Spontaneous Aberrant Bodies Formation in Human Pneumocytes Infected with Estrella lausannensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Bacterial Strains
2.2. Infection Procedure
2.3. Quantitative PCR
2.4. Immunofluorescence Staining and Confocal Microscopy
2.5. Electron Microscopy
3. Results
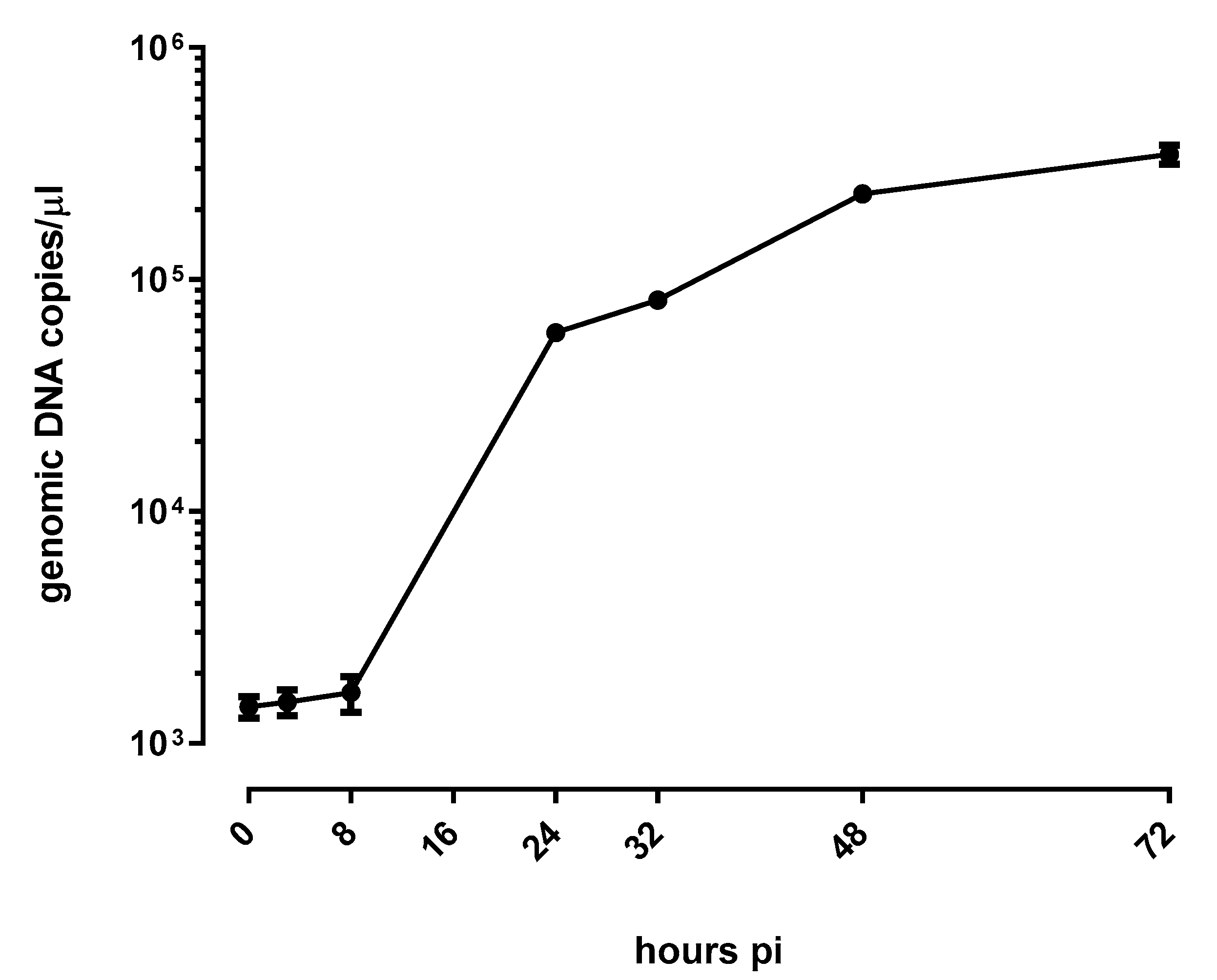
3.1. E. lausannensis Efficiently Replicates in Human Pneumocytes
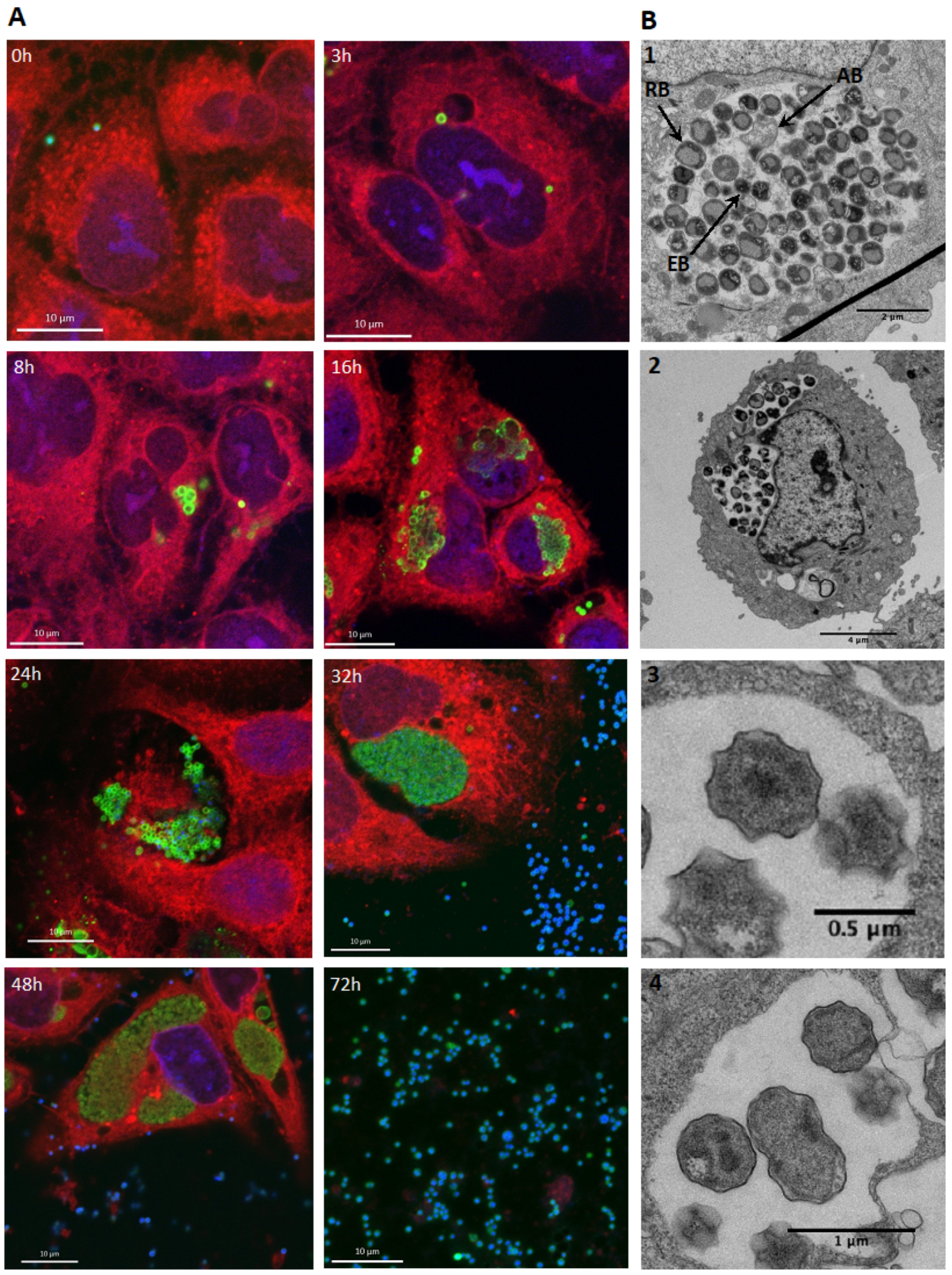
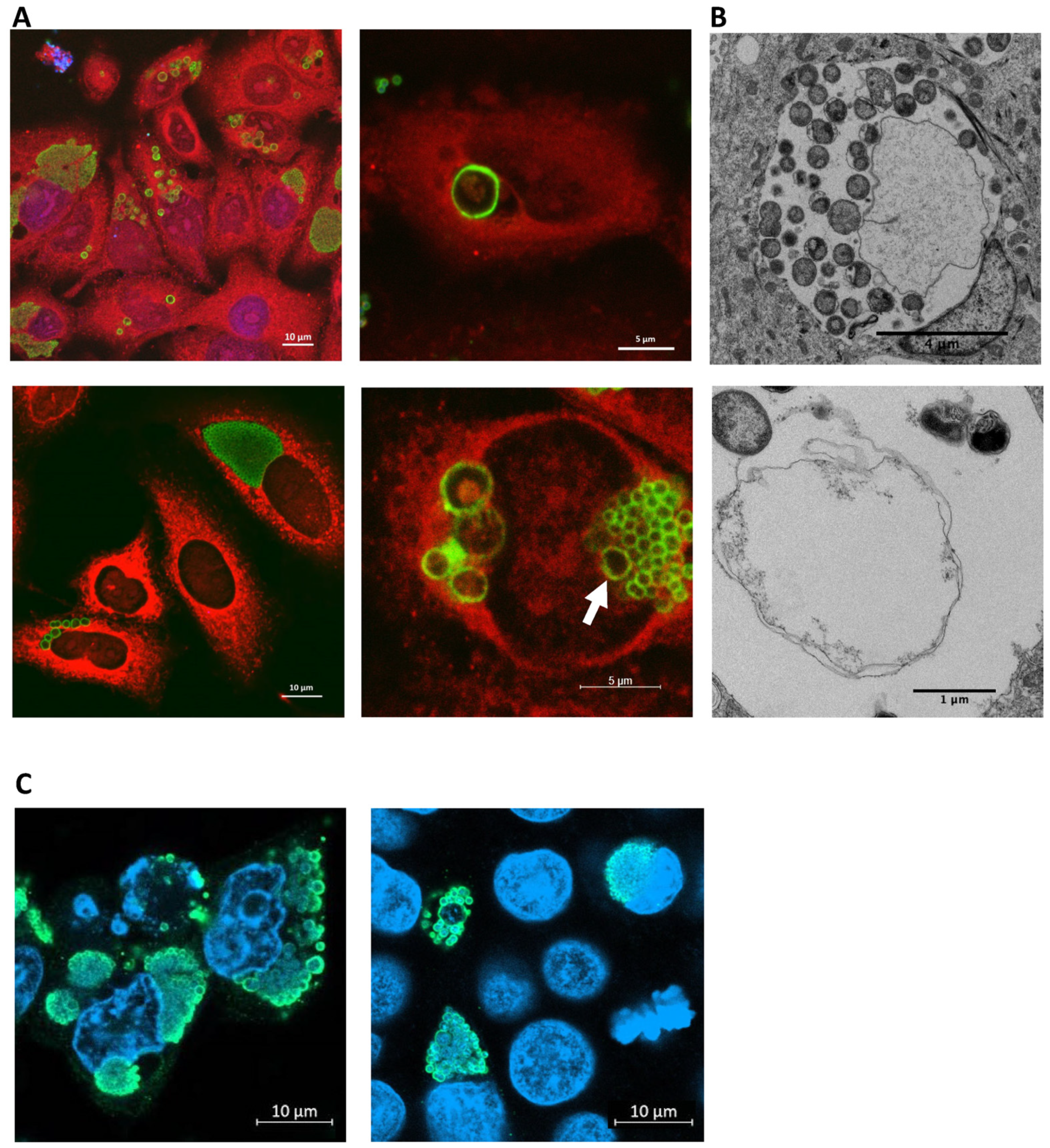
3.2. E. lausannensis Spontaneously Form Aberrant Bodies in Human Pneumocytes and Epithelial Cells
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillonel, T.; Bertelli, C.; Greub, G. Environmental Metagenomic Assemblies Reveal Seven New Highly Divergent Chlamydial Lineages and Hallmarks of a Conserved Intracellular Lifestyle. Front. Microbiol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Köstlbacher, S.; Collingro, A.; Halter, T.; Schulz, F.; Jungbluth, S.P.; Horn, M. Pangenomics reveals alternative environmental lifestyles among chlamydiae. Nat. Commun. 2021, 12, 4021. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.; Amadesi, S.; Djusse, M.E.; Foschi, C.; Gaspari, V.; Lazzarotto, T.; Gaibani, P. Whole Genome Sequencing of a Chlamydia trachomatis Strain Responsible for a Case of Rectal Lymphogranuloma Venereum in Italy. Curr. Issues Mol. Biol. 2023, 45, 1852–1859. [Google Scholar] [CrossRef]
- Pillonel, T.; Bertelli, C.; Aeby, S.; de Barsy, M.; Jacquier, N.; Kebbi-Beghdadi, C.; Mueller, L.; Vouga, M.; Greub, G. Sequencing the Obligate Intracellular Rhabdochlamydia helvetica within Its Tick Host Ixodes ricinus to Investigate Their Symbiotic Relationship. Genome Biol. Evol. 2019, 11, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Staub, E.; Marti, H.; Biondi, R.; Levi, A.; Donati, M.; Leonard, C.A.; Ley, S.D.; Pillonel, T.; Greub, G.; Seth-Smith, H.M.B.; et