Abstract
Phosphate-solubilizing bacteria (PSB) transform precipitated inorganic phosphorus into soluble orthophosphates. This study evaluated the efficiency of tricalcium and iron phosphate solubilization in Pikovskaya medium using five bacterial strains (A1, A2, A3, A5, and A6) cultured in acidic and alkaline pH levels. The bacterial strain that proved to be more efficient for P solubilization and was tolerant to pH variations was selected for assessing bacterial growth and P solubilization with glucose and sucrose in the culture medium. The bacterial strains were identified through 16S rRNA gene sequencing as Pseudomonas libanensis A1, Pseudomonas libanensis (A2), Bacillus pumilus (A3), Pseudomonas libanensis (A5), and Bacillus siamensis (A6). These five bacterial strains grew, tolerated pH changes, and solubilized inorganic phosphorus. The bacterial strain A3 solubilized FePO4 (4 mg L−1) and Ca3(PO4)2 (50 mg L−1). P solubilization was assayed with glucose and sucrose as carbon sources for A3 (Bacillus pumilus MN100586). After four culture days, Ca3(PO4)2 was solubilized, reaching 246 mg L−1 with sucrose in culture media. Using glucose as a carbon source, FePO4 was solubilized and reached 282 mg L−1 in six culture days. Our findings were: Pseudomonas libanensis, and Bacillus siamensis, as new bacteria, can be reported as P solubilizers with tolerance to acidic or alkaline pH levels. The bacterial strain B. pumilus grew using two sources of inorganic phosphorus and carbon, and it tolerated pH changes. For that reason, it is an ideal candidate for inorganic phosphorus solubilization and future production as a biofertilizer.
1. Introduction
Phosphorus (P) is a macronutrient that is present in all living things in its organic form as phytates, nucleic acids, and phospholipids. However, its bioavailability is very limited because in its inorganic form, and in an acidic media, it is found as iron or aluminum phosphate, and in an alkaline media, it is found as calcium phosphate [1,2].
In nature, P may be bioavailable through the action of phosphate-solubilizing bacteria (PSB) [3]. PSB have a key role in the P cycle as they make available precipitated P through the production of low-molecular-weight organic acids (i.e., malonic, gluconic, acetic, and lactic acid), which chelates metallic cations such as Ca+2, Fe+3, or Al+3, releasing orthophosphates as a result [4].
The bacterial solubilization of calcium phosphate is the most widely studied assay, and there are few studies exploring iron phosphate solubilization and even less at different pH ranges. Currently, the isolation of PSB is of major interest issue due to their potential use as biofertilizers, which could reduce the use of agrochemicals [5]. To consider a bacterial strain as biofertilizer for P solubilization, it is desirable for it to have good adaptation for growing in a wide range of pH values and the ability to solubilize several inorganic P sources [6].
PSB are heterotrophic microorganisms, and, therefore, to produce a bacterial biomass and enhance phosphate solubilization, there is a need to compare of the usage of different organic carbon sources in its culture media such that the bacterial strains show more growth [7]. The level of microbial activity for phosphate solubilization depends on the organic carbon degradation that sustains the PSB growth. This dependency on carbon degradation for PSB growth has not yet been studied nor reported widely. It is considered a key methodological step for PSB industrial production and application under field conditions to ensure their good performance [8].
The aims of this study were to evaluate five PSB strains through comparing their inorganic phosphorus solubilization under acidic and alkaline pH conditions, as well as to assess the effect of glucose and sucrose on their growth and P-solubilization.
2. Materials and Methods
2.1. Bacterial Strains Collection
Five phosphate solubilizer bacterial strains (PSB) were isolated from soil surface samples (at first, 15 to 20 cm in depth) from agricultural soils where potatoes (Solanum tuberosum) are grown at Toluca’s Nevado in the Municipality of Zinacantepec, State of Mexico, Mexico (GPS: longitude (dec): - 99.805278, latitude (dec): 19.161389). The PSB screening and isolation were as reported by Yañez-Ocampo et al. [9].
A plate assay method was performed on Pikovskaya (PVK) agar [10] as follows: glucose, 10 g; (NH4)2SO4, 0.5 g; NaCl, 0.2 g; KCl, 0.2 g; MgSO4·7H2O, 0.1 g; MnSO4·7H2O, 0.5 g; FeSO4·7H2O, 0.5 g; yeast extract, 0.5 g; and 15 g of agar in 1 L of distilled water (pH 7.2); with the addition of 3.0 g of tricalcium phosphate as an insoluble P source. All reagents used were of analytical grade. The bacterial strains were selected by the formation of a clear halo around the bacterial colony after five incubation days at 28 °C in PVK agar. The selected isolates were kept using pure cultures and stored at 4 °C.
