Antiviral Approach to Cytomegalovirus Infection: An Overview of Conventional and Novel Strategies
Abstract
1. Introduction
2. Antiviral Approach to HCMV Infection
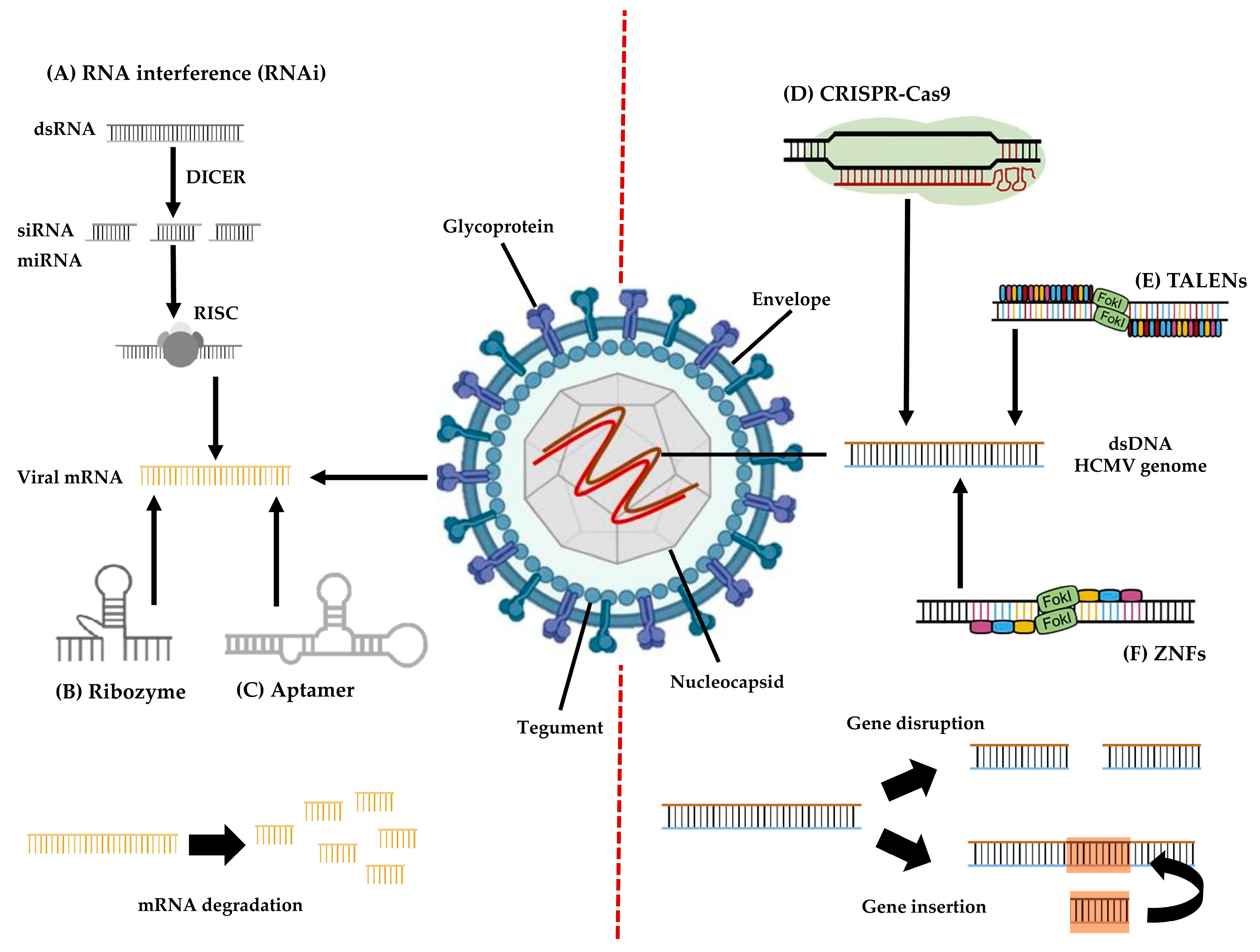
3. Genome-Based Approach to HCMV Infection
4. Immune-Based Approach to HCMV Infection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Ross, S.A.; Novak, Z.; Pati, S.; Boppana, S.B. Diagnosis of Cytomegalovirus Infections. Infect. Disord. Drug Targets 2011, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.; Van Loock, M. Functional annotation of human cytomegalovirus gene products: An update. Front. Microbiol. 2014, 5, 218. [Google Scholar] [CrossRef]
- Ye, L.; Qian, Y.; Yu, W.; Guo, G.; Wang, H.; Xue, X. Functional Profile of Human Cytomegalovirus Genes and Their Associated Diseases: A Review. Front. Microbiol. 2020, 11, 2104. [Google Scholar] [CrossRef] [PubMed]
- Bhella, D.; Rixon, F.J.; Dargan, D.J. Cryomicroscopy of human cytomegalovirus virions reveals more densely packed genomic DNA than in herpes simplex virus type 1. J. Mol. Biol. 2000, 295, 155–161. [Google Scholar] [CrossRef]
- Yu, X.; Jih, J.; Jiang, J.; Hong Zhou, Z. Atomic structure of the human cytomegalovirus capsid with its securing tegument layer of pp150 HHS Public Access. Science 2017, 356, eaam6892. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.J.; Aitken, J.; Mitchell, J.; Gowen, B.; Dargan, D.J. Structure of the human cytomegalovirus B capsid by electron cryomicroscopy and image reconstruction. J. Struct. Biol. 1998, 124, 70–76. [Google Scholar] [CrossRef]
- Tomtishen, J. Human cytomegalovirus tegument proteins (pp65, pp71, pp150, pp28). Virol. J. 2012, 9, 22. [Google Scholar] [CrossRef]
- Kalejta, R.F. Tegument Proteins of Human Cytomegalovirus. Microbiol. Mol. Biol. Rev. 2008, 72, 249–265. [Google Scholar] [CrossRef]
- Garoff, H.; Hewson, R.; Opstelten, D.-J.E. Virus Maturation by Budding. Microbiol. Mol. Biol. Rev. 1998, 62, 1171. [Google Scholar] [CrossRef]
- Homman-Loudiyi, M.; Hultenby, K.; Britt, W.; Söderberg-Nauclér, C. Envelopment of Human Cytomegalovirus Occurs by Budding into Golgi-Derived Vacuole Compartments Positive for gB, Rab 3, Trans-Golgi Network 46, and Mannosidase II. J. Virol. 2003, 77, 3191–3203. [Google Scholar] [CrossRef]
- Foglierini, M.; Marcandalli, J.; Perez, L. HCMV envelope glycoprotein diversity demystified. Front. Microbiol. 2019, 10, 1005. [Google Scholar] [CrossRef]
- Jean Beltran, P.M.; Cristea, I.M. The life cycle and pathogenesis of human cytomegalovirus infection: Lessons from proteomics. Expert Rev. Proteom. 2014, 11, 697–711. [Google Scholar] [CrossRef]
- Gerna, G.; Kabanova, A.; Lilleri, D. Human cytomegalovirus cell tropism and host cell receptors. Vaccines 2019, 7, 70. [Google Scholar] [CrossRef]
- Isomura, H.; Stinski, M.F.; Murata, T.; Yamashita, Y.; Kanda, T.; Toyokuni, S.; Tsurumi, T. The Human Cytomegalovirus Gene Products Essential for Late Viral Gene Expression Assemble into Prereplication Complexes before Viral DNA Replication. J. Virol. 2011, 85, 6629–6644. [Google Scholar] [CrossRef] [PubMed]
- Garci´a, J.J.; Garci´a-Rami´rez, G.; Rami´rez, R.; Ruchti, F.; Huang, H.; Simmen, K.; Angulo, A.; Ghazal, P. Dominance of Virus over Host Factors in Cross-Species Activation of Human Cytomegalovirus Early Gene Expression. J. Virol. 2001, 75, 26–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adamson, C.S.; Nevels, M.M. Bright and early: Inhibiting human cytomegalovirus by targeting major immediate-early gene expression or protein function. Viruses 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Omoto, S.; Mocarski, E.S. Transcription of True Late (γ2) Cytomegalovirus Genes Requires UL92 Function That Is Conserved among Beta- and Gammaherpesviruses. J. Virol. 2014, 88, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rozman, B.; Nachshon, A.; Levi Samia, R.; Lavi, M.; Schwartz, M.; Stern-Ginossar, N. Temporal dynamics of HCMV gene expression in lytic and latent infections. Cell Rep. 2022, 39, 110653. [Google Scholar] [CrossRef]
- Crough, T.; Khanna, R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef]
