Thiorhodovibrio frisius and Trv. litoralis spp. nov., Two Novel Members from a Clade of Fastidious Purple Sulfur Bacteria That Exhibit Unique Red-Shifted Light-Harvesting Capabilities
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Selective Enrichment and Isolation of a Novel Anoxygenic Phototroph
3.2. Phylogenomic Placement and Taxonomy of Strain 970
3.3. Phenotypic Differentiations and Their Genomic Basis within the Genus Thiorhodovibrio
3.3.1. General Morphological, Physiological, and Chemotaxonomical Characteristics
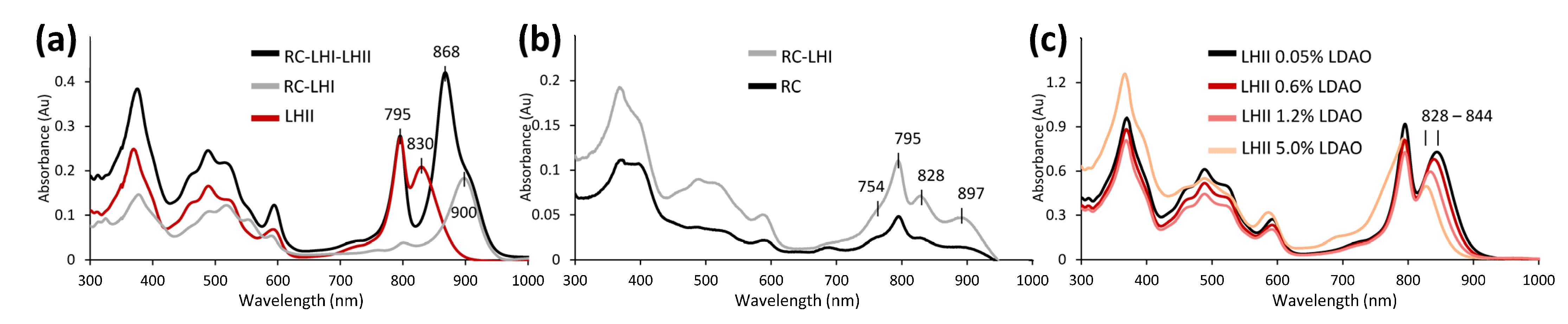
3.3.2. Unusual Light Harvesting
3.3.3. Sulfur and Hydrogen Metabolism
3.3.4. Carbon Metabolism
3.3.5. Nitrogen Metabolism
4. Conclusions
5. Emended Description of the Genus Thiorhodovibrio Overmann et al. 1992
6. Emended Description of the Species Thiorhodovibrio winogradskyi Overmann et al. 1992
7. Description of Thiorhodovibrio litoralis sp. nov.
8. Description of Thiorhodovibrio frisius sp. nov.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Overmann, J.; Garcia-Pichel, F. The phototrophic way of life. In The Prokaryotes, Prokaryotic Communities and Ecophysiology, 4th ed.; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: New York, NY, USA, 2013; pp. 203–257. [Google Scholar]
- Glaeser, J.; Overmann, J. Selective enrichment and characterization of Roseospirillum parvum, gen. nov. and sp. nov., a new purple nonsulfur bacterium with unusual light absorption properties. Arch. Microbiol. 1999, 171, 405–416. [Google Scholar] [CrossRef]
- Garcia, D.; Parot, P.; Verméglio, A.; Madigan, M.T. The light-harvesting complexes of a thermophilic purple sulfur photosynthetic bacterium Chromatium tepidum. Biochim. Biophys. Acta (Bioenerg.) 1986, 850, 390–395. [Google Scholar] [CrossRef]
- Pfennig, N.; Lünsdorf, H.; Süling, J.; Imhoff, J.F. Rhodospira trueperi gen. nov., spec. nov., a new phototrophic Proteobacterium of the alpha group. Arch. Microbiol. 1997, 168, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Permentier, H.P.; Neerken, S.; Overmann, J.; Amesz, J.A. A bacteriochlorophyll a antenna complex from purple bacteria absorbing at 963 nm. Biochemistry 2001, 40, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Rücker, O.; Köhler, A.; Behammer, B.; Sichau, K.; Overmann, J. Puf operon sequences and inferred structures of light-harvesting complexes of three closely related Chromatiaceae exhibiting different absorption characteristics. Arch. Microbiol. 2012, 194, 123–134. [Google Scholar] [CrossRef]
- Tani, K.; Kanno, R.; Makino, Y.; Hall, M.; Takenouchi, M.; Imanishi, M.; Yu, L.-J.; Overmann, J.; Madigan, M.T.; Kimura, Y.; et al. Cryo-EM structure of a Ca2+ bound photosynthetic LH1-RC complex containing multiple alpha/beta-polypeptides. Nature Comm. 2020, 11, 4955. [Google Scholar] [CrossRef]
- Overmann, J.; Fischer, U.; Pfennig, N. A new purple sulfur bacterium from saline littoral sediments, Thiorhodovibrio winogradskyi, gen. nov. and sp. nov. Arch. Microbiol. 1992, 157, 329–335. [Google Scholar] [CrossRef]