2.2. Phosphate Solubilization into Pikovskaya Liquid Medium
The liquid medium assays used 25 mL flasks with 10 mL of PVK medium and 3% (v/v) of the inoculum. Only the control flasks with sterile PVK liquid medium and without biomass were considered. The experiments were performed for 8 days at 28 °C and with 100 rpm agitation, after which measurements of the biomasses, orthophosphates, and pH levels were completed. The biomasses were measured spectrophotometrically by optical density at a wavelength of 600 nm. For determination of the orthophosphates, 5 mL aliquots of PVK medium were centrifuged to 1000× g for 25 min to obtain a biomass-free supernatant. The orthophosphates released in the PVK medium were determined using the molybdenum blue method [11,12], measuring the absorbance at a wavelength of 880 nm and converting this to concentration units using a standard curve. The pH levels of the PVK medium were recorded with a Multi 9620 IDS WTW® model pH meter. All measurements were recorded from three replicates, respectively.
2.3. Comparison of Phosphate Solubilization Efficiency by Bacterial Strains Cultured at Different pH Values
All five PSB bacterial strains were cultured in PVK medium with different pH levels considering two pH ranges: an acidic range (5 and 5.5 pH) using FePO4 (100 mg L−1) as a non-soluble source of inorganic phosphorus and an alkaline range (7.5 and 8.0 pH) using Ca3(PO4)2 (100 mg L−1) as a non-soluble source of inorganic phosphorus. The pH levels were adjusted with 0.1 M HCl and 0.1 M NaOH, respectively. Each experimental treatment had six replicates. The experiments were performed using flasks with 10 mL of PVK broth inoculated with 300 µL of bacterial inoculum (the biomasses were adjusted to 0.2 absorbance units at a wavelength of 600 nm). The control flasks were not inoculated. All experimental treatments were incubated at 28 °C for 8 days. The biomass, orthophosphate, and pH quantification techniques used on the PVK liquid medium were the same as those mentioned in the above section.
2.4. Bacterial Strain Identification by 16S rRNA Gene Sequencing Using Bioinformatic Tools
Five PSB bacterial strains were identified using a 16S rRNA gene-based molecular analysis method. The genomic DNA of each bacterial strain was extracted using a ZR Fungal/Bacterial DNA Kit TM. The 16S rRNA gene was amplified using the rD1 and fD1 oligonucleotides [13]. The amplification products were purified from electrophoresis gel using the GeneJET kit (Thermo Scientific, Waltham, MA, USA) and sequenced at the Institute of Biotechnology (IBT, Hong Kong) of the National University of Mexico (UNAM, Mexico City, Mexico). The obtained sequences were deposited in the GenBank of the National Center for Biotechnology Information (NCBI, Bethesda, MA, USA) with the following access numbers: (A1) ON679588, (A2) ON679589, (A3) MN100586, (A5) ON679590, and (A6) ON679591. The final sequences of the 16S ribosomal gene of each strain were compared with the 16S ribosomal genes deposited in the GenBank database using the BlastN bioinformatic tool. A matrix phylogenetic analysis was performed using the Kimura 2-parameter model with 1000 bootstrap using the Seaview 4.6.1 program [14].
2.5. Experimental Design to Assess the Bacterial Growth Optimization and Phosphate Solubilization by Comparing Two Carbon Sources
One bacterial strain was selected for its ability to tolerate both acidic and alkaline culture conditions. After the strain selection, the effect of two carbon sources (glucose and sucrose) in the culture medium was assessed with three factors: (1) using non-soluble phosphate sources (Ca₃(PO₄)2 and FePO₄) at initial concentrations of 1 g L−1; (2) using organic carbon sources (glucose and sucrose); and (3) days of incubation. The experiments featured factorial design. The treatments were: glucose plus tricalcium phosphate (GCa), sucrose plus tricalcium phosphate (SCa), glucose plus iron phosphate (GI), and sucrose plus iron phosphate (SI), and each one was performed in triplicate. A non-inoculated control was included in the experiments. Flasks with PVK liquid medium were inoculated with 300 µL of bacterial biomass (adjusted to 0.2 OD600) and incubated at 28 °C for 8 days. The pH variation, orthophosphates released, and biomass growth were quantified using the techniques mentioned in Section 2.2. All parameters were monitored at 0, 2, 4, 6, and 8 days.
2.6. Statistical Analysis
The phosphate solubilization results at both pH ranges (acidic and alkaline) for all five bacterial strains were analyzed using an ANOVA test and Tukey´s test. To optimize the bacterial growth and P-solubilization efficiency in the culture medium, two organic carbon sources and two non-soluble inorganic phosphorus sources were compared with a MANOVA test. Finally, the significant differences between treatments were observed using a Tukey´s test. A confidence level of 95% was applied in all experiments performed. Results were analyzed with the StatGraphics Centurion XV software.
3. Results and discussion
3.1. Phosphate Solubilization by Bacterial Strains Cultured in PVK Liquid Medium at pH Levels of 5.0 and 5.5
During this work, five bacterial strains were capable of solubilizing both calcium and iron phosphates in alkaline and acidic pH environments, respectively. Table 1 shows the iron phosphate solubilization in an acidic pH range (5.0 and 5.5). At a pH of 5.0, the bacterial strain A1 showed significant differences in solubilization (4.420 mg L−1) compared to the other four bacterial strains. However, at a pH of 5.5, the bacterial strain A5 solubilized 4.722 mg L−1, and this result was statistically different from all the other bacterial strains tested. The solubilization of iron phosphates was reported by Collavino and Sansberro [15] where Stenotrophomonas maltophilia solubilized 12 mg L−1. Likewise, Hernández–Leal et al. [16], using edaphic bacteria, found an iron phosphate solubilization of 4.28 mg L−1. Chen et al. [17] reported that Pantoea dispersa solubilized iron phosphate at 41.42 mg L−1. Finally, Anzuay et al. [18] reported iron phosphate solubilization values of 14.5 and 37.4 mg L−1 using different strains of the Serratia genus. These results suggest that the low outcomes were due to the P source since iron phosphate has a solubility of 1.78 x 10−9 g L−1 and, therefore, bacterial activity could be limited.