- Poole, E.; Wills, M.; Sinclair, J. Human Cytomegalovirus Latency: Targeting Differences in the Latently Infected Cell with a View to Clearing Latent Infection. New J. Sci. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, T.; Blázquez-Gamero, D.; Delforge, M.L.; Foulon, I.; Luck, S.; Modrow, S.; Leruez-Ville, M. Congenital Cytomegalovirus Infection: A Narrative Review of the Issues in Screening and Management From a Panel of European Experts. Front. Pediatr. 2020, 8, 13. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Gökçe, Ş. Human Cytomegalovirus Infection: Biological Features, Transmission, Symptoms, Diagnosis, and Treatment. In Human Herpesvirus Infection; Thomasini, R.L., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Chiavarini, M.; Bragetti, P.; Sensini, A.; Cenci, E.; Castronari, R.; Rossi, M.J.; Fantauzzi, A.; Minelli, L. Breastfeeding and transmission of cytomegalovirus to preterm infants. Case report and kinetic of CMV-DNA in breast milk. Ital. J. Pediatr. 2011, 37, 6. [Google Scholar] [CrossRef]
- Hyde, T.B.; Schmid, D.S.; Cannon, M.J. Cytomegalovirus seroconversion rates and risk factors: Implications for congenital CMV. Rev. Med. Virol. 2010, 20, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Rubinacci, V.; Fumagalli, M.; Meraviglia, G.; Gianolio, L.; Sala, A.; Stracuzzi, M.; Dighera, A.; Zuccotti, G.V.; Giacomet, V. Congenital CMV, Lights and Shadows on Its Management: The Experience of a Reference Center in Northern Italy. Children 2022, 9, 655. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pierrotti, L.C.; Abdala, E.; Costa, S.F.; Strabelli, T.M.V.; Campos, S.V.; Ramos, J.F.; Latif, A.Z.A.; Litvinov, N.; Maluf, N.Z.; et al. Cytomegalovirus infection in transplant recipients. Clinics 2015, 70, 515. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Higashida-Konishi, M.; Izumi, K.; Hama, S.; Oshige, T.; Oshima, H.; Okano, Y. Risk factors associated with cytomegalovirus reactivation in patients receiving immunosuppressive therapy for rheumatic diseases: A retrospective study. Sci. Rep. 2022, 12, 20926. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; Snydman, D. Cytomegalovirus in solid organ transplant recipients. Am. J. Transplant 2009, 9 (Suppl. 4), S78–S86. [Google Scholar] [CrossRef] [PubMed]
- van Zuylen, W.J.; Hamilton, S.T.; Naing, Z.; Hall, B.; Shand, A.; Rawlinson, W.D. Congenital cytomegalovirus infection: Clinical presentation, epidemiology, diagnosis and prevention. Obstet. Med. 2014, 7, 140–146. [Google Scholar] [CrossRef]
- Requião-Moura, L.R.; de Matos, A.C.C.; Pacheco-Silva, A. Cytomegalovirus infection in renal transplantation: Clinical aspects, management and the perspectives. Einstein 2015, 13, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Paya, C.V. Indirect effects of CMV in the solid organ transplant patient. Transpl. Infect. Dis. Off. J. Transplant. Soc. 1999, 1 (Suppl. 1), 8–12. [Google Scholar]
- Diena, D.; Allesina, A.; Fop, F.; Mella, A.; Cavallo, R.; Costa, C.; Dolla, C.; Gallo, E.; De Rosa, F.G.; Lavacca, A.; et al. Relationship between Cytomegalovirus Viremia and Long-Term Outcomes in Kidney Transplant Recipients with Different Donor Ages. Microorganisms 2023, 11, 458. [Google Scholar] [CrossRef]
- Razonable, R.R.; Inoue, N.; Pinninti, S.G.; Boppana, S.B.; Lazzarotto, T.; Gabrielli, L.; Simonazzi, G.; Pellett, P.E.; Schmid, D.S. Clinical Diagnostic Testing for Human Cytomegalovirus Infections. J. Infect. Dis. 2020, 221, S74. [Google Scholar] [CrossRef]
- Gu, J.; Ji, H.; Liu, T.; Chen, C.; Zhao, S.; Cao, Y.; Wang, N.; Xiao, M.; Chen, L.; Cai, H. Detection of cytomegalovirus (CMV) by digital PCR in stool samples for the non-invasive diagnosis of CMV gastroenteritis. Virol. J. 2022, 19, 183. [Google Scholar] [CrossRef]
- Novak, Z.; Chowdhury, N.; Ross, S.A.; Pati, S.K.; Fowler, K.; Boppana, S.B. Diagnostic consequences of cytomegalovirus glycoprotein B polymorphisms. J. Clin. Microbiol. 2011, 49, 3033–3035. [Google Scholar] [CrossRef]
- Rasmussen, L.; Geissler, A.; Cowan, C.; Chase, A.; Winters, M. The genes encoding the gCIII complex of human cytomegalovirus exist in highly diverse combinations in clinical isolates. J. Virol. 2002, 76, 10841–10848. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Ducroux, A.; Rouzioux, C. Exon 4 of the human cytomegalovirus (CMV) major immediate-early gene as a target for CMV real-time PCR. J. Clin. Microbiol. 2008, 46, 1571–1572. [Google Scholar] [CrossRef]
- Chou, S.; Dennison, K.M. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J. Infect. Dis. 1991, 163, 1229–1234. [Google Scholar] [CrossRef]
