Telacebec Interferes with Virulence Lipid Biosynthesis Protein Expression and Sensitizes to Other Antibiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. BCG Strains
2.2. Construction of the pfadD26-luxAB Plasmid
2.3. The pfadD26-luxAB-Transformed BCG
2.4. Bioluminescence Detection
2.5. Drug Susceptibility and Synergy Assessment
2.6. Protein Extraction
2.7. MS Analysis, SWATH Acquisition, and Data Interpretation
2.8. Lipid Analysis
2.9. Statistical Analysis
3. Results
3.1. Decreased Abundance of Proteins Required for PDIM/PGL Synthesis by Telacebec
3.2. Telacebec Decreased the fadD26 Promoter Activity
3.3. Telacebec Synergized with Vancomycin and Rifampicin
4. Discussion
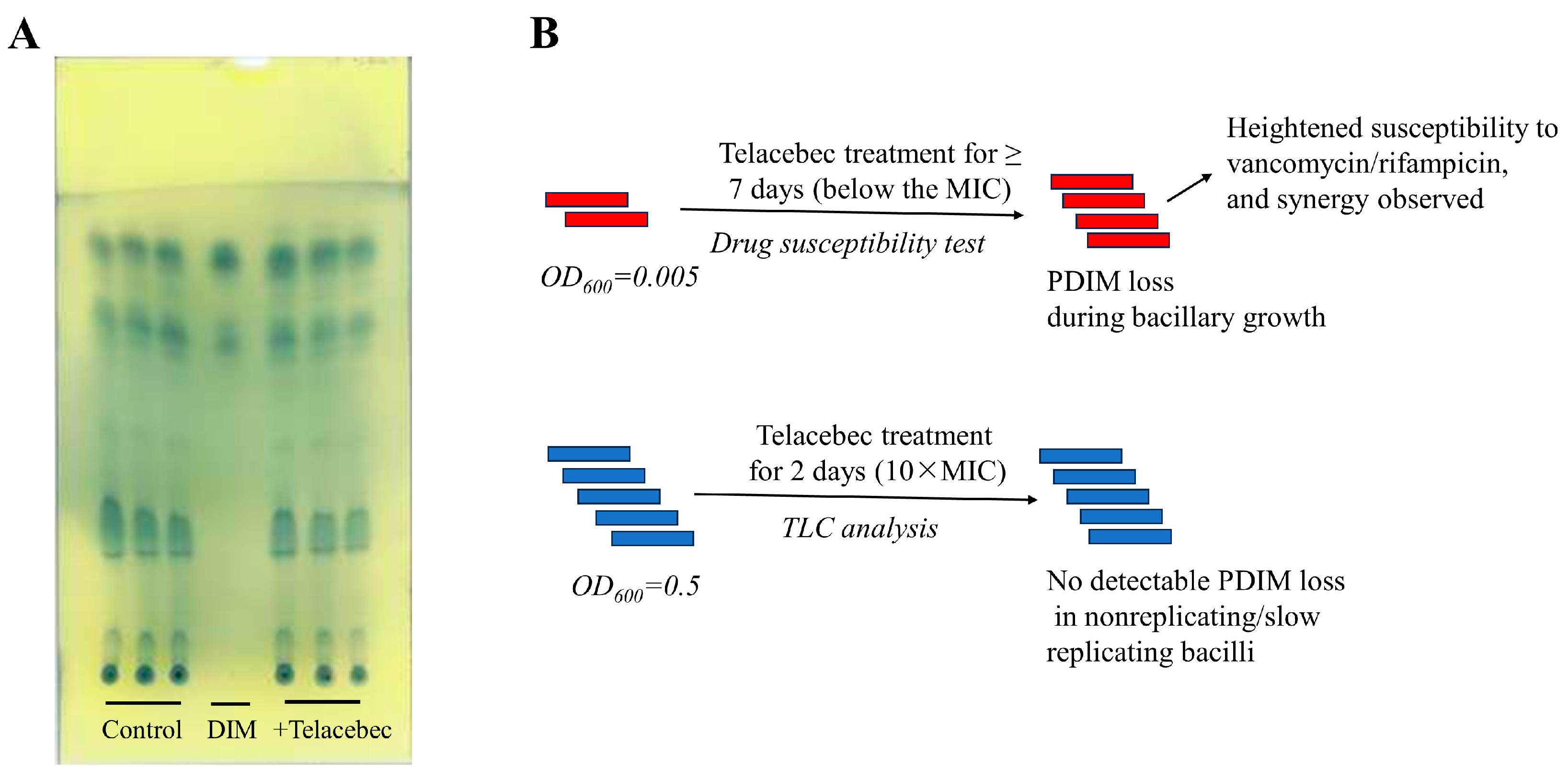
4.1. Effect of Telacebec on PDIMs: A Point of Metabolic Vulnerability
4.2. Other Proteomic Signatures by Telacebec
4.3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- Cook, G.M.; Hards, K.; Vilcheze, C.; Hartman, T.; Berney, M. Energetics of Respiration and Oxidative Phosphorylation in Mycobacteria. Microbiol. Spectr. 2014, 2, 389–409. [Google Scholar] [CrossRef]
- Pethe, K.; Bifani, P.; Jang, J.; Kang, S.; Park, S.; Ahn, S.; Jiricek, J.; Jung, J.; Jeon, H.K.; Cechetto, J.; et al. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat. Med. 2013, 19, 1157–1160. [Google Scholar] [CrossRef]
- Yanofsky, D.J.; Di Trani, J.M.; Krol, S.; Abdelaziz, R.; Bueler, S.A.; Imming, P.; Brzezinski, P.; Rubinstein, J.L. Structure of mycobacterial CIII(2)CIV(2) respiratory supercomplex bound to the tuberculosis drug candidate telacebec (Q203). Elife 2021, 10, e71959. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, W.; Zhou, X.; Zhang, Y.; Lai, Y.; Tang, Y.; Xu, J.; Li, D.; Lin, J.; Yang, X.; et al. Structure of Mycobacterium tuberculosis cytochrome bcc in complex with Q203 and TB47, two anti-TB drug candidates. Elife 2021, 10, e69418. [Google Scholar] [CrossRef]
- Lee, B.S.; Hards, K.; Engelhart, C.A.; Hasenoehrl, E.J.; Kalia, N.P.; Mackenzie, J.S.; Sviriaeva, E.; Chong, S.M.S.; Manimekalai, M.S.S.; Koh, V.H.; et al. Dual inhibition of the terminal oxidases eradicates antibiotic-tolerant Mycobacterium tuberculosis. EMBO Mol. Med. 2021, 13, e13207. [Google Scholar] [CrossRef]
