1 Protease: Mechanism and Drug Discovery. Org. Biomol. Chem. 2003, 1, 5–14. [Google Scholar] [CrossRef]
- Anstett, K.; Brenner, B.; Mesplede, T.; Wainberg, M.A. HIV Drug Resistance against Strand Transfer Integrase Inhibitors. Retrovirology 2017, 14, 36. [Google Scholar] [CrossRef]
- Delelis, O.; Carayon, K.; Saïb, A.; Deprez, E.; Mouscadet, J.-F. Integrase and Integration: Biochemical Activities of HIV-1 Integrase. Retrovirology 2008, 5, 114. [Google Scholar] [CrossRef] [Green Version]
- Quashie, P.K.; Mesplède, T.; Wainberg, M.A. HIV Drug Resistance and the Advent of Integrase Inhibitors. Curr. Infect. Dis. Rep. 2013, 15, 85–100. [Google Scholar] [CrossRef]
- Esté, J.A.; Telenti, A. HIV Entry Inhibitors. Lancet 2007, 370, 81–88. [Google Scholar] [CrossRef]
- Pugach, P.; Ketas, T.J.; Michael, E.; Moore, J.P. Neutralizing Antibody and Anti-Retroviral Drug Sensitivities of HIV-1 Isolates Resistant to Small Molecule CCR5 Inhibitors. Virology 2008, 377, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Qian, K.; Morris-Natschke, S.L.; Lee, K.-H. HIV Entry Inhibitors and Their Potential in HIV Therapy. Med. Res. Rev. 2009, 29, 369–393. [Google Scholar] [CrossRef]
- Dvory-Sobol, H.; Shaik, N.; Callebaut, C.; Rhee, M.S. Lenacapavir: A First-in-Class HIV-1 Capsid Inhibitor. Curr. Opin. HIV AIDS 2022, 17, 15–21. [Google Scholar] [CrossRef]
- Diamond, T.L.; Ngo, W.; Xu, M.; Goh, S.L.; Rodriguez, S.; Lai, M.-T.; Asante-Appiah, E.; Grobler, J.A. Islatravir Has a High Barrier to Resistance and Exhibits a Differentiated Resistance Profile from Approved Nucleoside Reverse Transcriptase Inhibitors (NRTIs). Antimicrob. Agents Chemother. 2022, 66, e00133-22. [Google Scholar] [CrossRef] [PubMed]
- Cane, P.A. Stability of Transmitted Drug-Resistant HIV-1 Species. Curr. Opin. Infect. Dis. 2005, 18, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Kitayimbwa, J.M.; Mugisha, J.Y.T.; Saenz, R.A. Estimation of the HIV-1 Backward Mutation Rate from Transmitted Drug-Resistant Strains. Popul. Biol. 2016, 112, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Abram, M.E.; Ferris, A.L.; Shao, W.; Alvord, W.G.; Hughes, S.H. Nature, Position, and Frequency of Mutations Made in a Single Cycle of HIV-1 Replication. J. Virol. 2010, 84, 9864–9878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansky, L.M. HIV Mutagenesis and the Evolution of Antiretroviral Drug Resistance. Drug Resist. Updat. 2002, 5, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and Characterization of Transmitted and Early Founder Virus Envelopes in Primary HIV-1 Infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7552–7557. [Google Scholar] [CrossRef] [Green Version]
- Nasir, A.; Dimitrijevic, M.; Romero-Severson, E.; Leitner, T. Large Evolutionary Rate Heterogeneity among and within HIV-1 Subtypes and CRFs. Viruses 2021, 13, 1689. [Google Scholar] [CrossRef]
- Levy, D.N.; Aldrovandi, G.M.; Kutsch, O.; Shaw, G.M. Dynamics of HIV-1 Recombination in Its Natural Target Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 4204–4209. [Google Scholar] [CrossRef] [Green Version]
- Hammer, S.M.; Saag, M.S.; Schechter, M.; Montaner, J.S.G.; Schooley, R.T.; Jacobsen, D.M.; Thompson, M.A.; Carpenter, C.C.J.; Fischl, M.A.; Gazzard, B.G.; et al. Treatment for Adult HIV Infection: 2006 Recommendations of the International AIDS Society-USA Panel. JAMA 2006, 296, 827–843. [Google Scholar] [CrossRef]