Table 1.
Phosphate solubilization by PSB in acidic pH regions after 8 days of incubation.
A decrease in the pH of the culture medium was observed, which could be indirect evidence of the presence of organic acids. Initially, the pH of the culture medium was adjusted to 5.0 and 5.5, and after 8 days incubation time and bacterial growth, the pH was decreased to the range of 3.5 and 4.4, respectively. There have been some reports where the acidification of culture medium is due to organic acids being produced by the PSB [19,20], as this is the main microbial mechanism for the solubilization of inorganic P [21]. Anzuay et al. [18] reported that culture medium acidification could have negative effects on P solubilization, suggesting that when there are pH decreases of 4.5 and 3.5, FePO4 solubility also decreases.
3.2. Phosphate Solubilization by Bacterial Strains Cultured in PVK Liquid Medium at pH levels of 7.5 and 8.0
After assessing P solubilization in an acidic pH range, we performed assays in an alkaline pH range (7.5 and 8.0) using tricalcium phosphate as a non-bioavailable P source. The highest phosphate solubilization value was 27.406 mg L−1, which was achieved at a pH of 7.5 and obtained by the bacterial strain A3 (Table 2). However, the bacterial strain A2 had significantly higher results, solubilizing 52.319 mg L−1 at a pH of 8.0. All bacterial strains showed better phosphate solubilization values at a pH of 8.0 than at a pH of 7.5. It is very remarkable that the concentration of solubilized P nearly doubled. All bacterial strains assayed showed better phosphate solubilization performances in the alkaline pH range than in the acidic pH range; therefore, tolerance was observed in their biological activities for both pH ranges. The solubilization of Ca3(PO4)2 has been reported in multiples studies. Ogut et al. [22] reported that Acinetobacter sp. WR326 solubilized 888 mg L−1 of Ca3(PO4)2. Another study by Prieto-Correal et al. [23] showed that Streptomyces T3A solubilized 600 mg L−1 of Ca3(PO4)2.

Table 2.
Phosphate solubilization by PSB in alkaline pH regions after 8 days of incubation.
Pande et al. [24] found that Burkholderia cepacia was able to solubilize 305 mg L−1 Ca3(PO4)2. Sowmya et al. [25] reported that Ca3(PO4)2 solubilization for the Pantoea and Enterobacter genus was 500 mg L−1 and 1200 mg L−1, respectively. The variations found in tricalcium phosphate solubilization rates could be associated with the metabolic characteristics of the bacteria analyzed. Prieto-Correal et al. [23], as a possible explanation for the low values of soluble phosphorus, posited that the phosphorus might be metabolized and immobilized as part of the microbial biomass. This may also explain the results of our research.
3.3. Bacterial Growth in PVK Liquid Medium in Both Acidic and Alkaline pH Ranges
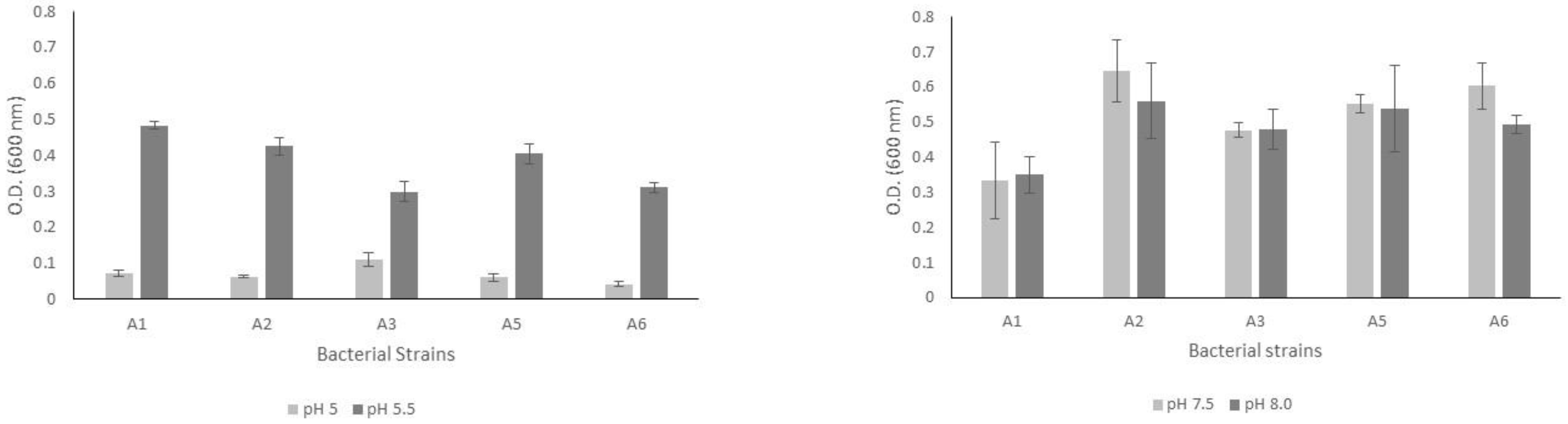
After eight culture days, the bacterial strains grew in both acidic and alkaline pH ranges (Figure 1). The bacterial biomasses reached 0.1 absorbance units when cultured at a pH of 5.0. In contrast, we observed higher growth rates of the biomasses when the bacterial strains were cultured at a pH of 5.5. Moreover, the five bacterial strains analyzed showed better adaptability for growing in an alkaline environment since the biomasses’ values were between 0.3 and 0.5 absorbance units.
