Lactobacillus helveticus Isolated from Raw Milk Improves Liver Function, Hepatic Steatosis, and Lipid Metabolism in Non-Alcoholic Fatty Liver Disease Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Culture, and Preparation of LAB Strains
2.2. Culture of HepG2 Cells
2.3. Treatments and Cell Viability Assays
2.4. Screening by Oil-Red-O Staining
2.5. Probiotic Characterization of the LAB Strains
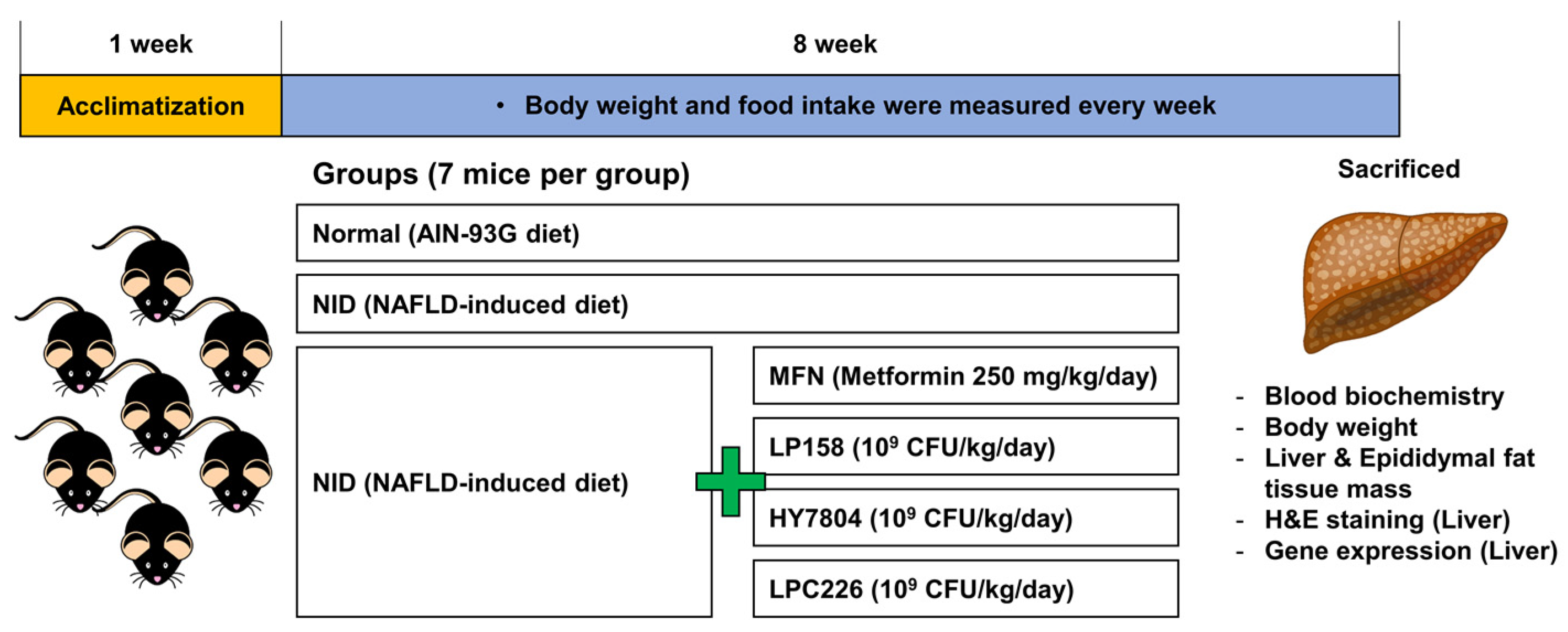
2.6. Animal Experiments
2.7. Blood Biochemistry Analysis
2.8. Histological Analysis
2.9. RNA Extraction, cDNA Synthesis, and Gene Expression Analysis
2.10. Statistical Analysis
3. Results
3.1. Screening of LAB Strains Isolated from Fresh Milk
3.2. Probiotic Characterization of LAB Strains
3.3. Effect of Probiotics on Gene Expression by PA-Induced HepG2 Cells
3.4. Animal Experiments
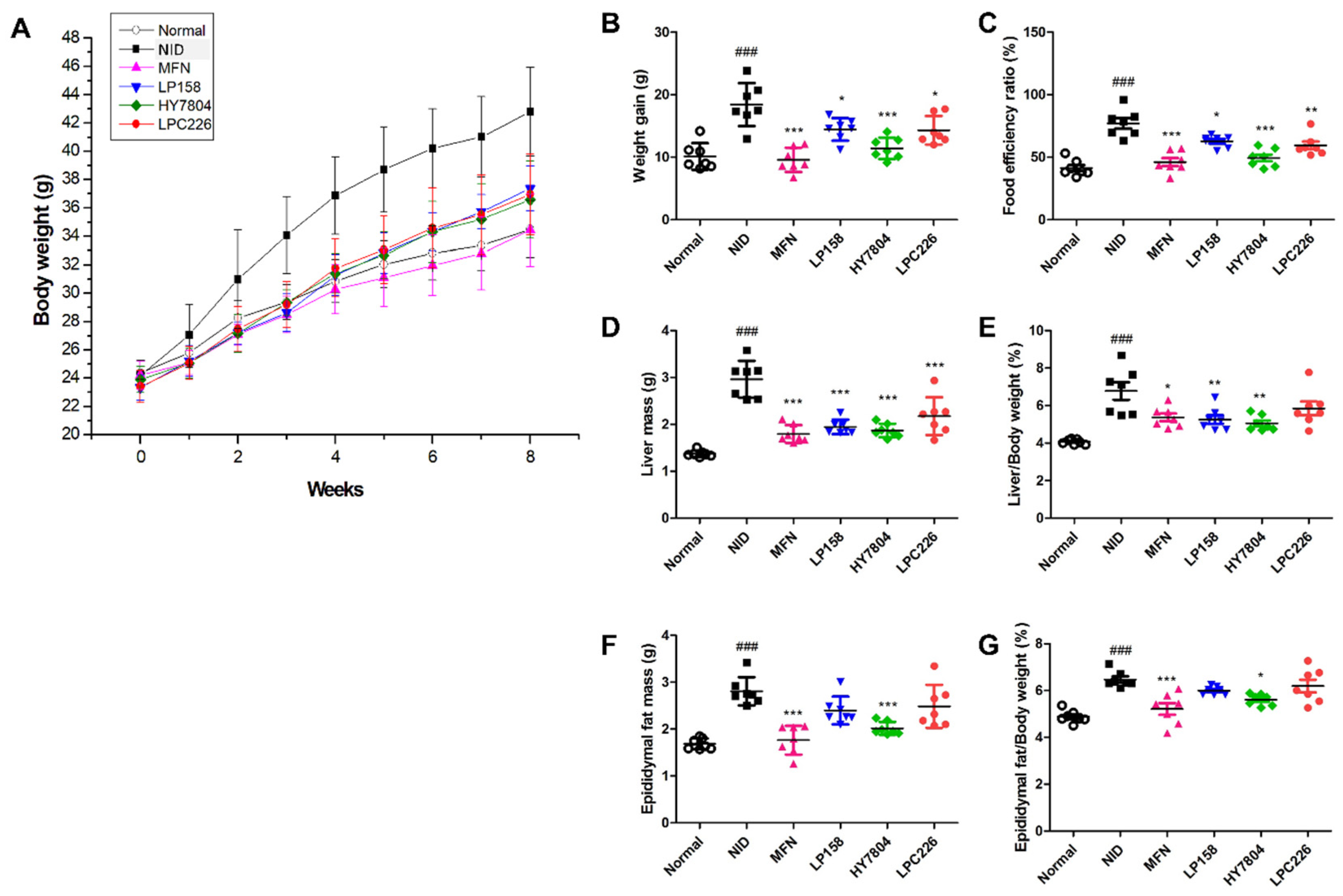
3.4.1. Effects of Probiotics on Body Weight and Food Efficiency Ratio in Mice Fed an NAFLD-Induced Diet
3.4.2. Effects of Probiotics on Liver Tissue Morphology and Liver and Epididymal Fat Mass in NAFLD-Induced-Diet Mice
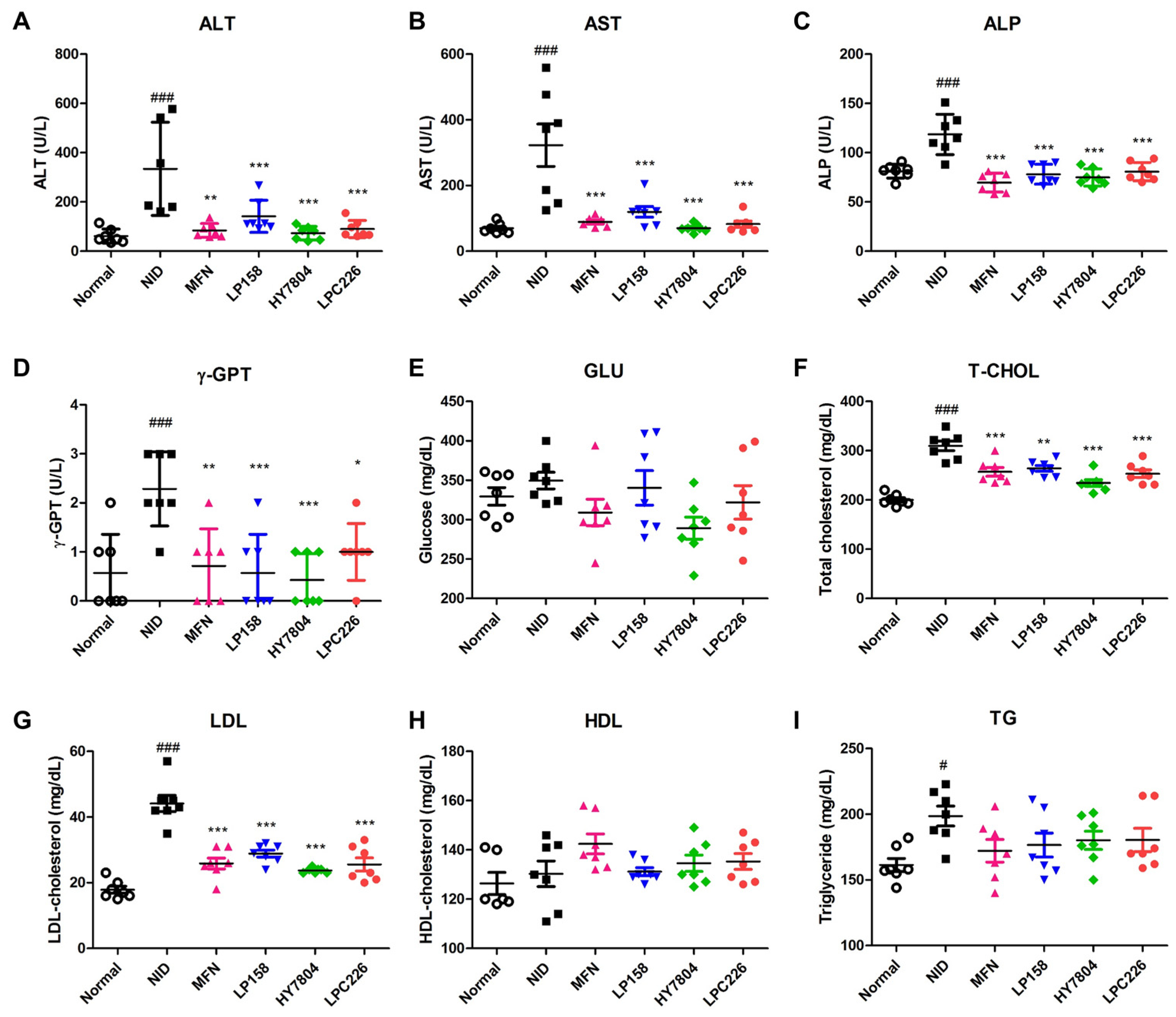
3.4.3. Effects of Probiotics on Serum Biochemistry
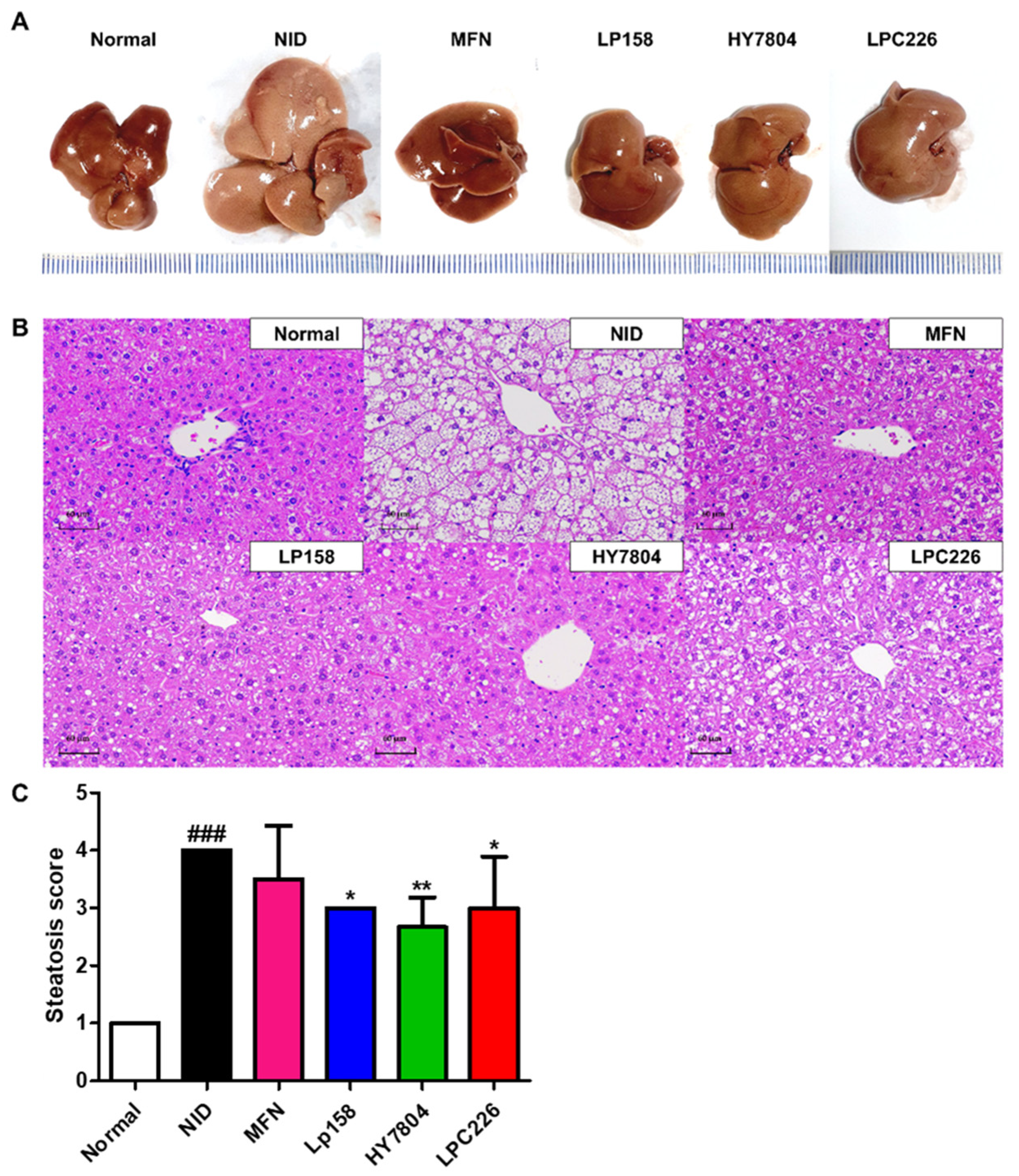
3.4.4. Effects of Probiotics on Liver Tissue Morphology and Histological Analysis of Liver Tissues from NAFLD Mice
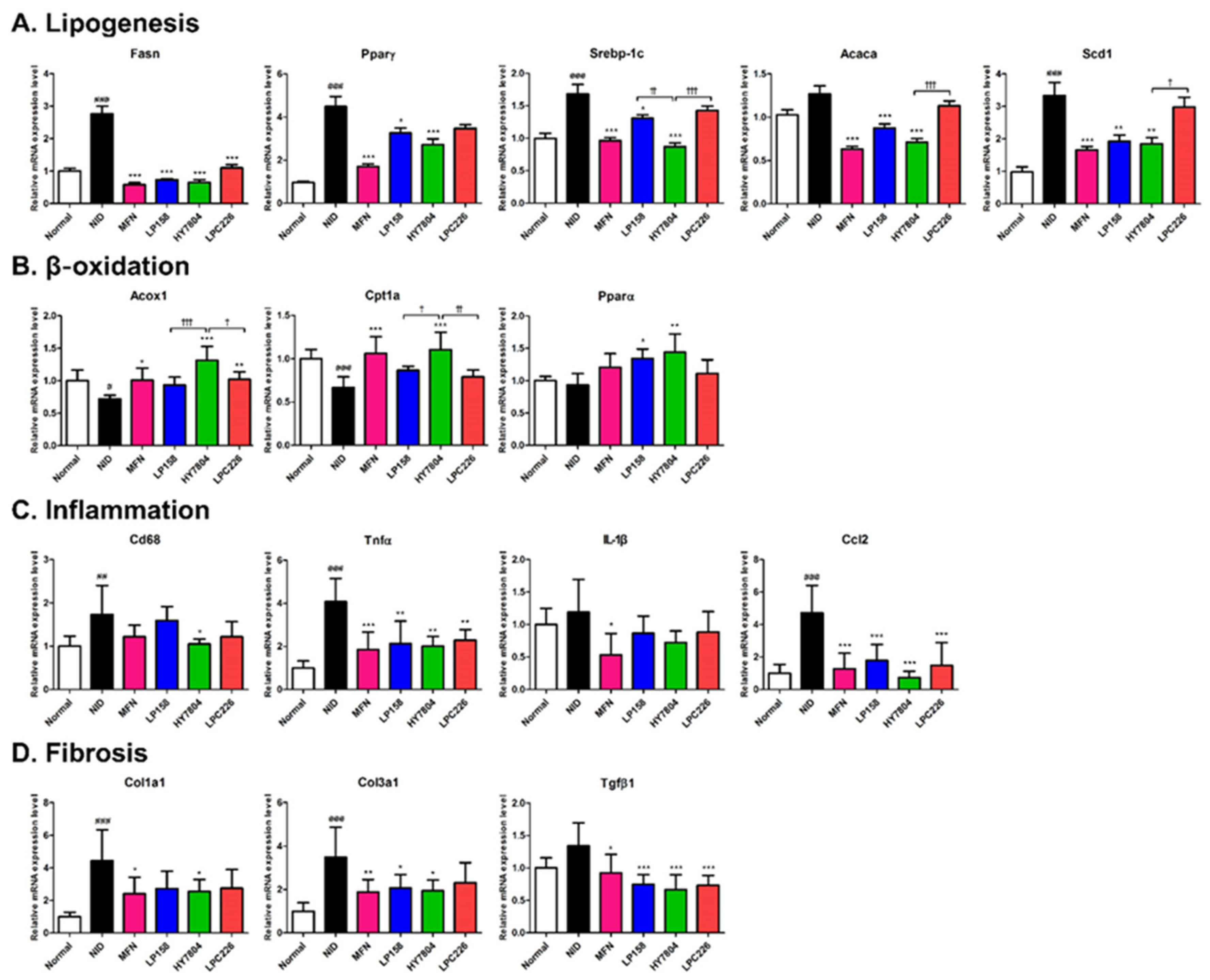
3.4.5. Effects of Probiotics on Gene Expression by NAFLD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Lirussi, F.; Mastropasqua, E.; Orando, S.; Orlando, R.; Group, C.H.B. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst. Rev. 1996, 2010, CD005165. [Google Scholar]