- Coffin, J.M. HIV Population Dynamics in Vivo: Implications for Genetic Variation, Pathogenesis, and Therapy. Science 1995, 267, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.L.; Ponte, R.; Jones, B.R.; Kinloch, N.N.; Omondi, F.H.; Jenabian, M.-A.; Dupuy, F.P.; Fromentin, R.; Brassard, P.; Mehraj, V.; et al. HIV Diversity and Genetic Compartmentalization in Blood and Testes during Suppressive Antiretroviral Therapy. J. Virol. 2019, 93, e00755-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perelson, A.S.; Ribeiro, R.M. Modeling the Within-Host Dynamics of HIV Infection. BMC Biol. 2013, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.W.; Shafer, R.W. HIV-1 Antiretroviral Resistance: Scientific Principles and Clinical Applications. Drugs 2012, 72, e1–e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Lan, Y.; Liang, S.; Wang, J.; Ni, M.; Zhang, X.; Yu, F.; Chen, M.; Zhang, H.; Yan, L.; et al. Prevalence of Doravirine Cross-Resistance in HIV-Infected Adults Who Failed First-Line ART in China, 2014-18. J. Antimicrob. Chemother. 2022, 77, 1119–1124. [Google Scholar] [CrossRef]
- Novak, R.M.; Chen, L.; MacArthur, R.D.; Baxter, J.D.; Huppler Hullsiek, K.; Peng, G.; Xiang, Y.; Henely, C.; Schmetter, B.; Uy, J.; et al. Prevalence of Antiretroviral Drug Resistance Mutations in Chronically HIV-Infected, Treatment-Naive Patients: Implications for Routine Resistance Screening before Initiation of Antiretroviral Therapy. Clin. Infect. Dis. 2005, 40, 468–474. [Google Scholar] [CrossRef]
- Wensing, A.M.J.; van de Vijver, D.A.; Angarano, G.; Asjö, B.; Balotta, C.; Boeri, E.; Camacho, R.; Chaix, M.-L.; Costagliola, D.; De Luca, A.; et al. Prevalence of Drug-Resistant HIV-1 Variants in Untreated Individuals in Europe: Implications for Clinical Management. J. Infect. Dis. 2005, 192, 958–966. [Google Scholar] [CrossRef] [Green Version]
- Brenner, B.G.; Coutsinos, D. The K65R Mutation in HIV-1 Reverse Transcriptase: Genetic Barriers, Resistance Profile and Clinical Implications. HIV 2009, 3, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Ross, L.; Elion, R.; Lanier, R.; Dejesus, E.; Cohen, C.; Redfield, R.R.; Gathe, J.C.; Hsu, R.K.; Yau, L.; Paulsen, D.; et al. Modulation of K65R Selection by Zidovudine Inclusion: Analysis of HIV Resistance Selection in Subjects with Virologic Failure Receiving Once-Daily Abacavir/Lamivudine/Zidovudine and Tenofovir DF (Study COL40263). AIDS Res. Hum. Retrovir. 2009, 25, 665–672. [Google Scholar] [CrossRef]
- Bazmi, H.Z.; Hammond, J.L.; Cavalcanti, S.C.; Chu, C.K.; Schinazi, R.F.; Mellors, J.W. In Vitro Selection of Mutations in the Human Immunodeficiency Virus Type 1 Reverse Transcriptase That Decrease Susceptibility to (-)-Beta-D-Dioxolane-Guanosine and Suppress Resistance to 3′-Azido-3′-Deoxythymidine. Antimicrob. Agents Chemother. 2000, 44, 1783–1788. [Google Scholar] [CrossRef]