- Overmann, J.; Pfennig, N. Continuous chemotrophic growth and respiration of Chromatiaceae species at low oxygen concentrations. Arch. Microbiol. 1992, 158, 59–67. [Google Scholar] [CrossRef]
- Watson, S.V.; Novitsky, T.J.; Quinby, H.L.; Valois, F.W. Determination of bacterial number and biomass in the marine environment. Appl. Environ. Microbiol. 1977, 33, 940–946. [Google Scholar] [CrossRef]
- Pfennig, N. Rhodocyclus purpureus gen nov. and spec. nov., a ring-shaped, vitamin B12-requiring member of the family Rhodopsirillaceae. Int. J. Syst. Bacteriol. 1978, 28, 283–288. [Google Scholar] [CrossRef]
- Siefert, E.; Pfennig, N. Convenient method to prepare neutral sulfide solution for cultivation of phototrophic sulfur bacteria. Arch. Microbiol. 1984, 139, 100–101. [Google Scholar] [CrossRef]
- Pfennig, N.; Wagener, S. An improved method of preparing wet mounts for photomicrographs of microorganisms. J. Microbiol. Methods 1986, 4, 303–306. [Google Scholar] [CrossRef]
- Steenbergen, C.L.M.; Korthals, H.J. Distribution of phototrophic microorganisms in the anaerobic and microaerophilic strata of Lake Vechten (The Netherlands). Pigment analysis and role in primary production. Limnol. Oceanogr. 1982, 27, 883–895. [Google Scholar] [CrossRef]
- Rizk, S.; Henke, P.; Santana-Molina, C.; Martens, G.; Gnädig, M.; Nguyen, N.A.; Devos, D.P.; Neumann-Schaal, M.; Saenz, J.P. Functional diversity of isoprenoid lipids in Methylobacterium extorquens PA1. Mol. Microbiol. 2021, 116, 1064–1078. [Google Scholar] [CrossRef]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids. In MIDI Technical Note 101; (Revised 2006); MIDI Inc: Newark, DE, USA, 1990; pp. 1–6. [Google Scholar]
- Vieira, S.; Huber, K.J.; Neumann-Schaal, M.; Geppert, A.; Luckner, M.; Wanner, G.; Overmann, J. Usitatibacter rugosus gen. nov., sp. nov. and Usitatibacter palustris sp. nov., novel members of Usitatibacteraceae fam. nov. within the order Nitrosomonadales isolated from soil. Int. J. Syst. Evol. Microbiol. 2021, 71, 004631. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.W.; Lambert-Fair, M.A. Location of double bonds in monounsaturated fatty acids of Campylobacter cryaerophila with dimethyl disulfide derivatives and combined gas chromatography-mass spectrometry. J. Clin. Microbiol. 1989, 27, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Schumann, P.; Kalensee, F.; Cao, J.; Criscuolo, A.; Clermont, D.; Köhler, J.M.; Meier-Kolthoff, J.P.; Neumann-Schaal, M.; Tindall, B.J.; Pukall, R. Reclassification of Haloactinobacterium glacieicola as Occultella glacieicola gen. nov., comb. nov., of Haloactinobacterium album as Ruania alba comb. nov, with an emended description of the genus Ruania, recognition that the genus names Haloactinobacterium and Ruania are heterotypic synonyms and description of Occultella aeris sp. nov., a halotolerant isolate from surface soil sampled at an ancient copper smelter. Int. J. Syst. Evol. Microbiol. 2021, 71, 004769. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Hofmann, M.; Norris, P.R.; Malik, L.; Schippers, A.; Schmidt, G.; Wolf, J.; Neumann-Schaal, M.; Hedrich, S. Metallosphaera javensis sp. nov., a novel species of thermoacidophilic archaea, isolated from a volcanic area. Int. J. Syst. Evol. Microbiol. 2022, 72, 005536. [Google Scholar] [CrossRef]