- Lazzarotto, T.; Chiereghin, A.; Piralla, A.; Piccirilli, G.; Girello, A.; Campanini, G.; Gabrielli, L.; Costa, C.; Prete, A.; Bonifazi, F.; et al. Cytomegalovirus and Epstein-Barr Virus DNA Kinetics in Whole Blood and Plasma of Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. 2018, 24, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, M.; Depka, D.; Gospodarek-Komkowska, E.; Bogiel, T. Whole Blood versus Plasma Samples-How Does the Type of Specimen Collected for Testing Affect the Monitoring of Cytomegalovirus Viremia? Pathogens 2022, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, M.; Depka, D.; Gospodarek-Komkowska, E.; Bogiel, T. Diagnostic Value of Whole-Blood and Plasma Samples in Epstein-Barr Virus Infections. Diagnostics 2023, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Beam, E.; Germer, J.J.; Lahr, B.; Yao, J.D.C.; Limper, A.H.; Binnicker, M.J.; Razonable, R.R. Cytomegalovirus (CMV) DNA quantification in bronchoalveolar lavage fluid of immunocompromised patients with CMV pneumonia. Clin. Transplant. 2018, 32, e13149. [Google Scholar] [CrossRef]
- Müller, J.; Flindt, J.; Pollmann, M.; Saschenbrecker, S.; Borchardt-Lohölter, V.; Warnecke, J.M. Efficiency of CMV serodiagnosis during pregnancy in daily laboratory routine. J. Virol. Methods 2023, 314, 114685. [Google Scholar] [CrossRef]
- Navarro, D.; Fernández-Ruiz, M.; Aguado, J.M.; Sandonís, V.; Pérez-Romero, P. Going beyond serology for stratifying the risk of CMV infection in transplant recipients. Rev. Med. Virol. 2019, 29, e2017. [Google Scholar] [CrossRef]
- Clari, M.Á.; Munoz-Cobo, B.; Solano, C.; Benet, I.; Costa, E.; Remigia, M.J.; Bravo, D.; Amat, P.; Navarro, D. Performance of the QuantiFERON-cytomegalovirus (CMV) assay for detection and estimation of the magnitude and functionality of the CMV-specific gamma interferon-producing CD8 + T-cell response in allogeneic stem cell transplant recipients. Clin. Vaccine Immunol. 2012, 19, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, K.H.; Ryu, J.H.; Choi, A.R.; Yu, J.H.; Lim, J.; Han, K.; Kim, S., II; Yang, C.W.; Chung, B.H.; et al. Cytomegalovirus (CMV) immune monitoring with ELISPOTand QuantiFERON-CMV assay in seropositive kidney transplant recipients. PLoS ONE 2017, 12, e0189488. [Google Scholar] [CrossRef]
- Callens, R.; Colman, S.; Delie, A.; Schauwvlieghe, A.; Lodewyck, T.; Selleslag, D.; Reynders, M.; Kerre, T.; Padalko, E. Immunologic Monitoring after Allogeneic Stem Cell Transplantation: T-SPOT.CMV and QuantiFERON-CMV, Are They the Same? Transplant. Cell. Ther. 2023, 29, 392.e1–392.e7. [Google Scholar] [CrossRef]
- Costa, C.; Balloco, C.; Sidoti, F.; Mantovani, S.; Rittà, M.; Piceghello, A.; Fop, F.; Messina, M.; Cavallo, R. Evaluation of CMV-specific cellular immune response by EliSPOT assay in kidney transplant patien. J. Clin. Virol. 2014, 61, 523–528. [Google Scholar] [CrossRef]
- Costa, C.; Astegiano, S.; Terlizzi, M.E.; Sidoti, F.; Curtoni, A.; Solidoro, P.; Baldi, S.; Bergallo, M.; Cavallo, R. Evaluation and significance of cytomegalovirus-specific cellular immune response in lung transplant recipients. Transplant. Proc. 2011, 43, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Boppana, S.B. Vaccination against the human cytomegalovirus. Vaccine 2019, 37, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Scarpini, S.; Morigi, F.; Betti, L.; Dondi, A.; Biagi, C.; Lanari, M. Development of a Vaccine against Human Cytomegalovirus: Advances, Barriers, and Implications for the Clinical Practice. Vaccines 2021, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Rozhnova, G.; Kretzschmar, M.E.; van der Klis, F.; van Baarle, D.; Korndewal, M.; Vossen, A.C.; van Boven, M. Short- and long-term impact of vaccination against cytomegalovirus: A modeling study. BMC Med. 2020, 18, 174. [Google Scholar] [CrossRef]
- Chen, S.J.; Wang, S.C.; Chen, Y.C. Antiviral agents as therapeutic strategies against cytomegalovirus infections. Viruses 2019, 12, 21. [Google Scholar] [CrossRef]
- Panda, K.; Parashar, D.; Viswanathan, R. An Update on Current Antiviral Strategies to Combat Human Cytomegalovirus Infection. Viruses 2023, 15, 1358. [Google Scholar] [CrossRef]
- Navarro, D.; San-Juan, R.; Manuel, O.; Giménez, E.; Fernández-Ruiz, M.; Hirsch, H.H.; Grossi, P.A.; Aguado, J.M. Cytomegalovirus infection management in solid organ transplant recipients across European centers in the time of molecular diagnostics: An ESGICH survey. Transpl. Infect. Dis. 2017, 19, e12773. [Google Scholar] [CrossRef]
- Yadav, D.K.; Adhikari, V.P.; Yadav, R.K.; Singh, A.; Huang, X.; Zhang, Q.; Pandit, P.; Ling, Q.; Liang, T. Antiviral prophylaxis or preemptive therapy for cytomegalovirus after liver transplantation?: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 953210. [Google Scholar] [CrossRef]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]
- Grossi, P.A.; Baldanti, F.; Andreoni, M.; Perno, C.F. CMV infection management in transplant patients in Italy. J. Clin. Virol. 2020, 123, 104211. [Google Scholar] [CrossRef]