al. Novel Chlamydia species isolated from snakes are temperature-sensitive and exhibit decreased susceptibility to azithromycin. Sci. Rep. 2018, 8, 5660. [Google Scholar] [CrossRef] [PubMed]
- Kebbi-Beghdadi, C.; Greub, G. Importance of amoebae as a tool to isolate amoeba-resisting microorganisms and for their ecology and evolution: The Chlamydia paradigm. Environ. Microbiol. Rep. 2014, 6, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Regan, L.; Greub, G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr. Opin. Infect. Dis. 2008, 21, 70–76. [Google Scholar] [CrossRef]
- Lienard, J.; Croxatto, A.; Prod’hom, G.; Greub, G. Estrella lausannensis, a new star in the Chlamydiales order. Microbes Infect. 2011, 13, 1232–1241. [Google Scholar] [CrossRef][Green Version]
- Fasoli, L.; Paldanius, M.; Don, M.; Valent, F.; Vetrugno, L.; Korppi, M.; Canciani, M. Simkania negevensis in community-acquired pneumonia in Italian children. Scand. J. Infect. Dis. 2008, 40, 269–272. [Google Scholar] [CrossRef]
- Haider, S.; Collingro, A.; Walochnik, J.; Wagner, M.; Horner, M. Chlamydia-like bacteria in respiratory samples of community-acquired pneumonia patients. FEMS Microbiol. Lett. 2008, 281, 198–202. [Google Scholar] [CrossRef]
- Baud, D.; Thomas, V.; Arafa, A.; Regan, L.; Greub, G. Waddlia chondrophila, a Potential Agent of Human Fetal Death. Emerg. Infect. Dis. 2007, 13, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Ammerdorffer, A.; Stojanov, M.; Greub, G.; Baud, D. Chlamydia trachomatis and chlamydia-like bacteria: New enemies of human pregnancies. Curr. Opin. Infect. Dis. 2017, 30, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hokynar, K.; Kurkela, S.; Nieminen, T.; Saxen, H.; Vesterinen, E.J.; Mannonen, L.; Pietikäinen, R.; Puolakkainen, M. Parachlamydia acanthamoebae Detected during a Pneumonia Outbreak in Southeastern Finland, in 2017–2018. Microorganisms 2019, 7, 141. [Google Scholar] [CrossRef]