- Dilling, W.; Liesack, W.; Pfennig, N. Rhabdochromatium marinum gen. nom. rev., sp. nov., a purple sulfur bacterium from a salt marsh microbial mat. Arch. Microbiol. 1995, 164, 125–131. [Google Scholar] [CrossRef]
- Imhoff, J.; Genus, X. Rhabdochromatium. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; Springer: New York, NY, USA, 2005; Volume 2. [Google Scholar]
- Schmidt, K.; Pfennig, N.; Jensen, S.L. Carotenoids of Thiorhodaceae IV. The carotenoid composition of 25 pure isolates. Arch. Mikrobiol. 1965, 52, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J. Genus XXIII. Thiospirillum. In Bergey´s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; Springer: New York, NY, USA, 2005; Volume 2, Part B; pp. 23–24. [Google Scholar]
- Imhoff, J.F.; Meyer, T.E.; Kyndt, J.A. The genome sequence of the giant phototrophic gammaproteobacterium Thiospirillum jenense gives insight into its physiological properties and phylogenetic relationships. Arch. Microbiol. 2021, 203, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef]
- Reimer, L.; Carbasse, J.S.; Koblitz, J.; Ebeling, C.; Podstawka, A.; Overmann, J. BacDive in 2022: The knowledge base for standardized bacterial and archaeal data. Nucleic Acids Res. 2022, 50, D741–D746. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, Y.O.; Yoon, S.H.; Ha, S.M.; Baek, I.; Chun, J. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. 2018, 56, 280–285. [Google Scholar] [CrossRef]
- Philippe, H. MUST, a computer package of Management Utilities for Sequences and Trees. Nucleic Acids Res. 1993, 21, 5264–5272. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Will, S.E.; Henke, P.; Boedeker, C.; Huang, S.; Brinkmann, H.; Rohde, M.; Jarek, M.; Friedl, T.; Seufert, S.; Schumacher, M.; et al. Day and night: Metabolic profiles and evolutionary relationships of six axenic non-marine cyanobacteria. Genome Biol. Evol. 2018, 11, 270–294. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Lim, J.M.; Kwon, S.J.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Brinkmann, H.; Göker, M.; Koblížek, M.; Wagner-Döbler, I.; Petersen, J. Horizontal operon transfer, plasmids, and the evolution of photosynthesis in Rhodobacteraceae. ISME J. 2018, 12, 1994–2010. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.; Zigann, R.; Bruce, D.; Chang, Y.J.; Detter, J.C.; Han, C.; Hauser, L.; Jeffries, C.D.; Land, M.; Munk, A.C.; et al. Complete genome sequence of Allochromatium vinosum DSM 180T. Stand. Genom. Sci. 2011, 5, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Oelze, J. Analysis of bacteriochlorophylls. Methods Microbiol. 1985, 18, 257–284. [Google Scholar]