- Ma, Y.-Y.; Li, L.; Yu, C.-H.; Shen, Z.; Chen, L.-H.; Li, Y.-M. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J. Gastroenterol. 2013, 19, 6911. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Takahashi, Y.; Fukusato, T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 15539. [Google Scholar] [CrossRef]
- Dharmalingam, M.; Yamasandhi, P.G. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2018, 22, 421. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Park, K.-G. Definition, pathogenesis, and natural progress of non-alcoholic fatty liver disease. J. Korean Diabetes 2014, 15, 65–70. [Google Scholar] [CrossRef]
- Vancells Lujan, P.; Viñas Esmel, E.; Sacanella Meseguer, E. Overview of non-alcoholic fatty liver disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Basaranoglu, G.; Bugianesi, E. Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surg. Nutr. 2015, 4, 109. [Google Scholar] [PubMed]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride metabolism in the liver. Compr. Physiol. 2017, 8, 1. [Google Scholar] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Abel, E.D. Free fatty acid oxidation in insulin resistance and obesity. Heart Metab. Manag. Coron. Patient 2010, 48, 5. [Google Scholar]
- Ruiz, R.; Jideonwo, V.; Ahn, M.; Surendran, S.; Tagliabracci, V.S.; Hou, Y.; Gamble, A.; Kerner, J.; Irimia-Dominguez, J.M.; Puchowicz, M.A. Sterol regulatory element-binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J. Biol. Chem. 2014, 289, 5510–5517. [Google Scholar] [CrossRef]