- Cilento, M.E.; Reeve, A.B.; Michailidis, E.; Ilina, T.V.; Nagy, E.; Mitsuya, H.; Parniak, M.A.; Tedbury, P.R.; Sarafianos, S.G. Development of Human Immunodeficiency Virus Type 1 Resistance to 4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine Starting with Wild-Type or Nucleoside Reverse Transcriptase Inhibitor-Resistant Strains. Antimicrob. Agents Chemother. 2021, 65, e0116721. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D. K65R, TAMs and Tenofovir. AIDS Rev. 2004, 6, 22–33. [Google Scholar] [PubMed]
- Luo, X.-L.; Mo, L.; Su, G.-S.; Huang, J.-P.; Wu, J.-Y.; Su, H.-Z.; Huang, W.-H.; Luo, S.; Ni, Z.-Y. Incidence and Types of HIV-1 Drug Resistance Mutation among Patients Failing First-Line Antiretroviral Therapy. J. Pharm. Sci. 2019, 139, 275–279. [Google Scholar] [CrossRef] [PubMed]
- McColl, D.J.; Chappey, C.; Parkin, N.T.; Miller, M.D. Prevalence, Genotypic Associations and Phenotypic Characterization of K65R, L74V and Other HIV-1 RT Resistance Mutations in a Commercial Database. Antivir. Ther. 2008, 13, 189–197. [Google Scholar] [CrossRef]
- Pozniak, A. Tenofovir: What Have over 1 Million Years of Patient Experience Taught Us? Int. J. Clin. Pract. 2008, 62, 1285–1293. [Google Scholar] [CrossRef]
- Wolf, K.; Walter, H.; Beerenwinkel, N.; Keulen, W.; Kaiser, R.; Hoffmann, D.; Lengauer, T.; Selbig, J.; Vandamme, A.-M.; Korn, K.; et al. Tenofovir Resistance and Resensitization. Antimicrob. Agents Chemother. 2003, 47, 3478–3484. [Google Scholar] [CrossRef] [Green Version]
- Lambert-Niclot, S.; Charpentier, C.; Storto, A.; Fofana, D.; Soulie, C.; Fourati, S.; Wirden, M.; Morand-Joubert, L.; Masquelier, B.; Flandre, P.; et al. Rilpivirine, Emtricitabine and Tenofovir Resistance in HIV-1-Infected Rilpivirine-Naive Patients Failing Antiretroviral Therapy. J. Antimicrob. Chemother. 2014, 69, 1086–1089. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, E.; Niubo, J.; Crespo, M.; Gatell, J.M.; Sanz, J.; Veloso, S.; Llibre, J.M.; Barrufet, P.; Sanchez, P.; Podzamczer, D.; et al. Genotypic Resistance in HIV-Infected Naive Patients Receiving Abacavir plus Lamivudine and Efavirenz. J. Acquir. Immune Defic. Syndr. 2007, 46, 253–255. [Google Scholar] [CrossRef] [Green Version]
- Galindo, J.; Amariles, P.; Mueses-Marín, H.F.; Hincapié, J.A.; González-Avendaño, S.; Galindo-Orrego, X. Effectiveness and Safety of Generic Version of Abacavir/Lamivudine and Efavirenz in Treatment Naïve HIV-Infected Patients: A Nonrandomized, Open-Label, Phase IV Study in Cali-Colombia, 2011-2012. BMC Infect. Dis. 2016, 16, 532. [Google Scholar] [CrossRef] [Green Version]
- Karkashadze, E.; Dvali, N.; Bolokadze, N.; Sharvadze, L.; Gabunia, P.; Karchava, M.; Tchelidze, T.; Tsertsvadze, T.; DeHovitz, J.; Del Rio, C.; et al. Epidemiology of Human Immunodeficiency Virus (HIV) Drug Resistance in HIV Patients with Virologic Failure of First-Line Therapy in the Country of Georgia. J. Med. Virol. 2019, 91, 235–240. [Google Scholar] [CrossRef]
- Ayitewala, A.; Kyeyune, F.; Ainembabazi, P.; Nabulime, E.; Kato, C.D.; Nankya, I. Comparison of HIV Drug Resistance Profiles across HIV-1 Subtypes A and D for Patients Receiving a Tenofovir-Based and Zidovudine-Based First Line Regimens in Uganda. AIDS Res. 2020, 17, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisic, M.; Mendieta, J.; Puertas, M.C.; Parera, M.; Martínez, M.A.; Martinez-Picado, J.; Menéndez-Arias, L. Mechanistic Basis of Zidovudine Hypersusceptibility and Lamivudine