- Singh, N.; Winston, D.J.; Razonable, R.R.; Lyon, G.M.; Silveira, F.P.; Wagener, M.M.; Stevens-Ayers, T.; Edmison, B.; Boeckh, M.; Limaye, A.P. Effect of Preemptive Therapy vs Antiviral Prophylaxis on Cytomegalovirus Disease in Seronegative Liver Transplant Recipients with Seropositive Donors: A Randomized Clinical Trial. JAMA 2020, 323, 1378. [Google Scholar] [CrossRef]
- Markham, A.; Faulds, D. Ganciclovir. An update of its therapeutic use in cytomegalovirus infection. Drugs 1994, 48, 455–484. [Google Scholar] [CrossRef]
- Crumpacker, C.S. Ganciclovir. N. Engl. J. Med. 1996, 335, 721–729. [Google Scholar] [CrossRef]
- Galar, A.; Valerio, M.; Catalán, P.; García-González, X.; Burillo, A.; Fernández-Cruz, A.; Zataráin, E.; Sousa-Casasnovas, I.; Anaya, F.; Rodríguez-Ferrero, M.L.; et al. Valganciclovir—Ganciclovir use and systematic therapeutic drug monitoring. An invitation to antiviral stewardship. Antibiotics 2021, 10, 77. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Åsberg, A.; Chou, S.; Snydman, D.R.; Allen, U.; Humar, A.; Emery, V.; Lautenschlager, I.; et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 2010, 89, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Lazzarotto, T.; Bonifazi, F.; Patriarca, F.; Irrera, G.; Ciceri, F.; Aversa, F.; Citterio, F.; Cillo, U.; Cozzi, E.; et al. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: A multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies. Clin. Transplant. 2019, 33, e13666. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.; McNaught, K.A.; Mulbah, J.L.; HajiAlilou, H.; Mody, V.; Cates, D.W. Antiviral drugs. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2022; Volume 44, pp. 291–301. ISBN 9780323989091. [Google Scholar]
- Boonsathorn, S.; Pasomsub, E.; Techasaensiri, C.; Apiwattanakul, N. Analysis of Ganciclovir-Resistant Cytomegalovirus Infection Caused by the UL97 Gene Mutation in Codons 460 and 520 in Pediatric Patients: A Case Series. Open Forum Infect. Dis. 2019, 6, ofz480. [Google Scholar] [CrossRef]
- Das, D.; Hong, J. Herpesvirus Polymerase Inhibitors. In Viral Polymerases; Academic Press: Cambridge, MA, USA, 2019; pp. 333–356. [Google Scholar] [CrossRef]
- Lea, A.P.; Bryson, H.M. Cidofovir. Drugs 1996, 52, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Paintsil, E.; Cheng, Y.C. Antiviral Agents. In Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2009; pp. 223–257. [Google Scholar] [CrossRef]
- Meesing, A.; Razonable, R.R. New Developments in the Management of Cytomegalovirus Infection after Transplantation. Drugs 2018, 78, 1085–1103. [Google Scholar] [CrossRef]
- Chou, S.; Komazin-Meredith, G.; Williams, J.D.; Bowlin, T.L. Cytomegalovirus mutants resistant to ganciclovir and cidofovir differ in susceptibilities to synguanol and its 6-ether and 6-thioether derivatives. Antimicrob. Agents Chemother. 2014, 58, 1809–1812. [Google Scholar] [CrossRef]
- Bogner, E.; Egorova, A.; Makarov, V. Small Molecules—Prospective Novel HCMV Inhibitors. Viruses 2021, 13, 474. [Google Scholar] [CrossRef]
- Salvaggio, M.R.; Gnann, J.W. Drugs for Herpesvirus Infections. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1309–1317.e1. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Bryson, H.M. Foscarnet: A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs 1994, 48, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Arav-Boger, R.; Marr, K.A.; Kraus, E.; Shoham, S.; Lees, L.; Trollinger, B.; Shah, P.; Ambinder, R.; Neofytos, D.; et al. Outcomes in Transplant Recipients Treated with Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation 2016, 100, e74–e80. [Google Scholar] [CrossRef]
- Chrisp, P.; Clissold, S.P. Foscarnet: A review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs 1991, 41, 104–129. [Google Scholar] [CrossRef]
- Chou, S. Foscarnet resistance mutations mapping to atypical domains of the cytomegalovirus DNA polymerase gene. Antivir. Res. 2017, 138, 57. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W. Antiviral Agents. In Principles and Practice of Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1583–1598.e6. [Google Scholar] [CrossRef]
- Lischka, P.; Hewlett, G.; Wunberg, T.; Baumeister, J.; Paulsen, D.; Goldner, T.; Ruebsamen-Schaeff, H.; Zimmermann, H. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob. Agents Chemother. 2010, 54, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Melendez, D.P.; Razonable, R.R. Letermovir and inhibitors of the terminase complex: A promising new class of investigational antiviral drugs against human cytomegalovirus. Infect. Drug Resist. 2015, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Raglow, Z.; Kaul, D.R. A New Antiviral Option for Cytomegalovirus Prevention after Kidney Transplant. JAMA 2023, 330, 27–29. [Google Scholar] [CrossRef]