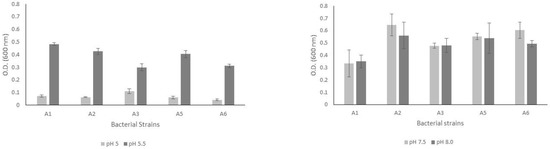
Figure 1.
Results of bacterial growth measured by optical density for the five bacterial strains cultured in PVK medium in both acidic (left graph) and alkaline (right graph) pH ranges.
3.4. Bacterial Strains Identification and Properties Reported
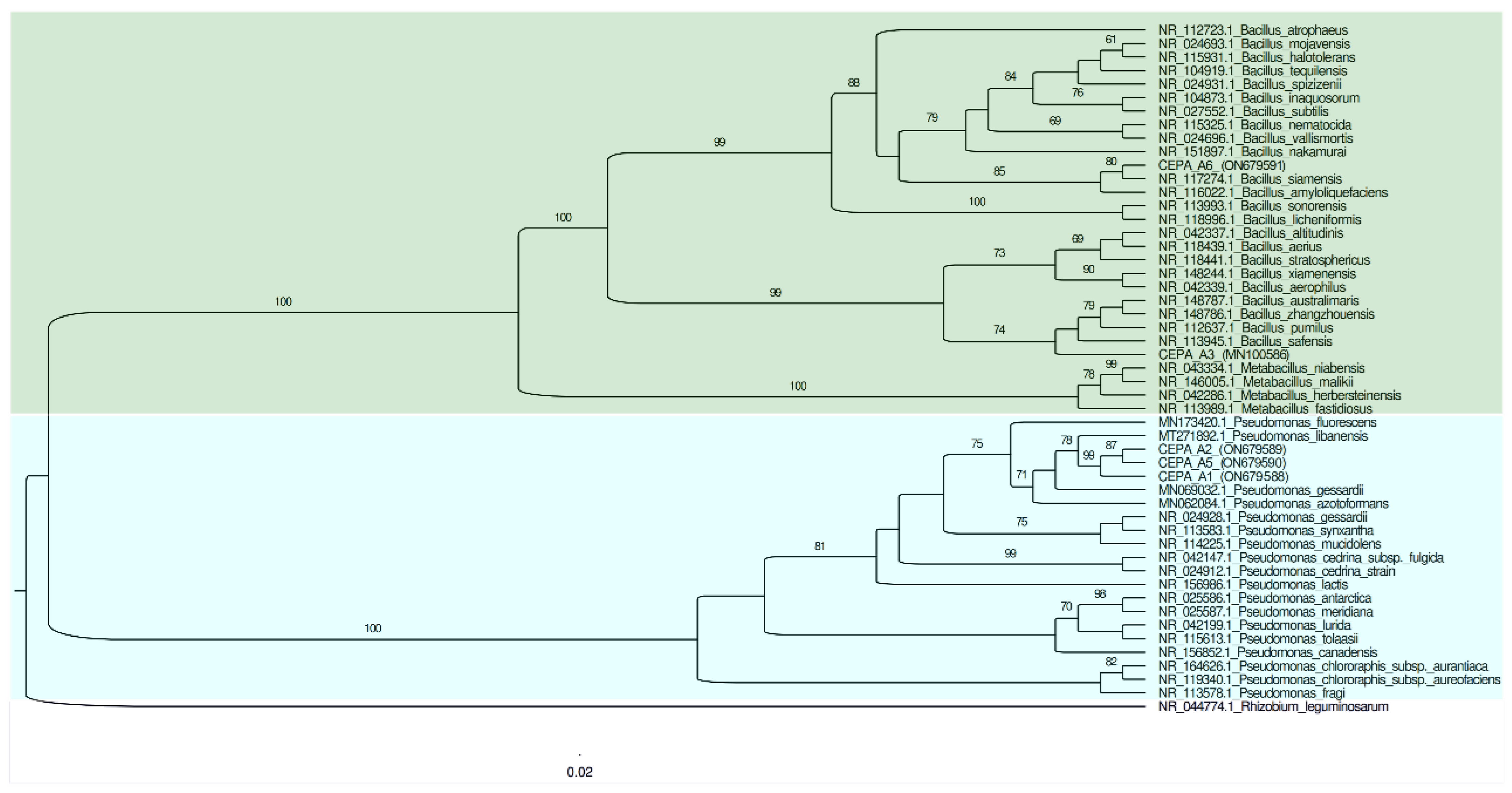
Table 3 shows the molecular identification results of the 16S ribosomal gene analysis, which were obtained using the sequences of the five bacterial strains. After BLAST analysis, it was observed that strains A1, A2, and A5 had more similarities with species of the Pseudomonas genus, while the bacterial strains A3 and A6 showed a relationship to the Bacillus genus.