- Zeng, S.; Soetaert, K.; Ravon, F.; Vandeput, M.; Bald, D.; Kauffmann, J.M.; Mathys, V.; Wattiez, R.; Fontaine, V. Isoniazid Bactericidal Activity Involves Electron Transport Chain Perturbation. Antimicrob. Agents Chemother. 2019, 63, e01841-18. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, J.; Sun, M.; Zhang, X.; Cook, G.M.; Zhang, T. Nitric Oxide-Dependent Electron Transport Chain Inhibition by the Cytochrome bc1 Inhibitor and Pretomanid Combination Kills Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e0095621. [Google Scholar] [CrossRef]
- Jeffreys, L.N.; Ardrey, A.; Hafiz, T.A.; Dyer, L.A.; Warman, A.J.; Mosallam, N.; Nixon, G.L.; Fisher, N.E.; Hong, W.D.; Leung, S.C.; et al. Identification of 2-Aryl-Quinolone Inhibitors of Cytochrome bd and Chemical Validation of Combination Strategies for Respiratory Inhibitors against Mycobacterium tuberculosis. ACS Infect. Dis. 2023, 9, 221–238. [Google Scholar] [CrossRef]
- Rens, C.; Laval, F.; Daffe, M.; Denis, O.; Frita, R.; Baulard, A.; Wattiez, R.; Lefevre, P.; Fontaine, V. Effects of Lipid-Lowering Drugs on Vancomycin Susceptibility of Mycobacteria. Antimicrob. Agents Chemother. 2016, 60, 6193–6199. [Google Scholar] [CrossRef]
- Zeng, S.; Constant, P.; Yang, D.; Baulard, A.; Lefevre, P.; Daffe, M.; Wattiez, R.; Fontaine, V. Cpn60.1 (GroEL1) Contributes to Mycobacterial Crabtree Effect: Implications for Biofilm Formation. Front. Microbiol. 2019, 10, 1149. [Google Scholar] [CrossRef]
- Soetaert, K.; Rens, C.; Wang, X.M.; De Bruyn, J.; Laneelle, M.A.; Laval, F.; Lemassu, A.; Daffe, M.; Bifani, P.; Fontaine, V.; et al. Increased Vancomycin Susceptibility in Mycobacteria: A New Approach To Identify Synergistic Activity against Multidrug-Resistant Mycobacteria. Antimicrob. Agents Chemother. 2015, 59, 5057–5060. [Google Scholar] [CrossRef] [PubMed]
- Dulberger, C.L.; Rubin, E.J.; Boutte, C.C. The mycobacterial cell envelope—A moving target. Nat. Rev. Microbiol. 2020, 18, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Cambier, C.J.; Banik, S.M.; Buonomo, J.A.; Bertozzi, C.R. Spreading of a mycobacterial cell-surface lipid into host epithelial membranes promotes infectivity. Elife 2020, 9, e60648. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.R.; Constant, P.; Raynaud, C.; Laneelle, M.A.; Triccas, J.A.; Gicquel, B.; Daffe, M.; Guilhot, C. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 2001, 276, 19845–19854. [Google Scholar] [CrossRef]
- De Majumdar, S.; Sikri, K.; Ghosh, P.; Jaisinghani, N.; Nandi, M.; Gandotra, S.; Mande, S.; Tyagi, J.S. Genome analysis identifies a spontaneous nonsense mutation in ppsD leading to attenuation of virulence in laboratory-manipulated Mycobacterium tuberculosis. BMC Genom. 2019, 20, 129. [Google Scholar] [CrossRef]