- Imhoff, J.F.; Rahn, T.; Künzel, S.; Keller, A.; Neulinger, S.C. Osmotic Adaptation and Compatible Solute Biosynthesis of Phototrophic Bacteria as Revealed from Genome Analyses. Microorganisms 2020, 9, 46. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Ebers, J. Taxonomic Parameters Revisited: Tarnished Gold Standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef]
- Imanishi, M.; Takenouchi, M.; Takaichi, S.; Nakagawa, S.; Saga, Y.; Takenaka, S.; Madigan, M.T.; Overmann, J.; Wang-Otomo, Z.-Y.; Kimura, Y. A dual role for Ca2+ in expanding the spectral diversity and stability of LH1-RC photocomplexes of purple phototrophic bacteria. Biochemistry 2019, 58, 2844–2852. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Nastasi, M.R.; Forte, E. ROS Defense Systems and Terminal Oxidases in Bacteria. Antioxidants 2021, 10, 839. [Google Scholar] [CrossRef]
- Ohnishi, K.; Fan, F.; Schoenhals, G.J.; Kihara, M.; Macnab, R.M. The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: Putative components for flagellar assembly. J. Bacteriol. 1997, 179, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Kapatral, V.; Olson, J.W.; Pepe, J.C.; Miller, V.L.; Minnich, S.A. Temperature-dependent regulation of Yersinia enterocolitica Class III flagellar genes. Mol. Microbiol. 1996, 19, 1061–1071. [Google Scholar] [CrossRef]
- Imhoff, J.F.; Pfennig, N. Thioflavicoccus mobilis gen. nov., sp. nov., a novel purple sulfur bacterium with bacteriochlorophyll b. Int. J. Syst. Evol. Microbiol. 2001, 51, 105–110. [Google Scholar] [CrossRef]
- Mothersole, D.J.; Jackson, P.J.; Vasilev, C.; Tucker, J.D.; Brindley, A.A.; Dickman, M.J.; Hunter, C.N. PucC and LhaA direct efficient assembly of thelight-harvesting complexes in Rhodobacter sphaeroides. Mol. Microbiol. 2016, 99, 307–327. [Google Scholar] [CrossRef] [PubMed]
- Sattley, W.M.; Swingley, W.D.; Burchell, B.M.; Dewey, E.D.; Hayward, M.K.; Renbarger, T.L.; Shafer, K.N.; Stokes, L.M.; Gurbani, S.A.; Kujawa, C.M.; et al. Complete genome of the thermophilic purple sulfur Bacterium Thermochromatium tepidum compared to Allochromatium vinosum and other Chromatiaceae. Photosynth. Res. 2022, 151, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F.; Rahn, T.; Künzel, S.; Neulinger, S.C. Phylogeny of Anoxygenic Photosynthesis Based on Sequences of Photosynthetic Reaction Center Proteins and a Key Enzyme in Bacteriochlorophyll Biosynthesis, the Chlorophyllide Reductase. Microorganisms 2019, 7, 576. [Google Scholar] [CrossRef]
- Tuschak, C.; Leung, M.M.; Beatty, J.T.; Overmann, J. The puf operon of the purple sulfur bacterium Amoebobacter purpureus: Structure, transcription and phylogenetic analysis. Arch. Microbiol. 2005, 183, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S.; Sasikala, C.; Ramana, C.V.; Okamura, K.; Hiraishi, A. Carotenoids in Rhodoplanes species: Variation of compositions and substrate specificity of predicted carotenogenesis enzymes. Curr. Microbiol. 2012, 65, 150–155. [Google Scholar] [CrossRef]
- Duzs, A.; Miklovics, N.; Paragi, G.; Rákhely, G.; Tóth, A. Insights into the catalytic mechanism of type VI sulfide: Quinone oxidoreductases. Biochim. Biophys. Acta Biomembr. 2021, 1862, 148337. [Google Scholar]