Table 3.
Bacterial strains identified through 16S rRNA gene analysis.
The phylogenetic tree was built using the 16S ribosomal gene sequences fragments to confirm the identities of the isolates at the genus level (Figure 2). The cladogram showed that the A1, A2, and A5 strains were related to Pseudomonas libanensis. These bacteria have been recently identified and reported as drought stress alleviators in wheat cultures grown under greenhouse conditions [26].
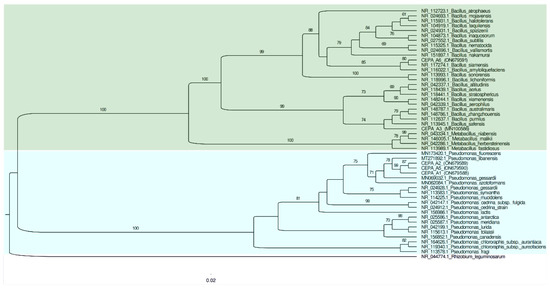
Figure 2.
Phylogenetic tree of the 16S rRNA gene sequencing for all five bacterial isolates with the ability to solubilize phosphate. The reference sequence accession numbers are shown after the species name.
Meanwhile, bacterial strain A3 was 96.03% related to Bacillus pumilus, and bacterial strain A6 was 99.07% related to Bacillus siamensis. Recently, Bacillus pumilus was recognized as a plant growth promoter rhizobacteria capable of synthesizing phytohormone precursors while also having an antagonistic activity against nematodes [27], in addition to chitinase activity in association with rice crop roots, enhancing carbohydrate metabolism and phenylpropanoid biosynthesis [28] and being a drought stress alleviator [29]. The bacterial species B. siamensis has been reported as the endophyte bacterium of Cicer arietinum L., showing antifungal activities [30].
The primary reports related to phosphate solubilizing with bacteria are those from the Bacillus genus and, more frequently, Bacillus subtillis. In the study by Hanif et al. [31], B. subtillis solubilized phosphate and increased the length and weight of both the roots and shoots in potatoes grown in soils with low phosphorus content. Similar results were reported by Ahmad et al. [32], where several phosphate and zinc solubilizer bacterial strains were identified through 16S rRNA sequencing, including Bacillus subtilis IA6 (accession # MN005922), Bacillus sp. IA16 (accession # MN005924), and Bacillus aryabhattai IA20 (accession # MN005925). They concluded that these bacterial isolates have promising uses as inoculants for improving cotton growth under semi-arid conditions.
Zaheer et al. [33] reported phosphorus solubilization by two bacterial isolates identified as Pseudomonas sp. strain AZ5 and Bacillus sp. strain AZ17. They also noted that both genera had positive effects on plants because of their multifunctional to P and Zn solubilization and production of IAA, phytase, organic acids, HCN, N-Acyl homoserine lactones, and siderophores, all of which are useful traits for plant growth. According to our results, Pseudomonas libanensis, P. gessardii, Bacillus pumilus, and B. siamensis are new contributions to the class of phosphate solubilizing bacteria (PSB).
3.5. Bacterial Growth and Phosphate Solubilization Comparison through the use of Glucose and Sucrose in PVK Culture Medium
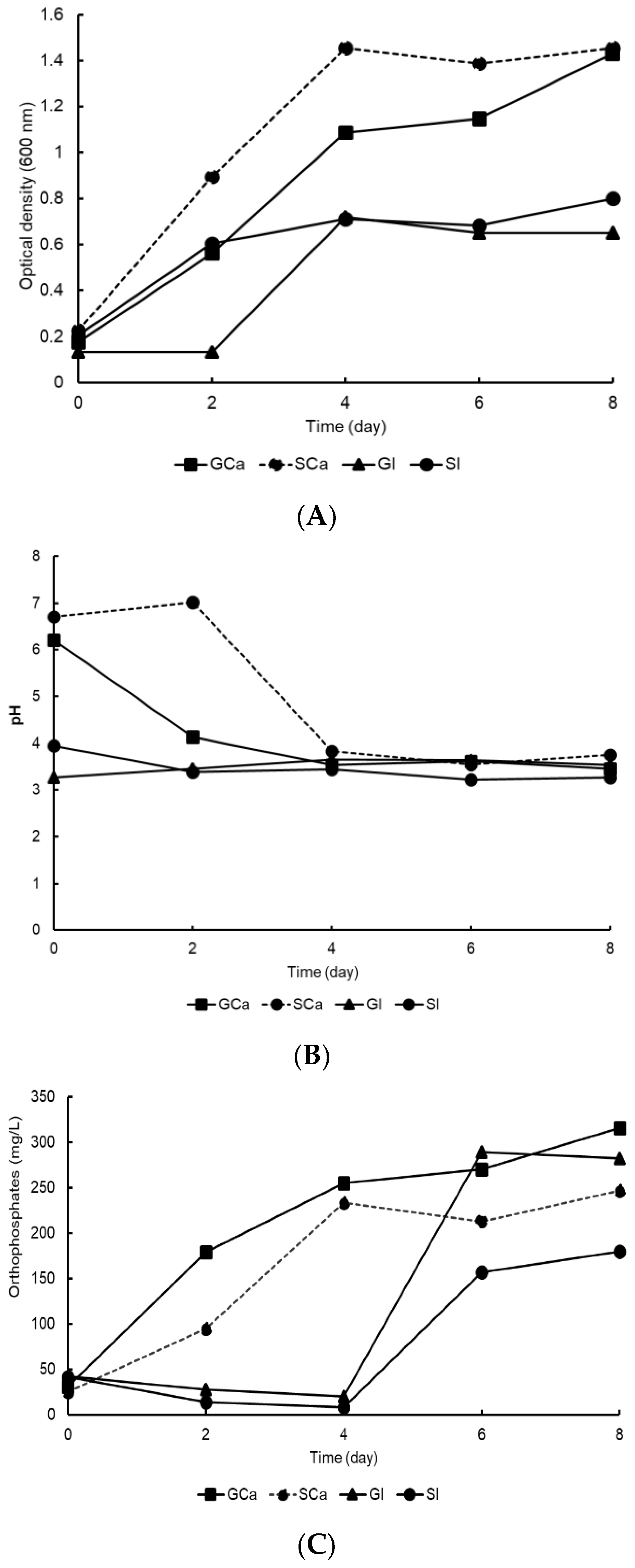