- Shigle, T.L.; Handy, V.W.; Chemaly, R.F. Letermovir and its role in the prevention of cytomegalovirus infection in seropositive patients receiving an allogeneic hematopoietic cell transplant. Ther. Adv. Hematol. 2020, 11, 204062072093715. [Google Scholar] [CrossRef]
- El Helou, G.; Razonable, R.R. Letermovir for the prevention of cytomegalovirus infection and disease in transplant recipients: An evidence-based review. Infect. Drug Resist. 2019, 12, 1481–1491. [Google Scholar] [CrossRef]
- Santos Bravo, M.; Tilloy, V.; Plault, N.; Palomino, S.S.; Mosquera, M.M.; Navarro Gabriel, M.; Fernández Avilés, F.; Suárez Lledó, M.; Rovira, M.; Moreno, A.; et al. Assessment of UL56 Mutations before Letermovir Therapy in Refractory Cytomegalovirus Transplant Recipients. Microbiol. Spectr. 2022, 10, e00191-22. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Sidler, D.; Dahdal, S.; Bittel, P.; Suter-Riniker, F.; Manuel, O.; Walti, L.N.; Hirzel, C. Emergence of letermovir resistance in solid organ transplant recipients with ganciclovir resistant cytomegalovirus infection: A case series and review of the literature. Transpl. Infect. Dis. 2021, 23, e13515. [Google Scholar] [CrossRef] [PubMed]
- Halpern-Cohen, V.; Blumberg, E.A. New Perspectives on Antimicrobial Agents: Maribavir. Antimicrob. Agents Chemother. 2022, 66, e02405-21. [Google Scholar] [CrossRef]
- Avery, R.K.; Alain, S.; Alexander, B.D.; Blumberg, E.A.; Chemaly, R.F.; Cordonnier, C.; Duarte, R.F.; Florescu, D.F.; Kamar, N.; Kumar, D.; et al. Maribavir for Refractory Cytomegalovirus Infections with or without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial. Clin. Infect. Dis. 2022, 75, 690–701. [Google Scholar] [CrossRef]
- Kang, C. Maribavir: First Approval. Drugs 2022, 82, 335–340. [Google Scholar] [CrossRef]
- Maertens, J.; Cordonnier, C.; Jaksch, P.; Poiré, X.; Uknis, M.; Wu, J.; Wijatyk, A.; Saliba, F.; Witzke, O.; Villano, S. Maribavir for Preemptive Treatment of Cytomegalovirus Reactivation. N. Engl. J. Med. 2019, 381, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Peck, R.W.; Yin, Y.; Allanson, J.; Wiggs, R.; Wire, M.B. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 2003, 47, 1334–1342. [Google Scholar] [CrossRef]
- Chou, S.; Alain, S.; Cervera, C.; Chemaly, R.F.; Kotton, C.N.; Lundgren, J.; Papanicolaou, G.A.; Pereira, M.R.; Wu, J.J.; Murray, R.A.; et al. Drug Resistance Assessed in a Phase 3 Clinical Trial of Maribavir Therapy for Refractory or Resistant Cytomegalovirus Infection in Transplant Recipients. J. Infect. Dis. 2023, jiad293. [Google Scholar] [CrossRef]
- Chou, S.; Song, K.; Wu, J.; Bo, T.; Crumpacker, C. Drug Resistance Mutations and Associated Phenotypes Detected in Clinical Trials of Maribavir for Treatment of Cytomegalovirus Infection. J. Infect. Dis. 2022, 226, 576–584. [Google Scholar] [CrossRef]
- Komazin, G.; Townsend, L.B.; Drach, J.C. Role of a Mutation in Human Cytomegalovirus Gene UL104 in Resistance to Benzimidazole Ribonucleosides. J. Virol. 2004, 78, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R.; Bernstein, D.I.; Mcvoy, M.A.; Stroup, G.; Bravo, F.; Creasy, B.; Mcgregor, A.; Henninger, K.; Hallenberger, S. The non-nucleoside antiviral, BAY 38-4766, protects against cytomegalovirus (CMV) disease and mortality in immunocompromised guinea pigs. Antivir. Res. 2005, 65, 35–43. [Google Scholar] [CrossRef]
- Weber, O.; Bender, W.; Eckenberg, P.; Goldmann, S.; Haerter, M.; Hallenberger, S.; Henninger, K.; Reefschläger, J.; Trappe, J.; Witt-Laido, A.; et al. Inhibition of murine cytomegalovirus and human cytomegalovirus by a novel non-nucleosidic compound in vivo. Antivir. Res. 2001, 49, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kosobucki, B.R.; Freeman, W.R. Retinal Disease in HIV-infected Patients. In Retina, 4th ed.; Mosby: Maryland Heights, MO, USA, 2006; Volume 2–3, pp. 1625–1672. [Google Scholar] [CrossRef]
- Townsend, L.B.; Devivar, R.V.; Turk, S.R.; Nassiri, M.R.; Drach, J.C. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J. Med. Chem. 1995, 38, 4098–4105. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.R.; Ferris, R.G.; Selleseth, D.W.; Davis, M.G.; Drach, J.C.; Townsend, L.B.; Biron, K.K.; Boyd, F.L. Mechanism of action of the ribopyranoside benzimidazole GW275175X against human cytomegalovirus. Antimicrob. Agents Chemother. 2004, 48, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- McVoy, M.A.; Nixon, D.E. Impact of 2-Bromo-5,6-Dichloro-1-β-d-Ribofuranosyl Benzimidazole Riboside and Inhibitors of DNA, RNA, and Protein Synthesis on Human Cytomegalovirus Genome Maturation. J. Virol. 2005, 79, 11115–11127. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.E.; McVoy, M.A. Dramatic effects of 2-bromo-5,6-dichloro-1-beta-D-ribofuranosyl benzimidazole riboside on the genome structure, packaging, and egress of guinea pig cytomegalovirus. J. Virol. 2004, 78, 1623–1635. [Google Scholar] [CrossRef]