- Chow, J.D.; Lawrence, R.T.; Healy, M.E.; Dominy, J.E.; Liao, J.A.; Breen, D.S.; Byrne, F.L.; Kenwood, B.M.; Lackner, C.; Okutsu, S. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol. Metab. 2014, 3, 419–431. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 2012, 18, 727. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Kim, D.-H.; Chun, K.-H. The role of hepatic macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Lab. Anim. Res. 2018, 34, 133–139. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef] [PubMed]
- Tien, M.-T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.-A.; Coppée, J.-Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pédron, T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.H.; Balakrishnan, K.; Thiagarajah, K.; Ismail, N.I.M.; Yin, O.S. Beneficial properties of probiotics. Trop. Life Sci. Res. 2016, 27, 73. [Google Scholar] [PubMed]
- Chatterjee, A.; Bhattacharya, H.; Kandwal, A. Probiotics in periodontal health and disease. J. Indian Soc. Periodontol. 2011, 15, 23. [Google Scholar] [PubMed]
- Paolella, G.; Mandato, C.; Pierri, L.; Poeta, M.; Di Stasi, M.; Vajro, P. Gut-liver axis and probiotics: Their role in non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 15518. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, H.; Kim, K.J.; Park, S.-Y.; Jung, S.K.; Park, S.H.; Lim, S.; Kim, J.Y. Antioxidant effect of wheat germ extracts and their antilipidemic effect in palmitic acid-induced steatosis in HepG2 and 3T3-L1 cells. Foods 2021, 10, 1061. [Google Scholar] [CrossRef]
- Jeung, W.H.; Shim, J.-J.; Woo, S.-W.; Sim, J.-H.; Lee, J.-L. Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 cell extracts inhibit adipogenesis in 3T3-L1 and HepG2 cells. J. Med. Food 2018, 21, 876–886. [Google Scholar] [CrossRef]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Leerapun, A.; Pinyopornpanish, K.; Chattipakorn, N. Effects of metformin on hepatic steatosis in adults with nonalcoholic fatty liver disease and diabetes: Insights from the cellular to patient levels. Gut Liver 2021, 15, 827. [Google Scholar] [CrossRef]
- Kumar, R.; Priyadarshi, R.N.; Anand, U. Non-alcoholic fatty liver disease: Growing burden, adverse outcomes and associations. J. Clin. Transl. Hepatol. 2020, 8, 76. [Google Scholar] [CrossRef]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.-F.; Caussy, C.; Loomba, R. Combination therapy for non-alcoholic steatohepatitis: Rationale, opportunities and challenges. Gut 2020, 69, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Negi, C.K.; Babica, P.; Bajard, L.; Bienertova-Vasku, J.; Tarantino, G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism 2022, 126, 154925. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Gu, M.; Werlinger, P.; Cho, J.-H.; Cheng, J.; Suh, J.-W. Lactobacillus sakei MJM60958 as a Potential Probiotic Alleviated Non-Alcoholic Fatty Liver Disease in Mice Fed a High-Fat Diet by Modulating Lipid Metabolism, Inflammation, and Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 13436. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic resistance among commercially available probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; G de los Reyes-Gavilán, C.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Courvalin, P. Antibiotic resistance: The pros and cons of probiotics. Dig. Liver Dis. 2006, 38, S261–S265. [Google Scholar] [CrossRef]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. 