- Cox, J.S.; Chen, B.; McNeil, M.; Jacobs, W.R., Jr. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 1999, 402, 79–83. [Google Scholar] [CrossRef]
- Osman, M.M.; Pagan, A.J.; Shanahan, J.K.; Ramakrishnan, L. Mycobacterium marinum phthiocerol dimycocerosates enhance macrophage phagosomal permeabilization and membrane damage. PLoS ONE 2020, 15, e0233252. [Google Scholar] [CrossRef]
- Astarie-Dequeker, C.; Le Guyader, L.; Malaga, W.; Seaphanh, F.K.; Chalut, C.; Lopez, A.; Guilhot, C. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 2009, 5, e1000289. [Google Scholar] [CrossRef]
- Quigley, J.; Hughitt, V.K.; Velikovsky, C.A.; Mariuzza, R.A.; El-Sayed, N.M.; Briken, V. The Cell Wall Lipid PDIM Contributes to Phagosomal Escape and Host Cell Exit of Mycobacterium tuberculosis. MBio 2017, 8, e00148-17. [Google Scholar] [CrossRef]
- Mohandas, P.; Budell, W.C.; Mueller, E.; Au, A.; Bythrow, G.V.; Quadri, L.E. Pleiotropic consequences of gene knockouts in the phthiocerol dimycocerosate and phenolic glycolipid biosynthetic gene cluster of the opportunistic human pathogen Mycobacterium marinum. FEMS Microbiol. Lett. 2016, 363, fnw016. [Google Scholar] [CrossRef]
- Nguyen, P.C.; Nguyen, V.S.; Martin, B.P.; Fourquet, P.; Camoin, L.; Spilling, C.D.; Cavalier, J.F.; Cambillau, C.; Canaan, S. Biochemical and Structural Characterization of TesA, a Major Thioesterase Required for Outer-Envelope Lipid Biosynthesis in Mycobacterium tuberculosis. J. Mol. Biol. 2018, 430, 5120–5136. [Google Scholar] [CrossRef]
- Snewin, V.A.; Gares, M.P.; Gaora, P.O.; Hasan, Z.; Brown, I.N.; Young, D.B. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect. Immun. 1999, 67, 4586–4593. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy assessed by checkerboard. A critical analysis. Diagn. Microbiol. Infect Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Deschoenmaeker, F.; Bayon-Vicente, G.; Sachdeva, N.; Depraetere, O.; Cabrera Pino, J.C.; Leroy, B.; Muylaert, K.; Wattiez, R. Impact of different nitrogen sources on the growth of Arthrospira sp. PCC 8005 under batch and continuous cultivation—A biochemical, transcriptomic and proteomic profile. Bioresour. Technol. 2017, 237, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, Y.; Kim, Y.; Kim, S.; Kim, J.J.; Kim, K.M.; Yoshizawa, J.; Fan, L.Y.; Cao, C.X.; Wong, D.T. Differential Proteomic Analysis of Human Saliva using Tandem Mass Tags Quantification for Gastric Cancer Detection. Sci. Rep. 2016, 6, 22165. [Google Scholar] [CrossRef]