Resistance Conferred by the Deletion of Codon 69 in the HIV-1 Reverse Transcriptase Coding Region. J. Mol. Biol. 2008, 382, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Stammers, D.K. Structural Basis for Drug Resistance Mechanisms for Non-Nucleoside Inhibitors of HIV Reverse Transcriptase. Virus Res. 2008, 134, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Zaccarelli, M.; Cingolani, A.; Forbici, F.; Rizzo, M.G.; Trotta, M.P.; Di Giambenedetto, S.; Narciso, P.; Ammassari, A.; Girardi, E.; et al. Cross-Resistance among Nonnucleoside Reverse Transcriptase Inhibitors Limits Recycling Efavirenz after Nevirapine Failure. AIDS Res. Hum. Retrovir. 2002, 18, 835–838. [Google Scholar] [CrossRef]
- Ruxrungtham, K.; Pedro, R.J.; Latiff, G.H.; Conradie, F.; Domingo, P.; Lupo, S.; Pumpradit, W.; Vingerhoets, J.H.; Peeters, M.; Peeters, I.; et al. Impact of Reverse Transcriptase Resistance on the Efficacy of TMC125 (Etravirine) with Two Nucleoside Reverse Transcriptase Inhibitors in Protease Inhibitor-Naïve, Nonnucleoside Reverse Transcriptase Inhibitor-Experienced Patients: Study TMC125-C227. HIV Med. 2008, 9, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Varghese, V.; Shahriar, R.; Rhee, S.-Y.; Liu, T.; Simen, B.B.; Egholm, M.; Hanczaruk, B.; Blake, L.A.; Gharizadeh, B.; Babrzadeh, F.; et al. Minority Variants Associated with Transmitted and Acquired HIV-1 Nonnucleoside Reverse Transcriptase Inhibitor Resistance: Implications for the Use of Second-Generation Nonnucleoside Reverse Transcriptase Inhibitors. J. Acquir. Immune Defic. Syndr. 2009, 52, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Melikian, G.L.; Rhee, S.-Y.; Varghese, V.; Porter, D.; White, K.; Taylor, J.; Towner, W.; Troia, P.; Burack, J.; Dejesus, E.; et al. Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) Cross-Resistance: Implications for Preclinical Evaluation of Novel NNRTIs and Clinical Genotypic Resistance Testing. J. Antimicrob. Chemother. 2014, 69, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, B.; Turner, D.; Oliveira, M.; Moisi, D.; Detorio, M.; Carobene, M.; Marlink, R.G.; Schapiro, J.; Roger, M.; Wainberg, M.A. A V106M Mutation in HIV-1 Clade C Viruses Exposed to Efavirenz Confers Cross-Resistance to Non-Nucleoside Reverse Transcriptase Inhibitors. AIDS 2003, 17, F1–F5. [Google Scholar] [CrossRef]
- Kolomeets, A.N.; Varghese, V.; Lemey, P.; Bobkova, M.R.; Shafer, R.W. A Uniquely Prevalent Nonnucleoside Reverse Transcriptase Inhibitor Resistance Mutation in Russian Subtype A HIV-1 Viruses. AIDS 2014, 28, F1–F8. [Google Scholar] [CrossRef] [Green Version]
- Sluis-Cremer, N.; Tachedjian, G. Mechanisms of Inhibition of HIV Replication by Non-Nucleoside Reverse Transcriptase Inhibitors. Virus Res. 2008, 134, 147–156. [Google Scholar] [CrossRef]