The results obtained in this research showed that bacterial strain A3 was capable of growing and solubilizing tricalcium phosphate and iron phosphate as it could tolerate both acidic and alkaline pH levels. Therefore, it was selected to compare its growth and P solubilization using glucose or sucrose in PVK medium as the main carbon source. Figure 3A shows that bacterial strain A3 grew in a PVK culture medium with both sources of carbon and phosphorus. In the PVK medium with added sucrose and tricalcium phosphate, the A3 biomass increased from 0.2 to 1.4 absorbance units. In contrast, the A3 cultured with iron phosphate and sucrose or glucose grew to only half of that treated with tricalcium phosphate.
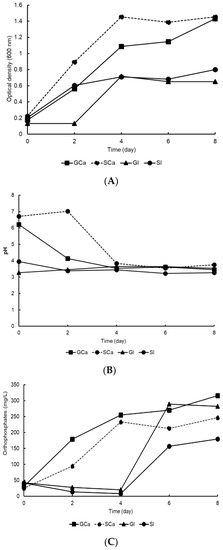
Figure 3.
Growth, pH, and P solubilization by the A3 bacterial strain, with glucose or sucrose in PVK medium. (A) Bacterial growth measured by optical density. (B) Dynamics of pH values. (C) P solubilization.
The behavior of pH throughout the experiment is reported in Figure 3B. The pH values at the beginning of the experiment in the treatments with tricalcium phosphate (GCa and SCa) tended towards neutrality. In the iron phosphate treatments (GI and SI), the initial pH values were acidic, and this remained constant throughout the experiment. After two culture days, the acidification process had initiated only in the treatments with tricalcium phosphate, indirectly indicating the production of organic acids by the A3 bacterial strain.
The solubilization of tricalcium phosphate began after two days of incubation, which was the same timeframe as the acidification process. The solubilization of iron phosphate did not begin until the fourth day of the experiment (Figure 3C). The highest soluble phosphorus values were recorded in the glucose treatments (GCa (315 mg L−1) and GI (289 mg L−1)). In the sucrose treatments with calcium (SCa) and iron (SI) phosphate, the soluble phosphorus values were 246 mg L−1 and 179 mg L−1, respectively.
Acidification is a key step towards Ca3(PO4)2 solubilization. Bacteria dissolve calcium phosphates through the production of gluconic and 2-ketogluconic acids through enzymes located in the periplasmic space [17]. Sowmya et al. [25] reported that there was a direct relationship between P solubilization and medium acidification. It has been reported that acidification modifies the precipitation/dissolution equilibrium of P and calcium sequestration by organic acids, and this results in P solubilizing and bioavailability [5,7]. Aliyat et al. [34] recently showed the decreasing pH of culture medium through organic acids production (formic, propionic, isobutyric, butyric, isovaleric, caproic, and heptanoic acids) by PSB strains. Benbrik et al. [19] pointed out that in a liquid culture medium, microbial biomass respiration generates CO2,-producing carbonic acid, creating acidic conditions and, consequently, bacterial growth inhibition, as well solubilization activity.
Mukhtar et al. [35] and McRae and Monreal [36] noted that PSBs use glucose and sucrose as carbon sources because these are the predominant sugars in rhizosphere exudates. Stefaoni et al. [37] noted that glucose increases solubilizing activity by serving as a substrate for the synthesis of gluconic acid. This acidifies the medium, allowing the phosphate solubilization. Glucose also favors the synthesis of oxalic acid, a key element for solubilizing Ca3(PO4)2, by acting as a calcium chelator.