- Xu, C.G.; Yang, Y.B.; Zhou, Y.H.; Hao, M.Q.; Ren, Y.Z.; Wang, X.T.; Chen, J.Q.; Muhammad, I.; Wang, S.; Liu, D.; et al. Comparative Proteomic Analysis Provides insight into the Key Proteins as Possible Targets Involved in Aspirin Inhibiting Biofilm Formation of Staphylococcus xylosus. Front. Pharmacol. 2017, 8, 543. [Google Scholar] [CrossRef]
- Cortes, T.; Schubert, O.T.; Rose, G.; Arnvig, K.B.; Comas, I.; Aebersold, R.; Young, D.B. Genome-wide mapping of transcriptional start sites defines an extensive leaderless transcriptome in Mycobacterium tuberculosis. Cell Rep. 2013, 5, 1121–1131. [Google Scholar] [CrossRef]
- Yu, J.; Tran, V.; Li, M.; Huang, X.; Niu, C.; Wang, D.; Zhu, J.; Wang, J.; Gao, Q.; Liu, J. Both phthiocerol dimycocerosates and phenolic glycolipids are required for virulence of Mycobacterium marinum. Infect. Immun. 2012, 80, 1381–1389. [Google Scholar] [CrossRef]
- Lamprecht, D.A.; Finin, P.M.; Rahman, M.A.; Cumming, B.M.; Russell, S.L.; Jonnala, S.R.; Adamson, J.H.; Steyn, A.J. Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat. Commun. 2016, 7, 12393. [Google Scholar] [CrossRef]
- Wang, Z.; Soni, V.; Marriner, G.; Kaneko, T.; Boshoff, H.I.M.; Barry, C.E., 3rd; Rhee, K.Y. Mode-of-action profiling reveals glutamine synthetase as a collateral metabolic vulnerability of M. tuberculosis to bedaquiline. Proc. Natl. Acad. Sci. USA 2019, 116, 19646–19651. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Lamprecht, D.A.; Asmal, R.; Adamson, J.H.; Borah, K.; Beste, D.J.V.; Lee, B.S.; Pethe, K.; Rousseau, S.; Krieger, I.; et al. Bedaquiline reprograms central metabolism to reveal glycolytic vulnerability in Mycobacterium tuberculosis. Nat. Commun. 2020, 11, 6092. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.P.; Hasenoehrl, E.J.; Ab Rahman, N.B.; Koh, V.H.; Ang, M.L.T.; Sajorda, D.R.; Hards, K.; Gruber, G.; Alonso, S.; Cook, G.M.; et al. Exploiting the synthetic lethality between terminal respiratory oxidases to kill Mycobacterium tuberculosis and clear host infection. Proc. Natl. Acad. Sci. USA 2017, 114, 7426–7431. [Google Scholar] [CrossRef] [PubMed]