- Casson, N.; Medico, N.; Bille, J.; Greub, G. Parachlamydia acanthamoebae enters and multiplies within pneumocytes and lung fibroblasts. Microbes Infect. 2006, 8, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Bayramova, F.; Jacquier, N.; Greub, G. Insight in the biology of Chlamydia-related bacteria. Microbes Infect. 2018, 20, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Greub, G. Lateral gene exchanges shape the genomes of amoeba-resisting microorganisms. Front. Cell Infect. Microbiol. 2012, 2, 110. [Google Scholar] [CrossRef]
- Dharamshi, J.E.; Köstlbacher, S.; Schön, M.E.; Collingro, A.; Ettema, T.J.G.; Horn, M. Gene gain facilitated endosymbiotic evolution of Chlamydiae. Nat. Microbiol. 2023, 8, 40–54. [Google Scholar] [CrossRef]
- Rusconi, B.; Kebbi-Beghdadi, C.; Greub, G. Trafficking of Estrella lausannensis in human macrophages. Pathog. Dis. 2015, 73, ftv027. [Google Scholar] [CrossRef]
- Kebbi-Beghdadi, C.; Cisse, O.; Greub, G. Permissivity of Vero cells, human pneumocytes and human endometrial cells to Waddlia chondrophila. Microbes Infect. 2011, 13, 566–574. [Google Scholar] [CrossRef]
- Kebbi-Beghdadi, C.; Fatton, M.; Greub, G. Permissivity of insect cells to Waddlia chondrophila, Estrella lausannensis and Parachlamydia acanthamoebae. Microbes Infect. 2015, 17, 749–754. [Google Scholar] [CrossRef]