- Vingerhoets, J.; Tambuyzer, L.; Azijn, H.; Hoogstoel, A.; Nijs, S.; Peeters, M.; de Béthune, M.-P.; De Smedt, G.; Woodfall, B.; Picchio, G. Resistance Profile of Etravirine: Combined Analysis of Baseline Genotypic and Phenotypic Data from the Randomized, Controlled Phase III Clinical Studies. AIDS 2010, 24, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.J.; Andrade-Villanueva, J.; Clotet, B.; Fourie, J.; Johnson, M.A.; Ruxrungtham, K.; Wu, H.; Zorrilla, C.; Crauwels, H.; Rimsky, L.T.; et al. Rilpivirine versus Efavirenz with Two Background Nucleoside or Nucleotide Reverse Transcriptase Inhibitors in Treatment-Naive Adults Infected with HIV-1 (THRIVE): A Phase 3, Randomised, Non-Inferiority Trial. Lancet 2011, 378, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Rimsky, L.; Vingerhoets, J.; Van Eygen, V.; Eron, J.; Clotet, B.; Hoogstoel, A.; Boven, K.; Picchio, G. Genotypic and Phenotypic Characterization of HIV-1 Isolates Obtained from Patients on Rilpivirine Therapy Experiencing Virologic Failure in the Phase 3 ECHO and THRIVE Studies: 48-Week Analysis. J. Acquir. Immune Defic. Syndr. 2012, 59, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Magistrelli, E.; Colangeli, V.; Manfredi, R.; Borderi, M.; Rossi, N.; Conti, M.; Mancini, R.; Viale, P. Dual Raltegravir-Etravirine Combination as Maintenance Regimen in Virologically Suppressed HIV-1-Infected Patients. AIDS Res. Hum. Retrovir. 2017, 33, 632–638. [Google Scholar] [CrossRef]
- Clutter, D.S.; Jordan, M.R.; Bertagnolio, S.; Shafer, R.W. HIV-1 Drug Resistance and Resistance Testing. Infect. Genet. Evol. 2016, 46, 292–307. [Google Scholar] [CrossRef] [Green Version]
- Picchio, G.; Vingerhoets, J.; Tambuyzer, L.; Coakley, E.; Haddad, M.; Witek, J. Short Communication Prevalence of Susceptibility to Etravirine by Genotype and Phenotype in Samples Received for Routine HIV Type 1 Resistance Testing in the United States. AIDS Res. Hum. Retrovir. 2011, 27, 1271–1275. [Google Scholar] [CrossRef]
- Tambuyzer, L.; Vingerhoets, J.; Azijn, H.; Daems, B.; Nijs, S.; de Béthune, M.-P.; Picchio, G. Characterization of Genotypic and Phenotypic Changes in HIV-1-Infected Patients with Virologic Failure on an Etravirine-Containing Regimen in the DUET-1 and DUET-2 Clinical Studies. AIDS Res. Hum. Retrovir. 2010, 26, 1197–1205. [Google Scholar] [CrossRef]
- Rock, A.E.; Lerner, J.; Badowski, M.E. Doravirine and Its Potential in the Treatment of HIV: An Evidence-Based Review of the Emerging Data. HIV 2020, 12, 201–210. [Google Scholar] [CrossRef]
- Stockdale, A.J.; Khoo, S. Doravirine: Its Role in HIV Treatment. Curr. Opin. HIV AIDS 2022, 17, 4–14. [Google Scholar] [CrossRef]
- Ali, A.; Bandaranayake, R.M.; Cai, Y.; King, N.M.; Kolli, M.; Mittal, S.; Murzycki, J.F.; Nalam, M.N.L.; Nalivaika, E.A.; Özen, A.; et al. Molecular Basis for Drug Resistance in HIV-1 Protease. Viruses 2010, 2, 2509–2535. [Google Scholar] [CrossRef]
- Munerato, P.; Sucupira, M.C.; Oliveros, M.P.R.; Janini, L.M.; de Souza, D.F.; Pereira, A.A.; Inocencio, L.A.; Diaz, R.S. HIV Type 1 Antiretroviral Resistance Mutations in Subtypes B, C, and F in the City of São Paulo, Brazil. AIDS Res. Hum. Retrovir. 2010, 26, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.C.; Covens, K.; Snoeck, J.; Vandamme, A.-M.; Camacho, R.J.; Van Laethem, K. HIV-1 Protease Mutation 82M Contributes to Phenotypic Resistance to Protease Inhibitors in Subtype G. J. Antimicrob. Chemother. 2012, 67, 1075–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulkes-Murzycki, J.E.; Scott, W.R.P.; Schiffer, C.A. Hydrophobic Sliding: A Possible Mechanism for Drug Resistance in Human Immunodeficiency Virus Type 1 Protease. Structure 2007, 15, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Fun, A.; Wensing, A.M.J.; Verheyen, J.; Nijhuis, M. Human Immunodeficiency Virus Gag and Protease: Partners in Resistance. Retrovirology 2012, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, K.M.; Nakka, P.; King, B.M.; Rhee, S.