2016, 91, 452–468. [Google Scholar] [CrossRef]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.E.; Lee, M.; Hardwick, J.P. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver Res. 2018, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, M.E.; Giles, D.A.; Stankiewicz, T.E.; Sheridan, R.; Karns, R.; Cappelletti, M.; Lampe, K.; Mukherjee, R.; Sina, C.; Sallese, A. Peroxisomal β-oxidation regulates whole body metabolism, inflammatory vigor, and pathogenesis of nonalcoholic fatty liver disease. JCI Insight 2018, 3, e93626. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kerner, J.; Hoppel, C.L. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J. Biol. Chem. 2011, 286, 25655–25662. [Google Scholar] [CrossRef]
- Sanyal, D.; Mukherjee, P.; Raychaudhuri, M.; Ghosh, S.; Mukherjee, S.; Chowdhury, S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J. Endocrinol. Metab. 2015, 19, 597. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Loomba, R. Nonalcoholic fatty liver disease: Indications for liver biopsy and noninvasive biomarkers. Clin. Liver Dis. 2012, 1, 104. [Google Scholar] [CrossRef]
- Feng, G.; Feng, L.; Zhao, Y. Association between ratio of γ-glutamyl transpeptidase to high-density lipoprotein cholesterol and prevalence of nonalcoholic fatty liver disease and metabolic syndrome: A cross-sectional study. Ann. Transl. Med. 2020, 8, 634. [Google Scholar] [CrossRef]
- Tu, L.N.; Showalter, M.R.; Cajka, T.; Fan, S.; Pillai, V.V.; Fiehn, O.; Selvaraj, V. Metabolomic characteristics of cholesterol-induced non-obese nonalcoholic fatty liver disease in mice. Sci. Rep. 2017, 7, 6120. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, E.; Rodríguez-Hernández, H.