- Krafft, T.; Bokranz, M.; Klimmek, O.; Schröder, I.; Fahrenholz, F.; Kojro, E.; Kröger, A. Cloning and nucleotide sequence of the psvA gene of Wolinella succinogenes polysulphide reductase. Eur. J. Biochem. 1992, 206, 503–510. [Google Scholar] [CrossRef]
- Hedderich, R.; Hamann, N.; Bennati, M. Heterodisulfide reductase from methanogenic archaea: A new catalytic role for an iron-sulfur cluster. Biol. Chem. 2005, 386, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Kappler, U.; Dahl, C. Enzymology and molecular biology of prokaryotic sulfite oxidation. FEMS Microbiol. Lett. 2001, 203, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Wynen, A.; Trüper, H.G.; Dahl, C. Characterization of the cys gene locus from Allochromatium vinosum indicates an unusual sulfate assimilation pathway. Mol. Biol. Rep. 2000, 27, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.G.; Bardischewsky, F.; Rother, D.; Quentmeier, A.; Fischer, J. Prokaryotic sulfur oxidation. Curr. Opin. Microbiol. 2005, 8, 253–259. [Google Scholar] [CrossRef]
- Dahl, C.; Franz, B.; Hensen, D.; Kesselheim, A.; Zigann, R. Sulfite oxidation in the purple sulfur bacterium Allochromatium vinosum: Identification of SoeABC as a major player and relevance of SoxYZ in the process. Microbiology 2013, 159, 2626–2638. [Google Scholar] [CrossRef]
- Prange, A.; Engelhardt, H.; Trüper, H.G.; Dahl, C. The role of the sulfur globule proteins of Allochromatium vinosum: Mutagenesis of the sulfur globule protein genes and expression studies by real-time RT-PCR. Arch. Microbiol. 2004, 182, 165–174. [Google Scholar] [CrossRef]
- Elferink, M.G.L.; Albers, S.V.; Konings, W.N.; Driessen, A.J.M. Sugar transport in Sulfolobus solfataricus is mediated by two families of binding protein-dependent ABC transporters. Mol. Microbiol. 2001, 39, 1494–1503. [Google Scholar] [CrossRef]
- Chevance, F.F.V.; Erhardt, M.; Lengsfeld, C.; Lee, S.J.; Boos, W. Mlc of Thermus thermophilus: A glucose-specific regulator for a glucose/mannose ABC transporter in the absence of the phosphotransferase system. J. Bacteriol. 2006, 188, 6561–6571. [Google Scholar] [CrossRef]
- Pavelka, M.S.; Hayes, S.F., Jr.; Silver, R.P. Characterization of KpsT, the ATP-binding Component of the ABC-Transporter Involved with the Export of Capsular Polysialic Acid in Escherichia coli K1. J. Biol. Chem. 1994, 269, 20149–20158. [Google Scholar] [CrossRef]
- Hosie, A.H.F.; Allaway, D.; Jones, M.A.; Walshaw, D.L.; Johnston, A.W.B.; Poole, P.S. Solute-binding protein-dependent ABC transporters are responsible for solute efflux in addition to solute uptake. Mol. Microbiol. 2001, 40, 1449–1459. [Google Scholar] [CrossRef]
- Grossmann, N.; Vakkasoglu, A.S.; Hulpke, S.; Abele, R.; Gaudet, R.; Tampé, R. Mechanistic determinants of the directionality and energetics of active export by a heterodimeric ABC transporter. Nat. Comm. 2014, 5, 5419. [Google Scholar] [CrossRef] [PubMed]