Lim et al. [38] and Shen et al. [39] reported that the solubilization of phosphates is related to the physiology, metabolism, and genetic constitution of each bacterial species, and depending on the time required for solubilizing inorganic P, PSBs can be classified into early and late solubilizers, with early solubilizers being those having a maximum P-solubilization value at 72 incubation hours and late solubilizers being those that solubilize P after four or five incubation days. According to this, the A3 bacterial strain could be considered a late solubilizer since it solubilized tricalcium and iron phosphate on the eighth and sixth incubation days, respectively.
4. Conclusions
The main findings of this research were that the bacterial strains here studied were tolerant to alkaline or acidic conditions and retained their phosphate solubilization activity. This is an important outcome because it demonstrates that these strains can be applied as biofertilizers in soils with similar pH levels. The bacteria Pseudomonas libanensis, and Bacillus siamensis were novel findings for their phosphate solubilization activities. Finally, Bacillus pumilus became an ideal candidate for promoting sustainable agriculture through its application as biofertilizer due to its proven ability to grow in both pH ranges, its metabolic versatility in using different carbon sources, and its efficiency in solubilizing iron phosphate and tricalcium phosphate.
Author Contributions
Conceptualization, M.E.S.-G., G.Y.-O. and J.L.; methodology, M.E.S.-G. and L.S.-P.; statistic software, R.V.-P. and P.D.A.; validation, M.E.S.-G.; formal analysis and data curation, R.V.-P.; writing—original draft preparation, M.E.S.-G. and M.E.M.-H.; writing—review and editing, N.D.L.P.-L. and A.W.-V.; supervision, G.Y.-O. and A.W.-V.; project administration, G.Y.-O. and J.L.; funding acquisition, G.Y.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dawwam, G.E.; Elbeltagy, A.; Emara, H.M.; Abbas, I.H.; Hassan, M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann. Agric. Sci. 2013, 58, 195–201. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, K.P.; Singh, V.; Singh, J.; Devi, S.; Tewari, A. Plant–microbe Symbiosis: Perspectives and applications. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N.K., Ed.; Springer: New Delhi, India, 2013; pp. 119–145. [Google Scholar] [CrossRef]
- Sashidhar, B.; Podile, A.R. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J. Appl. Microbiol. 2010, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust. J. Plant Physiol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Ingle, K.P.; Padole, D.A. Phosphate solubilizing microbes: An overview. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 844–852. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Prathap, M.R.; Kumari, B.D. A Critical Review on Plant Growth Promoting Rhizobacteria. J. Plant Pathol. Microb. 2015, 6, 266. [Google Scholar] [CrossRef]
- Trdan, S.; Vučajnk, F.; Bohinc, T.; Vidrih, M. The effect of a mixture of two plant growth-promoting bacteria from Argentina on the yield of potato, and occurrence of primary potato diseases and pest–short communication. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 89–94. [Google Scholar] [CrossRef]
- Yañez-Ocampo, G.; Mora-Herrera, M.E.; Wong-Villarreal, A.; de la Paz-Osorio, D.M.; de la Portilla-López, N.; Lugo, J.; Vaca-Paulín, R.; del Águila, P. Isolated phosphate-solubilizing soil bacteria promotes in vitro growth of Solanum tuberosum L. Polish J. Microbiol. 2020, 69, 357–365. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- He, Z.; Honeycutt, C.W. A modified molybdenum blue method for orthophosphate determination suitable for investigating enzymatic hydrolysis of organic phosphates. Commun. Soil Sci. Plant Anal. 2005, 36, 1373–1383. [Google Scholar] [CrossRef]
- Worsfold, P.; McKelvie, I.; Monbet, P. Determination of phosphorus in natural waters: A historical review. Anal. Chim. Acta. 2016, 918, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Collavino, M.M.; Sansberro, P.A. Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol. Fertil. Soils. 2010, 46, 727–738. [Google Scholar] [CrossRef]
- Hernández-Leal, T.I.; Carrión, G.; Heredia, G. In vitro phosphate solubilization by a strain of Paecilomyces lilacinus (Thom) Samson. Agrociencia 2011, 45, 881–892. [Google Scholar]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Anzuay, M.S.; Ciancio, M.G.R.; Ludueña, L.M.M.; Angelini, J.G.; Barros, G.; Pastor, N.; Taurian, A. Growth promotion of peanut (Arachis hypogaea L.) and maize (Zea mays L.) plants by single and mixed cultures of efficient phosphate solubilizing bacteria that are tolerant to abiotic stress and pesticides. Microbiol. Res. 2017, 199, 98–109. [Google Scholar] [CrossRef]