- Kebbi-Beghdadi, C.; Batista, C.; Greub, G. Permissivity of fish cell lines to three Chlamydia-related bacteria: Waddlia chondrophila, Estrella lausannensis and Parachlamydia acanthamoebae. FEMS Immunol. Med. Microbiol. 2011, 63, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- König, L.; Siegl, A.; Penz, T.; Haider, S.; Wentrup, C.; Polzin, J.; Mann, E.; Schmitz-Esser, S.; Domman, D.; Horn, M. Biphasic Metabolism and Host Interaction of a Chlamydial Symbiont. Msystems 2017, 2, e00202-16. [Google Scholar] [CrossRef] [PubMed]
- Scherler, A.; Jacquier, N.; Kebbi-Beghdadi, C.; Greub, G. Diverse Stress-Inducing Treatments cause Distinct Aberrant Body Morphologies in the Chlamydia-Related Bacterium, Waddlia chondrophila. Microorganisms 2020, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Aeby, S.; Chassot, B.; Clulow, J.; Hilfiker, O.; Rappo, S.; Ritzmann, S.; Schumacher, P.; Terrettaz, C.; Benaglio, P.; et al. Sequencing and characterizing the genome of Estrella lausannensis as an undergraduate project: Training students and biological insights. Front. Microbiol. 2015, 6, 101. [Google Scholar] [CrossRef]
- Rusconi, B.; Greub, G. Discovery of catalases in members of the Chlamydiales order. J. Bacteriol. 2013, 195, 3543–3551. [Google Scholar] [CrossRef]
- de Barsy, M.; Bottinelli, L.; Greub, G. Antibiotic susceptibility of Estrella lausannensis, a potential emerging pathogen. Microbes Infect. 2014, 16, 746–754. [Google Scholar] [CrossRef]
- Vouga, M.; Baud, D.; Greub, G. Simkania negevensis may produce long-lasting infections in human pneumocytes and endometrial cells. Pathog. Dis. 2017, 75, ftw115. [Google Scholar] [CrossRef][Green Version]
- Kebbi-Beghdadi, C.; Domröse, A.; Becker, E.; Cisse, O.H.; Hegemann, J.H.; Greub, G. OmpA family proteins and Pmp-like autotransporter: New adhesins of Waddlia chondrophila. Pathog. Dis. 2015, 73, ftv035. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakamura, S.; Matsuo, J.; Fukumoto, T.; Yoshida, M.; Takahashi, K.; Mizutani, Y.; Yao, T.; Yamaguchi, H. Host range of obligate intracellular bacterium Parachlamydia acanthamoebae. Microbiol. Immunol. 2010, 54, 707–713. [Google Scholar] [CrossRef]