- Gugliesi, F.; Coscia, A.; Griffante, G.; Galitska, G.; Pasquero, S.; Albano, C.; Biolatti, M. Where do we stand after decades of studying human cytomegalovirus? Microorganisms 2020, 8, 685. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Leading Edge Review Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Wiebusch, L.; Truss, M.; Hagemeier, C. Inhibition of human cytomegalovirus replication by small interfering RNAs. J. Gen. Virol. 2004, 85, 179–184. [Google Scholar] [CrossRef]
- Xiaofei, E.; Stadler, B.M.; Debatis, M.; Wang, S.; Lu, S.; Kowalik, T.F. RNA Interference-Mediated Targeting of Human Cytomegalovirus Immediate-Early or Early Gene Products Inhibits Viral Replication with Differential Effects on Cellular Functions. J. Virol. 2012, 86, 5660–5673. [Google Scholar] [CrossRef]
- Balakrishnan, K.N.; Abdullah, A.A.; Bala, J.A.; Jesse, F.F.A.; Abdullah, C.A.C.; Noordin, M.M.; Mohd-Azmi, M.L. Multiple gene targeting siRNAs for down regulation of Immediate Early-2 (Ie2) and DNA polymerase genes mediated inhibition of novel rat Cytomegalovirus (strain All-03). Virol. J. 2020, 17, 164. [Google Scholar] [CrossRef]
- Møller, R.; Schwarz, T.M.; Noriega, V.M.; Panis, M.; Sachs, D.; Tortorella, D.; tenOever, B.R. miRNA-mediated targeting of human cytomegalovirus reveals biological host and viral targets of IE2. Proc. Natl. Acad. Sci. USA 2018, 115, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Skilandat, M.; Zelger-Paulus, S.; Sigel, R.K.O. Ribozymes☆. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809633-8. [Google Scholar]
- Mulhbacher, J.; St-Pierre, P.; Lafontaine, D.A. Therapeutic applications of ribozymes and riboswitches. Curr. Opin. Pharmacol. 2010, 10, 551–556. [Google Scholar] [CrossRef] [PubMed]
- James, H.A.; Gibson, I. The Therapeutic Potential of Ribozymes. Blood 1998, 91, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Lilley, D.M.J. Classification of the nucleolytic ribozymes based upon catalytic mechanism [version 1; peer review: 3 approved]. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Kim, K.; Trang, P.; Umamoto, S.; Hai, R.; Liu, F. RNase P ribozyme inhibits cytomegalovirus replication by blocking the expression of viral capsid proteins. Nucleic Acids Res. 2004, 32, 3427–3434. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.; He, L.; Sheng, J.; Liu, Y.; Vu, G.P.; Yang, Z.; Li, W.; Trang, P.; Wang, Y.; et al. Inhibition of human cytomegalovirus immediate early gene expression and growth by a novel RNase P ribozyme variant. PLoS ONE 2017, 12, e0186791. [Google Scholar] [CrossRef]
- Yang, Z.; Reeves, M.; Ye, J.; Trang, P.; Zhu, L.; Sheng, J.; Wang, Y.; Zen, K.; Wu, J.; Liu, F. RNase P Ribozymes Inhibit the Replication of Human Cytomegalovirus by Targeting Essential Viral Capsid Proteins. Viruses 2015, 7, 3345–3360. [Google Scholar] [CrossRef]
- Ni, X.; Castanares, M.; Mukherjee, A.; Lupold, S.E. Nucleic Acid Aptamers: Clinical Applications and Promising New Horizons. Curr. Med. Chem. 2011, 18, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.; Lischka, P.; Wagenknecht, N.; Stamminger, T. Inhibition of Human Cytomegalovirus Replication via Peptide Aptamers Directed against the Nonconventional Nuclear Localization Signal of the Essential Viral Replication Factor pUL84. J. Virol. 2009, 83, 11902–11913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mondal, D. Fomivirsen. Ref. Modul. Biomed. Sci. 2016. [Google Scholar] [CrossRef]
- Kozak, I.; Allen McCutchan, J.; Freeman, W.R. HIV-Associated Infections. In Retina, 5th ed.; Saunders: Philadelphia, PA, USA, 2013; pp. 1441–1472. [Google Scholar] [CrossRef]
- Ede, D.R.; Farhang, N.; Stover, J.D.; Bowles, R.D. 4.32 Gene Editing Tools. Compr. Biomater. II 2017, 4, 589–599. [Google Scholar] [CrossRef]
- Becker, S.; Boch, J. TALE and TALEN genome editing technologies. Gene Genome Ed. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Nain, V. TALENs-an indispensable tool in the era of CRISPR: A mini review. J. Genet. Eng. Biotechnol. 2021, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas Iii, C.F. ZFN, TALEN and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Khan, S.H. Genome-Editing Technologies: Concept, Pros, and Cons of Various Genome-Editing Techniques and Bioethical Concerns for Clinical Application. Mol. Ther. Nucleic Acids 2019, 16, 326–334. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef]