- Joerger, R.D.; Jacobson, M.R.; Premakumar, R.; Wolfinger, E.D.; Bishop, P.E. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J. Bacteriol. Res. 1989, 171, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, C.M.; Ludden, P.W. Regulation of Biological Nitrogen Fixation. J. Nutr. 2000, 130, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Schmehl, M.; Jahn, A.; Vilsendorf, A.M.Z.; Hennecke, S.; Masepohl, B.; Schuppler, M.; Marxer, M.; Oelze, J.; Klipp, W. Identification of a new class of nitrogen fixation genes in Rhodobacter capsulatus: A putative membrane complex involved in electron transport to nitrogenase. Mol. Gen. Genet. 1993, 241, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.L.; Bovee, R.J.; Thiel, V.; Sattin, S.R.; Mohr, W.; Schaperdoth, I.; Vogl, K.; Gilhooly, I.I.I.W.P.; Lyons, T.W.; Tomsho, L.P.; et al. Coupled reductive and oxidative sulfur cycling in the phototrophic plate of a meromictic lake. Geobiology 2014, 12, 451–468. [Google Scholar] [CrossRef]



| Genome Features | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Accession No. | CP099568 | CP099569 | CP099570 | GCA_016583795 | GCA_014145225 |
| Size (Mbp) | 5.42 | 5.48 | 5.47 | 4.36 | 3.22 |
| No. of Contigs | 1 | 1 | 1 | 214 | 128 |
| Coverage | 911× | 1589× | 528× | 123× | 56× |
| Sequencing platform | PacBio Sequel IIe | PacBio Sequel IIe | PacBio Sequel IIe | Illumina MiSeq | Illumina MiniSeq |
| GC content (mol %) | 60.2 | 60.6 | 61.4 | 59.7 | 48.7 |
| No. of plasmids | 0 | 0 | 0 | n.d. | n.d. |
| No. of Genes | 5032 | 5000 | 4940 | 3881 | 2967 |
| No. of CDS | 4922 | 4833 | 4834 | 3825 | 2911 |
| No. of tRNA | 51 | 47 | 48 | 46 | 47 |
| No. of rRNA | 6 | 6 | 6 | 6 | 5 |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Cell morphology | Short spirillum, vibrioid | Vibrio-spirilloid | Short spirillum | Long rods | Curved rods, sigmoid, or spiral |
| Cell width (±S.D.) | 0.9 (±0.1) µm | 1.4 (±0.2) µm | 0.8 (±0.1) µm | 1.5–1.7 µm | 2.5–4.0 µm |
| Cell length (±S.D.) | 3.6 (±0.8) µm | 3.3 (±0.7) µm | 3.2 (±0.7) µm | 16–32 µm | 30–100 µm |
| Motility | − (lost) | + | + | + | + |
| Flagellation | − | Monopolar, | Monopolar, | Bipolar, | Monopolar |
| monotrichous | monotrichous | polytrichous | (rarely bipolar), | ||
| polytrichous | |||||
| Carotenoid composition (%) | |||||
| Lycopene | − 1 | 6.4 | 14.9 | 83.3 | 12 |
| Rhodopin | 2.4 1 | 47.4 | 55.3 | 4.8 | 88 |
| Carotenoids of the normal spirilloxanthin pathway | |||||
| Anhydrorhodovibrin | − | 13.6 | 10.2 | 3.5 | − |
| Rhodovibrin | − | 4.1 | 0 | 8.3 | − |
| Spirilloxanthin | − | 28.5 | 19.6 | 0 | − |
| Carotenoids of the unusual spirilloxanthin pathway | |||||
| 3,4-Dihydroanhydrorhodovibrin | 8.1 1 | − | − | − | − |
| 3′,4′-Dihydrorhodovibrin | 1.9 1 | − | − | − | − |
| 3,4,3′,4′-Tetrahydrospirilloxanthin | 87.6 1 | − | − | − | − |
| Chemotaxonomy 2,3 | |||||
| Quinones (%) | Q8 (84.4), MK8 | Q8 (87.4), MK8 | (71.0), MK8 | n.d. | n.d. |
| (15.6) | (12.6) | Q8 (29.0) | |||
| Lipids | PE, LPE, PG, | PE, LPE, PG, | PE, LPE, PG, | n.d. | n.d. |
| LPG, DPG, | LPG, DPG, | LPG, DPG, | |||
| glykolipids | glykolipids | glykolipids | |||