- Kintner, J.; Lajoie, D.; Hall, J.; Whittimore, J.; Schoborg, R.V. Commonly prescribed beta-lactam antibiotics induce C. trachomatis persistence/stress in culture at physiologically relevant concentrations. Front. Cell. Infect. Microbiol. 2014, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.L.; Byrne, G.I.; Morrison, R.P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 1993, 90, 3998–4002. [Google Scholar] [CrossRef]
- Raulston, J.E. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect. Immun. 1997, 65, 4539–4547. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.M.; Reynolds, D.J.; Harper, A.; Devitt, A.; Pearce, J.H. Low-nutrient induction of abnormal chlamydial development: A novel component of chlamydial pathogenesis? FEMS Microbiol. Lett. 1993, 106, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Deka, S.; Vanover, J.; Dessus-Babus, S.; Whittimore, J.; Howett, M.K.; Wyrick, P.B.; Schoborg, R.V. Chlamydia trachomatis enters a viable but non-cultivable (persistent) state within herpes simplex virus type 2 (HSV-2) co-infected host cells. Cell. Microbiol. 2006, 8, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Wyrick, P.B. Chlamydia trachomatis persistence in vitro: An overview. J. Infect. Dis. 2010, 201 (Suppl. S2), S88–S95. [Google Scholar] [CrossRef]
- Panzetta, M.E.; Valdivia, R.H.; Saka, H.A. Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo. Front. Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef]