- Benbrik, B.; Elabed, A.; El Modafar, C.; Douira, A.; Amir, S.; Filali-Maltouf, A.; El Abed, S.; El Gachtouli, N.; Mohammed, I.; Koraichi, S.I. Reusing phosphate sludge enriched by phosphate solubilizing bacteria as biofertilizer: Growth promotion of Zea Mays. Biocatal. Agric. Biotechnol. 2020, 30, 101825. [Google Scholar] [CrossRef]
- Hamdali, H.; Bouizgarne, B.; Hafidi, M.; Lebrihi, A.; Virolle, M.J.; Ouhdouch, Y. Screening for rock phosphate solubilizing Actinomycetes from Moroccan phosphate mines. Appl. Soil Ecol. 2008, 38, 12–19. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Ogut, M.; Er, F.; Kandemir, N. Phosphate solubilization potentials of soil Acinetobacter strains. Biol. Fertil. Soils 2010, 46, 707–715. [Google Scholar] [CrossRef]
- Prieto-Correal, G.C.; Prada-Salcedo, L.D.; Cuervo-Patiño, C.L.; Franco -Correa, M. Evaluación de la producción de ácidos orgánicos por Streptomyces spp. y solubilización de tres fuentes de fósforo por la cepa T3A. Rev. Colomb. Biotecnol. 2015, 17, 111–121. [Google Scholar] [CrossRef]
- Pande, A.; Pandey, P.; Mehra, S.; Singh, M.; Kaushik, S. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J. Genet. Eng. Biotechnol. 2017, 15, 379–391. [Google Scholar] [CrossRef]
- Sowmya, S.; Rekha, P.D.; Yashodhara, I.; Karunakara, N.; Arun, A.B. Uranium tolerant phosphate solubilizing bacteria isolated from Gogi, a proposed uranium mining site in South India. Appl. Geochem. 2020, 114, 104523. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Sheikh, I.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium. Proc. Natl. Acad. Sci. USA 2020, 90, 785–795. [Google Scholar] [CrossRef]
- Okazaki, S.; Sano, N.; Yamada, T.; Ishii, K.; Kojima, K.; Djedidi, S.; Ramírez, M.D.A.; Yuan, K.; Kanekatsu, M.; Ohkama-Ohtsu, N.; et al. Complete genome sequence of plant growth-promoting Bacillus pumilus TUAT1. Microbiol. Resour. Announc. 2019, 8, 23–24. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Xu, W.; Zeng, J.; Li, L.; Li, S.; Gao, Z. Bacillus pumilus LZP02 promotes rice root growth by improving carbohydrate metabolism and phenylpropanoid biosynthesis. Mol. Plant-Microbe Interact. 2020, 33, 1222–1231. [Google Scholar] [CrossRef]
- Xie, Z.; Chu, Y.; Zhang, W.; Lang, D.; Zhang, X. Bacillus pumilus alleviates drought stress and increases metabolite accumulation in Glycyrrhiza uralensis Fisch. Environ. Exp. Bot. 2019, 158, 99–106. [Google Scholar] [CrossRef]
- Gorai, P.S.; Ghosh, R.; Mandal, S.; Ghosh, S.; Chatterjee, S.; Gond, S.K.; Mandal, N.C. Bacillus siamensis CNE6-a multifaceted plant growth promoting endophyte of Cicer arietinum L. having broad spectrum antifungal activities and host colonizing potential. Microbiol. Res. 2021, 252, 126859. [Google Scholar] [CrossRef]
- Hanif, M.K.; Hameed, S.; Imran, A.; Naqqash, T.; Shahid, M.; Van Elsas, J.D. Isolation and characterization of a β-propeller gene containing phosphobacterium Bacillus subtilis strain KPS-11 for growth promotion of potato (Solanum tuberosum L.). Front. Microbiol. 2015, 6, 583. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, M.; Hussain, A.; Jamil, M. Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: A promising approach for improving cotton growth. Folia Microbiol. 2021, 66, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, A.; Malik, A.; Sher, A.; Qaisrani, M.M.; Mehmood, A.; Khan, S.U.; Ashraf, M.; Mirza, Z.; Karim, S.; Rasool, M. Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J. Biol. Sci. 2019, 26, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Aliyat, F.Z.; Maldani, M.; El Guilli, M.; Nassiri, L.; Ibijbijen, J. Phosphate-solubilizing bacteria isolated from phosphate solid sludge and their ability to solubilize three inorganic phosphate forms: Calcium, iron, and aluminum phosphates. Microorganisms 2022, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Shahid, I.; Mehnaz, S.; Malik, K.A. Assessment of two carrier materials for phosphate solubilizing biofertilizers and their effect on growth of wheat (Triticum aestivum L.). Microbiol. Res. 2017, 205, 107–117. [Google Scholar] [CrossRef]
- McRae, G.; Monreal, C.M. LC-MS/MS quantitative analysis of reducing carbohydrates in soil solutions extracted from crop rhizospheres. Anal. Bioanal. Chem. 2011, 400, 2205–2215. [Google Scholar] [CrossRef]
- Stefanoni-Rubio, P.J.; Godoy, M.S.; Della-Mónica, I.F.; Pettinari, M.J.; Godeas, A.M.; Scervino, J.M. Carbon and nitrogen sources influence tricalcium phosphate solubilization and extracellular phosphatase activity by Talaromyces flavus. Curr. Microbiol. 2016, 72, 41–47. [Google Scholar] [CrossRef]
- Lim, B.L.; Yeung, P.; Cheng, C.; Hill, J.E. Distribution and diversity of phytate-mineralizing bacteria. ISME J. 2007, 1, 321–330. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).