| Fatty acids (%) | 18:1ω7c (34.8), | 18:1ω7c (46.1), | 18:1ω7c (37.2), | n.d. | n.d. |
| 16:0 (30.7), | 16:0 (25.6), | 16:0 (28.7), | |||
| 16:1ω7c (25.7), | 16:1ω7c (17.8), | 16:1ω7c (22.6), | |||
| 18:0 (2.5), 14:0 | 18:0 (4.3), 11 | 18:0 (4.5), 11 | |||
| (1.3) | methyl 18:1ω7c | methyl 18:1ω7c | |||
| (1.7), 12:0 (1.1) | (1.8), 12:0 (1.6) | ||||
| Physiology | |||||
| pH optimum | 7.3 | 7.2 | 7.2 | 7.2–7.3 | 7.0 |
| pH range | 6.8–8.3 | 7.0–7.4 | 7.0–7.4 | n.d. | 6.5–7.5 |
| Temperature optimum (°C) | 27–30 | 33 | 33 | 30 | 20–25 |
| Temperature range (°C) | 15–37 | 14–37 | 14–37 | 8.0–35 | n.d. |
| NaCl optimum (% w/v) | 1.5–2.1 | 2.2–3.2 | 2.2–3.2 | 1.5–5.0 | 0 |
| NaCl range (% w/v) | 1.1–5.3 | 2.2–7.2 | 2.2–7.2 | 1.0–6.5 | 0 |
| Vitamin B12 required | − | − | − | − | + |
| Electron donors of photosynthetic growth (final concentrations tested) | |||||
| H2S (1.25 mM) | + | + | + | + | + |
| Sulfur (in excess) | + | + | + | + | + |
| Na2S2O3 (5 mM) | − | − | + | + | n.d. |
| Hydrogen (head space) | + | + 3 | + 3 | + | − |
| Carbon sources assimilated during phototrophic growth (final concentrations tested) | |||||
| Acetate (5 mM) | + | + | + | + | + |
| Propionate (1 mM) | − | + | − | + | n.d. |
| Pyruvate (5 mM) | + | + | + | + | n.d. |
| Glucose (5 mM) | + 4 | − | − | − | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Methner, A.; Kuzyk, S.B.; Petersen, J.; Bauer, S.; Brinkmann, H.; Sichau, K.; Wanner, G.; Wolf, J.; Neumann-Schaal, M.; Henke, P.; et al. Thiorhodovibrio frisius and Trv. litoralis spp. nov., Two Novel Members from a Clade of Fastidious Purple Sulfur Bacteria That Exhibit Unique Red-Shifted Light-Harvesting Capabilities. Microorganisms 2023, 11, 2394. https://doi.org/10.3390/microorganisms11102394
Methner A, Kuzyk SB, Petersen J, Bauer S, Brinkmann H, Sichau K, Wanner G, Wolf J, Neumann-Schaal M, Henke P, et al. Thiorhodovibrio frisius and Trv. litoralis spp. nov., Two Novel Members from a Clade of Fastidious Purple Sulfur Bacteria That Exhibit Unique Red-Shifted Light-Harvesting Capabilities. Microorganisms. 2023; 11(10):2394. https://doi.org/10.3390/microorganisms11102394
Chicago/Turabian StyleMethner, Anika, Steven B. Kuzyk, Jörn Petersen, Sabine Bauer, Henner Brinkmann, Katja Sichau, Gerhard Wanner, Jacqueline Wolf, Meina Neumann-Schaal, Petra Henke, and et al. 2023. "Thiorhodovibrio frisius and Trv. litoralis spp. nov., Two Novel Members from a Clade of Fastidious Purple Sulfur Bacteria That Exhibit Unique Red-Shifted Light-Harvesting Capabilities" Microorganisms 11, no. 10: 2394. https://doi.org/10.3390/microorganisms11102394
APA StyleMethner, A., Kuzyk, S. B., Petersen, J., Bauer, S., Brinkmann, H., Sichau, K., Wanner, G., Wolf, J., Neumann-Schaal, M., Henke, P., Tank, M., Spröer, C., Bunk, B., & Overmann, J. (2023). Thiorhodovibrio frisius and Trv. litoralis spp. nov., Two Novel Members from a Clade of Fastidious Purple Sulfur Bacteria That Exhibit Unique Red-Shifted Light-Harvesting Capabilities. Microorganisms, 11(10), 2394. https://doi.org/10.3390/microorganisms11102394