- Lupien, A.; Foo, C.S.; Savina, S.; Vocat, A.; Piton, J.; Monakhova, N.; Benjak, A.; Lamprecht, D.A.; Steyn, A.J.C.; Pethe, K.; et al. New 2-Ethylthio-4-methylaminoquinazoline derivatives inhibiting two subunits of cytochrome bc1 in Mycobacterium tuberculosis. PLoS Pathog. 2020, 16, e1008270. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.C.; Lukey, P.T.; Robb, L.C.; McAdam, R.A.; Duncan, K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 2002, 43, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Gengenbacher, M.; Rao, S.P.S.; Pethe, K.; Dick, T. Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology 2010, 156, 81–87. [Google Scholar] [CrossRef]
- Koul, A.; Vranckx, L.; Dhar, N.; Gohlmann, H.W.; Ozdemir, E.; Neefs, J.M.; Schulz, M.; Lu, P.; Mortz, E.; McKinney, J.D.; et al. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat. Commun. 2014, 5, 3369. [Google Scholar] [CrossRef]
- Flentie, K.N.; Stallings, C.L.; Turk, J.; Minnaard, A.J.; Hsu, F.F. Characterization of phthiocerol and phthiodiolone dimycocerosate esters of M. tuberculosis by multiple-stage linear ion-trap MS. J. Lipid. Res. 2016, 57, 142–155. [Google Scholar] [CrossRef]
- Block, A.M.; Namugenyi, S.B.; Palani, N.P.; Brokaw, A.M.; Zhang, L.; Beckman, K.B.; Tischler, A.D. Mycobacterium tuberculosis Requires the Outer Membrane Lipid Phthiocerol Dimycocerosate for Starvation-Induced Antibiotic Tolerance. mSystems 2023, 8, e0069922. [Google Scholar] [CrossRef]
- Woodworth, J.S.; Clemmensen, H.S.; Battey, H.; Dijkman, K.; Lindenstrom, T.; Laureano, R.S.; Taplitz, R.; Morgan, J.; Aagaard, C.; Rosenkrands, I.; et al. A Mycobacterium tuberculosis-specific subunit vaccine that provides synergistic immunity upon co-administration with Bacillus Calmette-Guerin. Nat. Commun. 2021, 12, 6658. [Google Scholar] [CrossRef]
- Cambier, C.J.; Takaki, K.K.; Larson, R.P.; Hernandez, R.E.; Tobin, D.M.; Urdahl, K.B.; Cosma, C.L.; Ramakrishnan, L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 2014, 505, 218–222. [Google Scholar] [CrossRef]
- de Jager, V.R.; Dawson, R.; van Niekerk, C.; Hutchings, J.; Kim, J.; Vanker, N.; van der Merwe, L.; Choi, J.; Nam, K.; Diacon, A.H. Telacebec (Q203), a New Antituberculosis Agent. N. Engl. J. Med. 2020, 382, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Slayden, R.A.; Knudson, D.L.; Belisle, J.T. Identification of cell cycle regulators in Mycobacterium tuberculosis by inhibition of septum formation and global transcriptional analysis. Microbiology 2006, 152, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Mavrici, D.; Marakalala, M.J.; Holton, J.M.; Prigozhin, D.M.; Gee, C.L.; Zhang, Y.J.; Rubin, E.J.; Alber, T. Mycobacterium tuberculosis FtsX extracellular domain activates the peptidoglycan hydrolase, RipC. Proc. Natl. Acad. Sci. USA 2014, 111, 8037–8042. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Lun, S.; Guo, H.; Ammerman, N.C.; Geiman, D.E.; Bishai, W.R. Rv2190c, an NlpC/P60 family protein, is required for full virulence of Mycobacterium tuberculosis. PLoS ONE 2012, 7, e43429. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Guinn, K.M.; Harrell, M.I.; Liao, R.; Voskuil, M.I.; Tompa, M.; Schoolnik, G.K.; Sherman, D.R. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 2003, 48, 833–843. [Google Scholar] [CrossRef]
- Boon, C.; Dick, T. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 2002, 184, 6760–6767. [Google Scholar] [CrossRef]
- Zheng, H.; Colvin, C.J.; Johnson, B.K.; Kirchhoff, P.D.; Wilson, M.; Jorgensen-Muga, K.; Larsen, S.D.; Abramovitch, R.B. Inhibitors of Mycobacterium tuberculosis DosRST signaling and persistence. Nat. Chem. Biol. 2017, 13, 218–225. [Google Scholar] [CrossRef]
- Kaur, D.; Kutum, R.; Dash, D.; Brahmachari, S.K. Data Intensive Genome Level Analysis for Identifying Novel, Non-Toxic Drug Targets for Multi Drug Resistant Mycobacterium tuberculosis. Sci. Rep. 2017, 7, 46595. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Shi, W.; Liu, W.; Zhang, W.; Zhang, Y. Mutations in panD encoding aspartate decarboxylase are associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg. Microbes. Infect. 2013, 2, e34. [Google Scholar] [CrossRef]
- Leonardi, R.; Jackowski, S. Biosynthesis of Pantothenic Acid and Coenzyme A. EcoSal. Plus 2007, 2, 10-1128. [Google Scholar] [CrossRef]
- Foo, C.S.; Lupien, A.; Kienle, M.; Vocat, A.; Benjak, A.; Sommer, R.; Lamprecht, D.A.; Steyn, A.J.C.; Pethe, K.; Piton, J.; et al. Arylvinylpiperazine Amides, a New Class of Potent Inhibitors Targeting QcrB of Mycobacterium tuberculosis. mBio 2018, 9, e01276-18. [Google Scholar] [CrossRef] [PubMed]
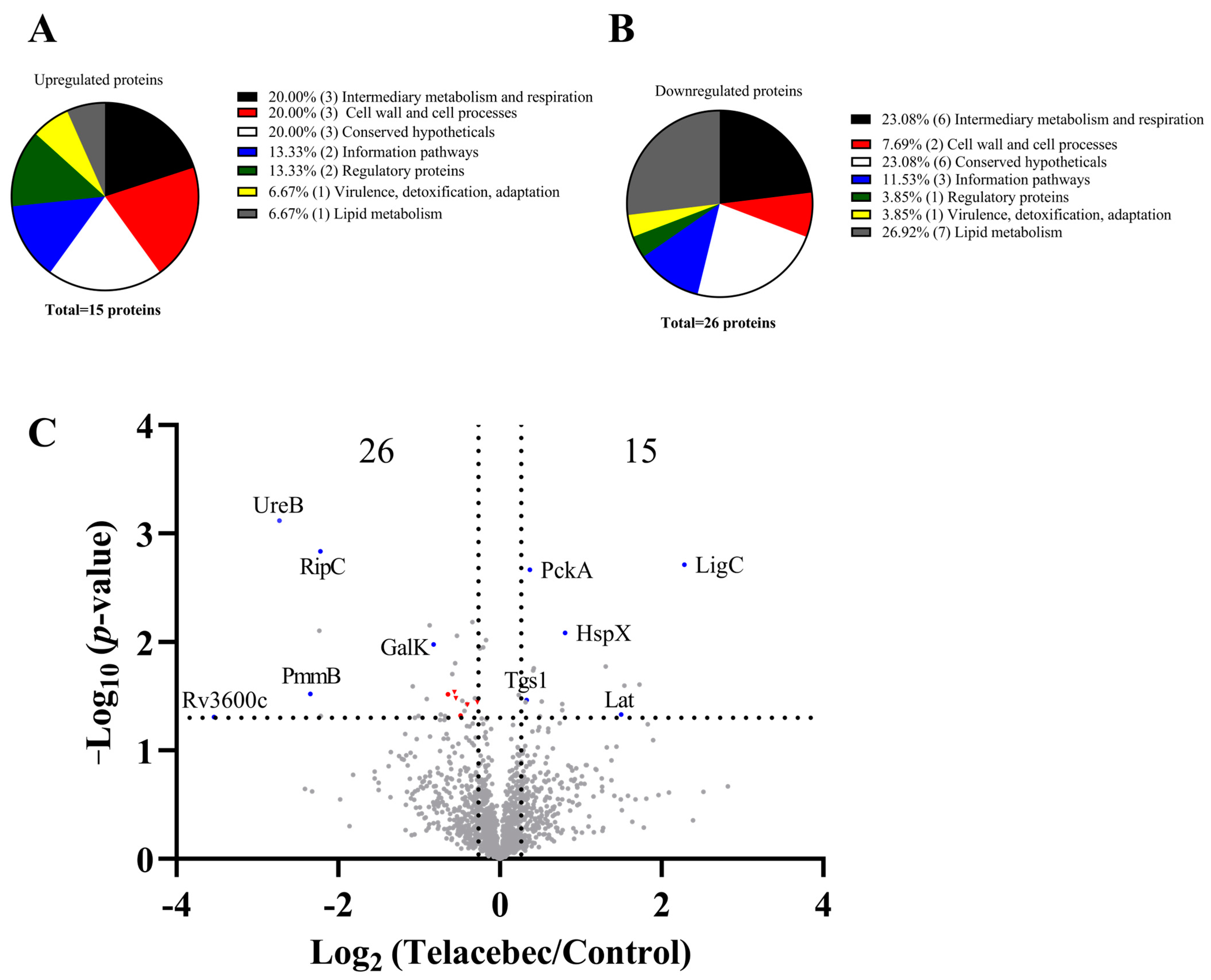

| BCG Homology Proteins in H37Rv | Name | Description | p-Value | Fold Change (Telacebec/Control) | |
|---|---|---|---|---|---|
| Cell wall biosynthesis and cell process | Rv2950c | FadD29 | Long-chain-fatty-acid-AMP ligase | 0.02887 | 0.677371423 |
| Rv2247 | AccD6 | Acetyl-/propionyl-CoA carboxylase subunit beta | 0.03024 | 0.641045938 | |
| Rv2930 | FadD26 | Fatty-acid-AMP ligase | 0.03297 | 0.685370986 | |
| Rv3130c | Tgs1 | Diacylglycerol O-acyltransferase | 0.03431 | 1.258329713 | |