-Y.; Holmes, S.P.; Shafer, R.W.; Radhakrishnan, M.L. A Multifaceted Analysis of HIV-1 Protease Multidrug Resistance Phenotypes. BMC Bioinform. 2011, 12, 477. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.-Y.; Taylor, J.; Fessel, W.J.; Kaufman, D.; Towner, W.; Troia, P.; Ruane, P.; Hellinger, J.; Shirvani, V.; Zolopa, A.; et al. HIV-1 Protease Mutations and Protease Inhibitor Cross-Resistance. Antimicrob. Agents Chemother. 2010, 54, 4253–4261. [Google Scholar] [CrossRef] [Green Version]
- Barber, T.J.; Harrison, L.; Asboe, D.; Williams, I.; Kirk, S.; Gilson, R.; Bansi, L.; Pillay, D.; Dunn, D. UK HIV Drug Resistance Database and UK Collaborative HIV Cohort (UK CHIC) Study Steering Committees Frequency and Patterns of Protease Gene Resistance Mutations in HIV-Infected Patients Treated with Lopinavir/Ritonavir as Their First Protease Inhibitor. J. Antimicrob. Chemother. 2012, 67, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Dolling, D.I.; Dunn, D.T.; Sutherland, K.A.; Pillay, D.; Mbisa, J.L.; Parry, C.M.; Post, F.A.; Sabin, C.A.; Cane, P.A.; UK HIV Drug Resistance Database (UKHDRD); et al. Low Frequency of Genotypic Resistance in HIV-1-Infected Patients Failing an Atazanavir-Containing Regimen: A Clinical Cohort Study. J. Antimicrob. Chemother. 2013, 68, 2339–2343. [Google Scholar] [CrossRef] [Green Version]
- El Bouzidi, K.; White, E.; Mbisa, J.L.; Phillips, A.; Mackie, N.; Pozniak, A.; Dunn, D. Protease Mutations Emerging on Darunavir in Protease Inhibitor-Naïve and Experienced Patients in the UK. J. Int. AIDS Soc. 2014, 17, 19739. [Google Scholar] [CrossRef]
- Lathouwers, E.; De Meyer, S.; Dierynck, I.; Van de Casteele, T.; Lavreys, L.; de Béthune, M.-P.; Picchio, G. Virological Characterization of Patients Failing Darunavir/Ritonavir or Lopinavir/Ritonavir Treatment in the ARTEMIS Study: 96-Week Analysis. Antivir. Ther. 2011, 16, 99–108. [Google Scholar] [CrossRef]
- Molina, J.-M.; Cahn, P.; Grinsztejn, B.; Lazzarin, A.; Mills, A.; Saag, M.; Supparatpinyo, K.; Walmsley, S.; Crauwels, H.; Rimsky, L.T.; et al. Rilpivirine versus Efavirenz with Tenofovir and Emtricitabine in Treatment-Naive Adults Infected with HIV-1 (ECHO): A Phase 3 Randomised Double-Blind Active-Controlled Trial. Lancet 2011, 378, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Mollan, K.; Daar, E.S.; Sax, P.E.; Balamane, M.; Collier, A.C.; Fischl, M.A.; Lalama, C.M.; Bosch, R.J.; Tierney, C.; Katzenstein, D.; et al. HIV-1 Amino Acid Changes among Participants with Virologic Failure: Associations with First-Line Efavirenz or Atazanavir plus Ritonavir and Disease Status. J. Infect. Dis. 2012, 206, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, G.U.; Liu, T.F.; Claassen, M.; Engelbrecht, S.; de Oliveira, T.; Preiser, W.; Wood, N.T.; Travers, S.; Shafer, R.W. Trends in Genotypic HIV-1 Antiretroviral Resistance between 2006 and 2012 in South African Patients Receiving First- and Second-Line Antiretroviral Treatment Regimens. PLoS ONE 2013, 8, e67188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaner, J.S.G.; Schutz, M.; Schwartz, R.; Jayaweera, D.T.; Burnside, A.F.; Walmsley, S.; Saag, M.S. Efficacy, Safety and Pharmacokinetics of Once-Daily Saquinavir Soft-Gelatin Capsule/Ritonavir in Antiretroviral-Naive, HIV-Infected Patients. MedGenMed 2006, 8, 36. [Google Scholar] [CrossRef]