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of triglycerides and glucose as biomarker for screening simple steatosis and NASH in asymptomatic women. Ann. Hepatol. 2017, 15, 715–720. [Google Scholar]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Park, M.; Yoo, J.-H.; Lee, Y.-S.; Lee, H.-J. Lonicera caerulea extract attenuates non-alcoholic fatty liver disease in free fatty acid-induced HepG2 hepatocytes and in high fat diet-fed mice. Nutrients 2019, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gomez, G.; Gray, S.; Vidal-Puig, A. Adipogenesis and lipotoxicity: Role of peroxisome proliferator-activated receptor γ (PPARγ) and PPARγcoactivator-1 (PGC1). Public Health Nutr. 2007, 10, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Vajreswari, A. Stearoyl-CoA desaturase 1: A potential target for non-alcoholic fatty liver disease?-perspective on emerging experimental evidence. World J. Hepatol. 2022, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Régnier, M.; Polizzi, A.; Smati, S.; Lukowicz, C.; Fougerat, A.; Lippi, Y.; Fouché, E.; Lasserre, F.; Naylies, C.; Bétoulières, C. Hepatocyte-specific deletion of Pparα promotes NAFLD in the context of obesity. Sci. Rep. 2020, 10, 6489. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of inflammatory cytokines with non-alcoholic fatty liver disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Pan, X.; Chiwanda Kaminga, A.; Liu, A.; Wen, S.W.; Chen, J.; Luo, J. Chemokines in non-alcoholic fatty liver disease: A systematic review and network meta-analysis. Front. Immunol. 2020, 11, 1802. [Google Scholar] [CrossRef]