- Perez, E.E.; Wang, J.; Miller, J.C.; Jouvenot, Y.; Kim, K.A.; Liu, O.; Wang, N.; Lee, G.; Bartsevich, V.V.; Lee, Y.L.; et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 808. [Google Scholar] [CrossRef]
- Lista, M.J.; Witney, A.A.; Nichols, J.; Davison, A.J.; Wilson, H.; Latham, K.A.; Ravenhill, B.J.; Nightingale, K.; Stanton, R.J.; Weekes, M.P.; et al. Strain-Dependent Restriction of Human Cytomegalovirus by Zinc Finger Antiviral Proteins. J. Virol. 2023, 97, e01846-22. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, Y.-C. Potential Application of TALENs against Murine Cytomegalovirus Latent Infections. Viruses 2019, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child.-Educ. Pract. 2016, 101, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Deng, J.; Zhang, Q.; Ma, P.; Lv, L.; Zhang, Y.; Li, C.; Zhang, Y. Targeting human cytomegalovirus IE genes by CRISPR/Cas9 nuclease effectively inhibits viral replication and reactivation. Arch. Virol. 2020, 165, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Natesan, J.S. Suganthini Krishnan Using CRISPR technology to inhibit the replication of human cytomegalovirus by deletion of a gene promoter. J. Emerg. Investig. 2021, 4, 1–5. [Google Scholar]
- King, M.W.; Munger, J. Editing the human cytomegalovirus genome with the CRISPR/Cas9 system. Virology 2019, 529, 186–194. [Google Scholar] [CrossRef]
- Esposito, S.; Amirthalingam, G.; Bassetti, M.; Blasi, F.; De Rosa, F.G.; Halasa, N.B.; Hung, I.; Osterhaus, A.; Tan, T.; Torres, J.P.; et al. Monoclonal antibodies for prophylaxis and therapy of respiratory syncytial virus, SARS-CoV-2, human immunodeficiency virus, rabies and bacterial infections: An update from the World Association of Infectious Diseases and Immunological Disorders and the It. Front. Immunol. 2023, 14, 1162342. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: Development strategies and progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef]
- Crowe, J.E. Human Antibodies for Viral Infections. Annu. Rev. Immunol. 2022, 40, 349–386. [Google Scholar] [CrossRef]
- Maertens, J.; Logan, A.C.; Jang, J.; Long, G.; Tang, J.-L.; Hwang, W.Y.K.; Koh, L.P.; Chemaly, R.; Gerbitz, A.; Winkler, J.; et al. Phase 2 Study of Anti-Human Cytomegalovirus Monoclonal Antibodies for Prophylaxis in Hematopoietic Cell Transplantation. Antimicrob. Agents Chemother. 2020, 64, 10–128. [Google Scholar] [CrossRef]
- Britt, W.J.; Jarvis, M.A.; Drummond, D.D.; Mach, M. Antigenic domain 1 is required for oligomerization of human cytomegalovirus glycoprotein B. J. Virol. 2005, 79, 4066–4079. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okamoto, M.; Kurino, R.; Miura, R.; Takada, K. A fully human neutralizing monoclonal antibody targeting a highly conserved epitope of the human cytomegalovirus glycoprotein B. PLoS ONE 2023, 18, e0285672. [Google Scholar] [CrossRef]
- Parsons, A.J.; Ophir, S.I.; Duty, J.A.; Kraus, T.A.; Stein, K.R.; Moran, T.M.; Tortorella, D. Development of broadly neutralizing antibodies targeting the cytomegalovirus subdominant antigen gH. Commun. Biol. 2022, 5, 387. [Google Scholar] [CrossRef] [PubMed]
- McVoy, M.A. Cytomegalovirus vaccines. Clin. Infect. Dis. 2013, 57 (Suppl. 4), S196–S199. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.; Klenerman, P.; Kadambari, S. Indirect effects of cytomegalovirus infection: Implications for vaccine development. Rev. Med. Virol. 2023, 33, e2405. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Stanton, A.; McCarrell, E.; Smith, C.; Osman, M.; Harber, M.; Davenport, A.; Jones, G.; Wheeler, D.C.; O’Beirne, J.; et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo-controlled trial. Lancet 2011, 377, 1256–1263. [Google Scholar] [CrossRef]
- Gomes, A.C.; Baraniak, I.A.; Lankina, A.; Moulder, Z.; Holenya, P.; Atkinson, C.; Tang, G.; Mahungu, T.; Kern, F.; Griffiths, P.D.; et al. The cytomegalovirus gB/MF59 vaccine candidate induces antibodies against an antigenic domain controlling cell-to-cell spread. Nat. Commun. 2023, 14, 1041. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Wang, D.; Oualim, A.; Diamond, D.J.; Kotton, C.N.; Mossman, S.; Carfi, A.; Anderson, D.; Dormitzer, P.R. The Status of Vaccine Development Against the Human Cytomegalovirus. J. Infect. Dis. 2020, 221, S113–S122. [Google Scholar] [CrossRef]
- Goodrum, F. Human Cytomegalovirus Latency: Approaching the Gordian Knot. Annu. Rev. Virol. 2016, 3, 333–357. [Google Scholar] [CrossRef]
- Krishna, B.A.; Wills, M.R.; Sinclair, J.H. Advances in the treatment of cytomegalovirus. Br. Med. Bull. 2019, 131, 5–17. [Google Scholar] [CrossRef] [PubMed]