- Jayaswal, A.; Mishra, A.; Mishra, H.; Shah, K. Evaluation of Novel Saquinavir Analogs for Resistance Mutation Compatibility and Potential as an HIV-Protease Inhibitor Drug. Bioinformation 2014, 10, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Ross, L.L.; Cotton, M.F.; Cassim, H.; Voronin, E.; Givens, N.; Sievers, J.; Cheng, K.Y.; APV29005 & APV20002 Pediatric Study Groups. Treatment-Emergent Mutations and Resistance in HIV-Infected Children Treated with Fosamprenavir-Containing Antiretroviral Regimens. Open AIDS J. 2015, 9, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, V.; Verdina, A.; Manente, L.; Spugnini, E.P.; Viglietti, R.; Parrella, R.; Pagliano, P.; Parrella, G.; Galati, R.; De Luca, A.; et al. Amprenavir Inhibits the Migration in Human Hepatocarcinoma Cell and the Growth of Xenografts. J. Cell Physiol. 2013, 228, 640–645. [Google Scholar] [CrossRef]
- Blanco, J.-L.; Varghese, V.; Rhee, S.-Y.; Gatell, J.M.; Shafer, R.W. HIV-1 Integrase Inhibitor Resistance and Its Clinical Implications. J. Infect. Dis. 2011, 203, 1204–1214. [Google Scholar] [CrossRef]
- Geretti, A.M.; Armenia, D.; Ceccherini-Silberstein, F. Emerging Patterns and Implications of HIV-1 Integrase Inhibitor Resistance. Curr. Opin. Infect. Dis. 2012, 25, 677–686. [Google Scholar] [CrossRef]
- Cooper, D.A.; Steigbigel, R.T.; Gatell, J.M.; Rockstroh, J.K.; Katlama, C.; Yeni, P.; Lazzarin, A.; Clotet, B.; Kumar, P.N.; Eron, J.E.; et al. Subgroup and Resistance Analyses of Raltegravir for Resistant HIV-1 Infection. N. Engl. J. Med. 2008, 359, 355–365. [Google Scholar] [CrossRef]
- Lennox, J.L.; DeJesus, E.; Lazzarin, A.; Pollard, R.B.; Madruga, J.V.R.; Berger, D.S.; Zhao, J.; Xu, X.; Williams-Diaz, A.; Rodgers, A.J.; et al. Safety and Efficacy of Raltegravir-Based versus Efavirenz-Based Combination Therapy in Treatment-Naive Patients with HIV-1 Infection: A Multicentre, Double-Blind Randomised Controlled Trial. Lancet 2009, 374, 796–806. [Google Scholar] [CrossRef]
- Han, Y.-S.; Mesplède, T.; Wainberg, M.A. Differences among HIV-1 Subtypes in Drug Resistance against Integrase Inhibitors. Infect. Genet. Evol. 2016, 46, 286–291. [Google Scholar] [CrossRef]
- Trkola, A.; Kuhmann, S.E.; Strizki, J.M.; Maxwell, E.; Ketas, T.; Morgan, T.; Pugach, P.; Xu, S.; Wojcik, L.; Tagat, J.; et al. HIV-1 Escape from a Small Molecule, CCR5-Specific Entry Inhibitor Does Not Involve CXCR4 Use. Proc. Natl. Acad. Sci. USA 2002, 99, 395–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.P.; Kuritzkes, D.R. A Pièce de Resistance: How HIV-1 Escapes Small Molecule CCR5 Inhibitors. Curr. Opin. HIV AIDS 2009, 4, 118–124. [Google Scholar] [CrossRef]