- Pellicano, A.J.; Spahn, K.; Zhou, P.; Goldberg, I.D.; Narayan, P. Collagen characterization in a model of nonalcoholic steatohepatitis with fibrosis; a call for development of targeted therapeutics. Molecules 2021, 26, 3316. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef]


| Gene | Gene Name | Catalog Number |
|---|---|---|
| Human | ||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs99999905_m1 |
| FASN | Fatty acid synthase | Hs00188012_m1 |
| SREBP-1 | Sterol regulatory element-binding protein 1 | Hs01088691_m1 |
| PPARγ | Peroxisome proliferator-activated receptor gamma | Hs01115513_m1 |
| C/EBPa | CCAAT/enhancer-binding protein alpha | Hs00269972_s1 |
| ACACA | Acetyl-CoA carboxylase alpha | Hs01046047_m1 |
| CPT1A | Carnitine palmitoyltransferase 1A | Hs00912671_m1 |
| ACOX1 | Acyl-CoA oxidase 1 | Hs01074241_m1 |
| Mouse | ||
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | Mm99999915_g1 |
| Fasn | Fatty acid synthase | Mm00433237_m1 |
| Srebp-1 | Sterol regulatory element-binding protein 1 | Mm00550338_m1 |
| Pparγ | Peroxisome proliferator-activated receptor gamma | Mm00440945_m1 |
| C/ebpα | CCAAT/enhancer-binding protein alpha | Mm00514283_m1 |
| Acaca | Acetyl-CoA carboxylase alpha | Mm01304257_m1 |
| Scd1 | Steroyl-Coenzyme A desaturease 1 | Mm00772290_m1 |
| Cpt1a | Carnitine palmitoyltransferase 1a | Mm01231183_m1 |
| Acox1 | Acyl-CoA oxidase 1 | Mm01246831_m1 |
| Col1a1 | Collagen type III alpha 1 | Mm00801666_g1 |
| Co13a1 | Collagen type I alpha 1 | Mm00802300_m1 |
| Tgfβ1 | Transforming growth factor beta 1 | Mm01178820_m1 |
| Cd68 | CD68 molecule | Mm03047343_m1 |
| Ccl2 | C-C motif chemokine ligand 2 | Mm00441242_m1 |
| Tnf | Tumor necrosis factor | Mm00443258_m1 |