- Pospischil, A.; Borel, N.; Chowdhury, E.H.; Guscetti, F. Aberrant chlamydial developmental forms in the gastrointestinal tract of pigs spontaneously and experimentally infected with Chlamydia suis. Vet. Microbiol. 2009, 135, 147–156. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Muñoz, G.; Sánchez, R.; Henkel, R.; Gallegos-Avila, G.; Díaz-Gutierrez, O.; Vigil, P.; Vásquez, F.; Kortebani, G.; Mazzolli, A.; et al. Update on the impact of Chlamydia trachomatis infection on male fertility. Andrologia 2004, 36, 1–23. [Google Scholar] [CrossRef]
| Estrella | Simkania | Waddlia | Chlamydia | |
| lausannensis | negevensis | chondrophila | trachomatis | |
| [20] and this work | [28] | [20] | [28] | |
| H. sapiens pneumocytes | 3.94 | 12.89 | 1.9 | nd |
| H. sapiens macrophages | 2.75 | nd | 2.71 | nd |
| C. aethiops epithelial | 2.53 | nd | 1.19 | 11.42 |
| S. frugiperda | 3.39 | 21.07 | 3.78 | nd |
| A. albopictus | 3.5 | nd | 4.31 | nd |
| A. castellanii | 2.71 | 16.38 | 1.95 | nd |
| D. discoideum | 14.45 | nd | 10.91 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovero, A.; Kebbi-Beghdadi, C.; Greub, G. Spontaneous Aberrant Bodies Formation in Human Pneumocytes Infected with Estrella lausannensis. Microorganisms 2023, 11, 2368. https://doi.org/10.3390/microorganisms11102368
Rovero A, Kebbi-Beghdadi C, Greub G. Spontaneous Aberrant Bodies Formation in Human Pneumocytes Infected with Estrella lausannensis. Microorganisms. 2023; 11(10):2368. https://doi.org/10.3390/microorganisms11102368
Chicago/Turabian StyleRovero, Aurelien, Carole Kebbi-Beghdadi, and Gilbert Greub. 2023. "Spontaneous Aberrant Bodies Formation in Human Pneumocytes Infected with Estrella lausannensis" Microorganisms 11, no. 10: 2368. https://doi.org/10.3390/microorganisms11102368
APA StyleRovero, A., Kebbi-Beghdadi, C., & Greub, G. (2023). Spontaneous Aberrant Bodies Formation in Human Pneumocytes Infected with Estrella lausannensis. Microorganisms, 11(10), 2368. https://doi.org/10.3390/microorganisms11102368