- Lalezari, J.P.; Eron, J.J.; Carlson, M.; Cohen, C.; DeJesus, E.; Arduino, R.C.; Gallant, J.E.; Volberding, P.; Murphy, R.L.; Valentine, F.; et al. A Phase II Clinical Study of the Long-Term Safety and Antiviral Activity of Enfuvirtide-Based Antiretroviral Therapy. AIDS 2003, 17, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Marfil, S.; García, E.; Martinez-Picado, J.; Bonjoch, A.; Bofill, M.; Moreno, S.; Ribera, E.; Domingo, P.; Clotet, B.; et al. Genetic Evolution of Gp41 Reveals a Highly Exclusive Relationship between Codons 36, 38 and 43 in Gp41 under Long-Term Enfuvirtide-Containing Salvage Regimen. AIDS 2006, 20, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Deeks, S.G.; Hoh, R.; Beatty, G.; Kuritzkes, B.A.; Martin, J.N.; Kuritzkes, D.R. Rapid Emergence of Enfuvirtide Resistance in HIV-1-Infected Patients: Results of a Clonal Analysis. J. Acquir. Immune Defic. Syndr. 2006, 43, 60–64. [Google Scholar] [CrossRef]
- Marcelin, A.-G.; Reynes, J.; Yerly, S.; Ktorza, N.; Segondy, M.; Piot, J.-C.; Delfraissy, J.-F.; Kaiser, L.; Perrin, L.; Katlama, C.; et al. Characterization of Genotypic Determinants in HR-1 and HR-2 Gp41 Domains in Individuals with Persistent HIV Viraemia under T-20. AIDS 2004, 18, 1340–1342. [Google Scholar] [CrossRef]
- Menzo, S.; Castagna, A.; Monachetti, A.; Hasson, H.; Danise, A.; Carini, E.; Bagnarelli, P.; Lazzarin, A.; Clementi, M. Genotype and Phenotype Patterns of Human Immunodeficiency Virus Type 1 Resistance to Enfuvirtide during Long-Term Treatment. Antimicrob. Agents Chemother. 2004, 48, 3253–3259. [Google Scholar] [CrossRef] [Green Version]
- Sista, P.R.; Melby, T.; Davison, D.; Jin, L.; Mosier, S.; Mink, M.; Nelson, E.L.; DeMasi, R.; Cammack, N.; Salgo, M.P.; et al. Characterization of Determinants of Genotypic and Phenotypic Resistance to Enfuvirtide in Baseline and On-Treatment HIV-1 Isolates. AIDS 2004, 18, 1787–1794. [Google Scholar] [CrossRef]
- Chang, L.; Zhao, J.; Guo, F.; Ji, H.; Zhang, L.; Jiang, X.; Wang, L. HIV-1 Gp41 Genetic Diversity and Enfuvirtide Resistance-Associated Mutations among Enfuvirtide-Naïve Patients in Southern China. Virus Res. 2021, 292, 198215. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Amolong Hinay, A.; Telan, E.F.O.; Samonte, G.M.J.; Leano, P.S.A.; Tsuneki-Tokunaga, A.; Kanai, K. Intrinsic Replication Competences of HIV Strains After Zidovudine/Lamivudine/Nevirapine Treatment in the Philippines. J. Int. Assoc. Provid. AIDS Care 2019, 18, 2325958219856579. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastri, B.M.; Pagliano, P.; Zannella, C.; Folliero, V.; Masullo, A.; Rinaldi, L.; Galdiero, M.; Franci, G. HIV and Drug-Resistant Subtypes. Microorganisms 2023, 11, 221. https://doi.org/10.3390/microorganisms11010221
Nastri BM, Pagliano P, Zannella C, Folliero V, Masullo A, Rinaldi L, Galdiero M, Franci G. HIV and Drug-Resistant Subtypes. Microorganisms. 2023; 11(1):221. https://doi.org/10.3390/microorganisms11010221
Chicago/Turabian StyleNastri, Bianca Maria, Pasquale Pagliano, Carla Zannella, Veronica Folliero, Alfonso Masullo, Luca Rinaldi, Massimiliano Galdiero, and Gianluigi Franci. 2023. "HIV and Drug-Resistant Subtypes" Microorganisms 11, no. 1: 221. https://doi.org/10.3390/microorganisms11010221
APA StyleNastri, B. M., Pagliano, P., Zannella, C., Folliero, V., Masullo, A., Rinaldi, L., Galdiero, M., & Franci, G. (2023). HIV and Drug-Resistant Subtypes. Microorganisms, 11(1), 221. https://doi.org/10.3390/microorganisms11010221