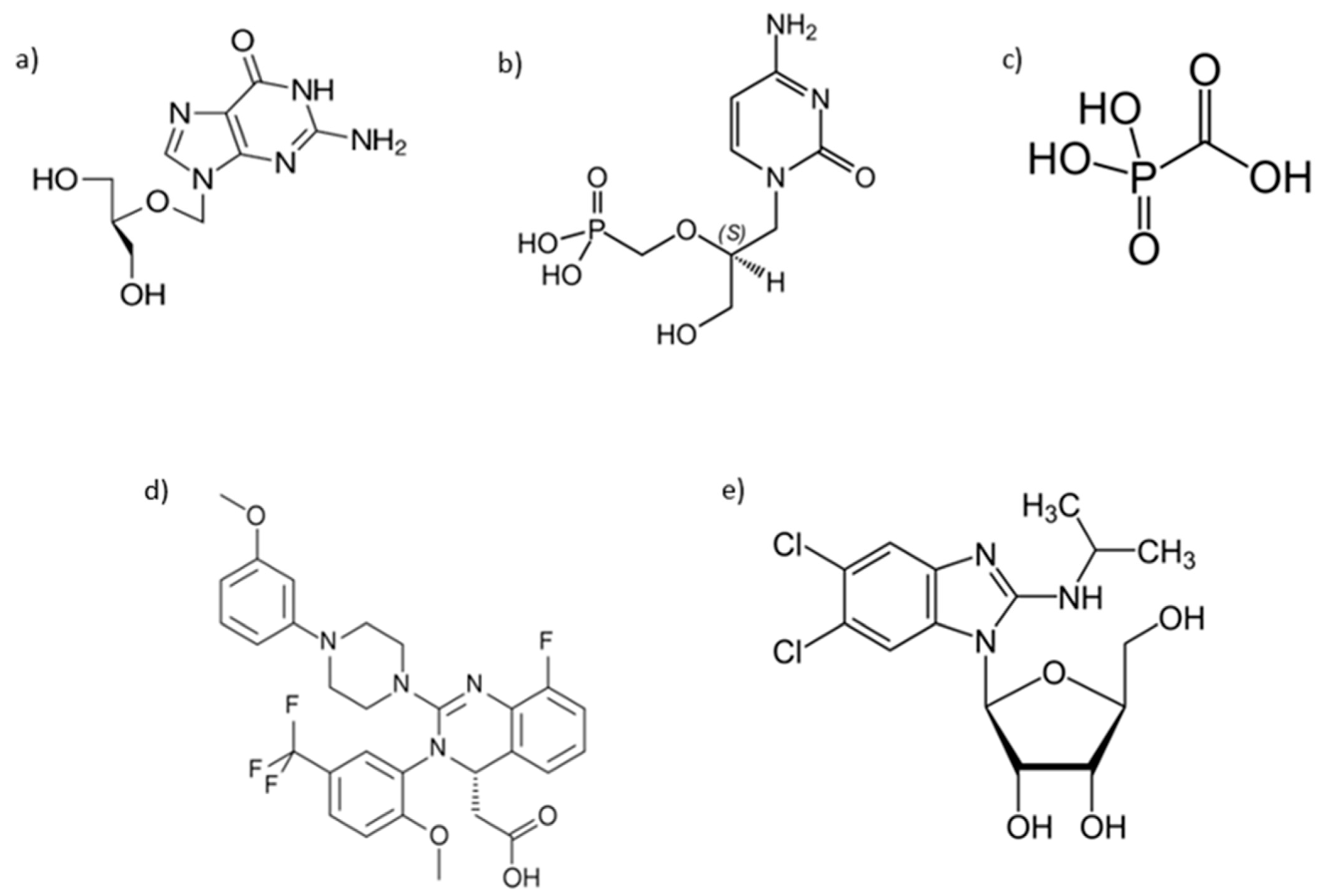
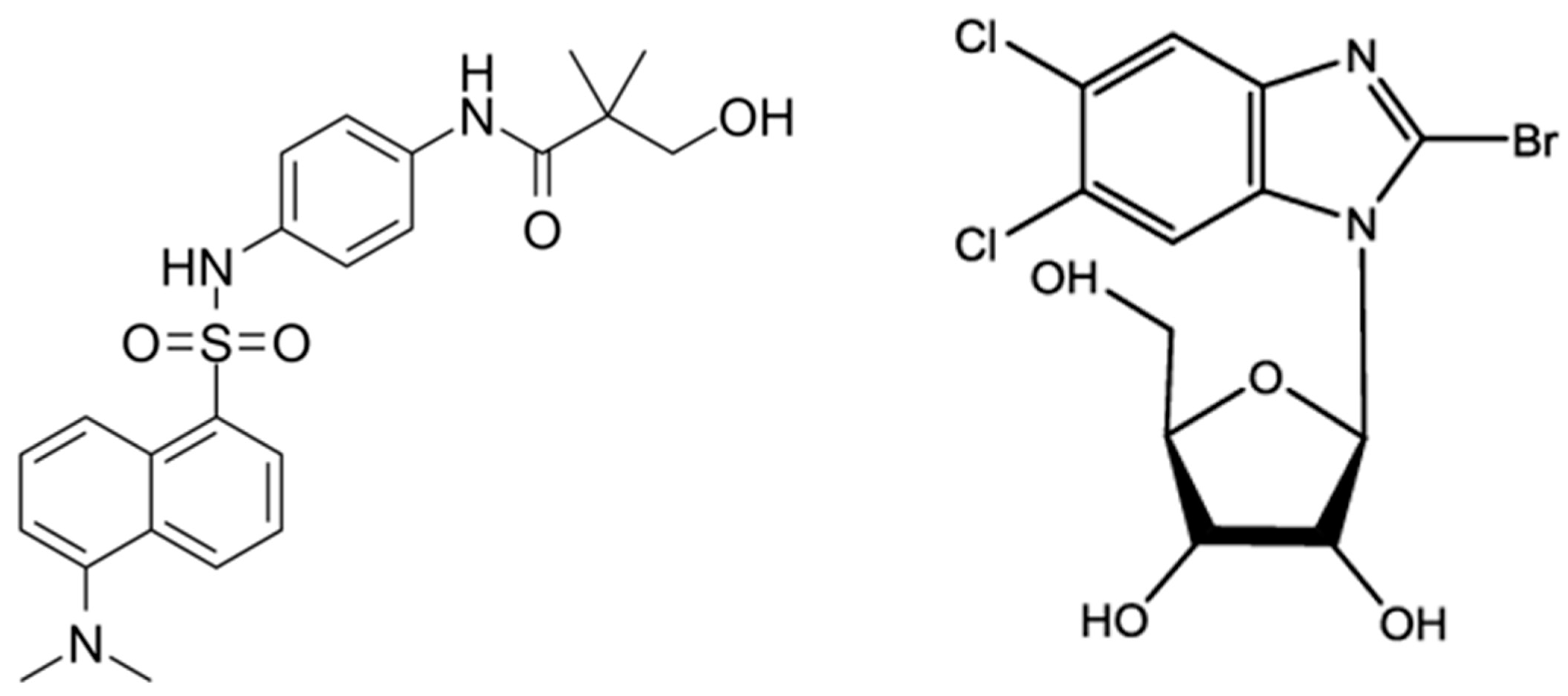
| Drug | Class | Status | Commercial Name | Mechanism of Action | Route of Administration | Posology | Resistance Mechanism | Side Effects |
|---|---|---|---|---|---|---|---|---|
| Ganciclovir | Purine nucleoside | Clinical use (first line), FDA approval (1989) | Cytovene® | Competitive inhibition of viral DNA polymerase | Intravenous | Induction: 1.25 mg/Kg to 5.0 mg/Kg, twice daily (7 to 14 days) Maintenance: 0.625 to 5.0 mg/Kg die Prophylaxis: 5.0 mg/Kg die (7 days per week) of 6.0 mg/kg die (5 days per week) | Mutations on UL97 kinase and UL54 DNA polymerase genes | Bone marrow suppression (leukopenia) |
| Valganciclovir | Purine nucleoside, modified to improve oral bioavailability | Clinical use (first line), FDA approval (2001) | Valcyte® | Competitive inhibition of viral DNA polymerase | Oral | Induction: 900 mg twice daily (21 days) Maintenance: 900 mg once daily Prophylaxis: 900 mg/Kg once daily | Mutations on UL97 kinase and UL54 DNA polymerase genes | Bone marrow suppression (leukopenia) |
| Cidofovir | Purine nucleoside | Clinical use for treatment of HCMV ganciclovir-resistant, FDA approval (1996) | Vistide® | Competitive inhibition of viral DNA polymerase | Intravenous, in combination with oral probenecid | Induction: 5.0 mg/Kg, week (per 14 days) Maintenance: 5.0 mg/Kg, week Prophylaxis: not recommended | Mutations on UL54 DNA polymerase, added to pre-existing UL97kinase mutations | Nephrotoxicity |
| Brincidofovir | Purine nucleoside | Phase III trials, discontinued | NA | Competitive inhibition of viral DNA polymerase | Oral | NA | NA | Gastrointestinal, elevations of serum transaminases |
| Foscarnet | Pyrophosphate analog | Clinical use for treatment of HCMV ganciclovir-resistant, FDA approval (1991) | Foscavir® | Noncompetitive inhibition of viral DNA polymerase (All Herpesvirus) | Intravenous | Induction: 60 mg/Kg every 8 h or 90 mg/Kg, every 12 h (14 to 21 days) Maintenance: 90–120 mg/Kg die Prophylaxis: not recommended | Mutations on UL54 DNA polymerase, cross-resistance with Ganciclovir, Valganciclovir and Cidofovir | Nephrotoxicity, myelosuppression, mucosal ulcerations, electrolyte alterations |
| Letermovir | Quinazoline derivative | Clinical use, FDA approval (2017) | Prevymis® | Inhibition of viral terminase enzyme complex | Oral, intravenous | Induction: not Recommended Maintenance: not Recommended Prophylaxis: 480 mg once daily (0–28 to 100 days after transplantation) | Mutations on UL56, UL51 and UL89 genes | Gastrointestinal |
| Maribavir | Benzimidazole riboside | Clinical use for treatment of HCMV Ganciclovir, Vfoscarnetalganciclovir, Cidofovir or Foscarnet resistant, FDA approval (2021) | Livtencity® | Competitive inhibition of viral kinase | Oral | Post-transplant HCMV infection/disease refractory to treatment (with or without genotypic resistance): 400 mg twice daily | Mutations on UL97 kinase gene | Gastrointestinal, dysgeusia |
| Tomeglovir | Naphthalene derivative | Phase II trials, discontinued | NA | Inhibition of viral terminase enzyme complex | Oral | NA | NA | NA |
| BDCRD * | Benzimidazole riboside | Phase I trials, discontinued | NA | Inhibition of viral terminase enzyme complex | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottino, P.; Pastrone, L.; Curtoni, A.; Bondi, A.; Sidoti, F.; Zanotto, E.; Cavallo, R.; Solidoro, P.; Costa, C. Antiviral Approach to Cytomegalovirus Infection: An Overview of Conventional and Novel Strategies. Microorganisms 2023, 11, 2372. https://doi.org/10.3390/microorganisms11102372
Bottino P, Pastrone L, Curtoni A, Bondi A, Sidoti F, Zanotto E, Cavallo R, Solidoro P, Costa C. Antiviral Approach to Cytomegalovirus Infection: An Overview of Conventional and Novel Strategies. Microorganisms. 2023; 11(10):2372. https://doi.org/10.3390/microorganisms11102372
Chicago/Turabian StyleBottino, Paolo, Lisa Pastrone, Antonio Curtoni, Alessandro Bondi, Francesca Sidoti, Elisa Zanotto, Rossana Cavallo, Paolo Solidoro, and Cristina Costa. 2023. "Antiviral Approach to Cytomegalovirus Infection: An Overview of Conventional and Novel Strategies" Microorganisms 11, no. 10: 2372. https://doi.org/10.3390/microorganisms11102372
APA StyleBottino, P., Pastrone, L., Curtoni, A., Bondi, A., Sidoti, F., Zanotto, E., Cavallo, R., Solidoro, P., & Costa, C. (2023). Antiviral Approach to Cytomegalovirus Infection: An Overview of Conventional and Novel Strategies. Microorganisms, 11(10), 2372. https://doi.org/10.3390/microorganisms11102372