| Il-1b | Interleukin 1 beta | Mm00434228_m1 |
| Pparα | Peroxisome proliferator-activated receptor alpha | Mm00440939_m1 |
| Antibiotic | EFSA 1 | LP158 | EFSA | HY7804 | EFSA | LPC226 |
|---|---|---|---|---|---|---|
| Ampicillin | 2 | 0.032 | 1 | 0.125 | 4 | 0.5 |
| Vancomycin | n.r. 2 | n.r. | 2 | 0.5 | n.r. | n.r. |
| Gentamycin | 16 | 12 | 16 | 16 | 32 | 16 |
| Kanamycin | 64 | 64 | 16 | 16 | 64 | 64 |
| Streptomycin | n.r. | n.r. | 16 | 16 | 64 | 64 |
| Erythromycin | 1 | 0.75 | 1 | 0.25 | 1 | 1 |
| Clindamycin | 2 | 0.75 | 1 | 0.75 | 1 | 0.25 |
| Tetracyclin | 32 | 8 | 4 | 2 | 4 | 2 |
| Chloramphenicol | 8 | 6 | 4 | 4 | 4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Lee, K.; Kim, J.-Y.; Shim, J.-J.; Lim, J.; Kim, J.-Y.; Lee, J.-L. Lactobacillus helveticus Isolated from Raw Milk Improves Liver Function, Hepatic Steatosis, and Lipid Metabolism in Non-Alcoholic Fatty Liver Disease Mouse Model. Microorganisms 2023, 11, 2466. https://doi.org/10.3390/microorganisms11102466
Kim H, Lee K, Kim J-Y, Shim J-J, Lim J, Kim J-Y, Lee J-L. Lactobacillus helveticus Isolated from Raw Milk Improves Liver Function, Hepatic Steatosis, and Lipid Metabolism in Non-Alcoholic Fatty Liver Disease Mouse Model. Microorganisms. 2023; 11(10):2466. https://doi.org/10.3390/microorganisms11102466
Chicago/Turabian StyleKim, Hyeonji, Kippeum Lee, Ju-Yeon Kim, Jae-Jung Shim, Junghyun Lim, Joo-Yun Kim, and Jung-Lyoul Lee. 2023. "Lactobacillus helveticus Isolated from Raw Milk Improves Liver Function, Hepatic Steatosis, and Lipid Metabolism in Non-Alcoholic Fatty Liver Disease Mouse Model" Microorganisms 11, no. 10: 2466. https://doi.org/10.3390/microorganisms11102466