| Rv2524c | Fas | Fatty acid synthase | 0.03723 | 0.754112466 | |
| Rv2932 | PpsB | Phthiocerol synthesis polyketide synthase type I | 0.03774 | 0.755835038 | |
| Rv2190c | RipC | Peptidoglycan peptidase | 0.00146 | 0.214892227 | |
| Rv3484 | CpsA2 | Polysaccharide transfer to peptidoglycan | 0.00653 | 0.789836749 | |
| Rv3835 | NA | Hypothetical protein in the cell wall | 0.00786 | 0.212690355 | |
| Rv3915 | NA | Peptidoglycan hydrolase | 0.01965 | 0.664403424 | |
| Metabolic pathways | Rv0211 | PckA | Phosphoenolpyruvate carboxykinase | 0.00217 | 1.294218998 |
| Rv0620 | GalK | Galactokinase | 0.01055 | 0.566918099 | |
| Rv3308 | PmmB | Phosphomannomutase; converts D-mannose 1-phosphate to D-mannose 6-phosphate | 0.0301 | 0.196754059 | |
| Rv3600c | NA | Type III pantothenate kinase catalyzing the phosphorylation of pantothenate, the first step in CoA biosynthesis | 0.04899 | 0.086255116 | |
| Rv1589 | BioB | Biotin synthetase | 0.01568 | 0.681640846 | |
| Rv0991c | NA | Redox-regulated molecular chaperone | 0.00875 | 0.693003332 | |
| Rv0758 | PhoR | Two-component system response sensor kinase | 0.0358 | 1.247705251 | |
| Virulence | Rv0485 | NA | Transcriptional regulator of pe13 and ppe18 gene pair | 0.04811 | 0.497470112 |
| Rv0981 | MprA | Two-component response regulator | 0.04824 | 1.407815183 | |
| Rv2031c | HspX | Alpha-crystallin | 0.00824 | 1.749009088 | |
| Rv0726c | NA | Possible methyltransferase associated with isoniazid resistance | 0.00699 | 0.547160643 | |
| Rv3867 | EspH | ESX-1 secretion-associated protein | 0.04805 | 0.625076945 | |
| Rv3614c | EspD | ESX-1 secretion-associated protein | 0.03731 | 1.704031265 | |
| DNA/RNA metabolism | Rv1629 | PolA | DNA polymerase I | 0.01742 | 1.338907985 |
| Rv1641 | InfC | Initiation factor IF-3 | 0.03486 | 0.725560396 | |
| Rv3731 | LigC | DNA ligase C | 0.00194 | 4.862120018 | |
| Others | Rv1287 | NA | HTH-type transcriptional regulator | 0.02526 | 2.906914209 |
| Rv3200c | NA | Transmembrane cation transporter | 0.01681 | 2.480604421 | |
| Rv1072 | NA | Transmembrane protein | 0.0353 | 1.432802446 | |
| Rv1849 | UreB | Urease subunit beta | 0.00076 | 0.150994385 | |
| Rv3011c | GatA | Glutamyl-tRNA amidotransferase subunit A | 0.04319 | 0.738761056 | |
| Rv3290c | Lat | L-lysine-epsilon aminotransferase; catalytic activity: L-lysine + 2-oxoglutarate = 2-aminoadipate 6-semialdehyde + L-glutamate | 0.04644 | 2.83040088 |
| Treatment | MIC/FIC/FICI | |
|---|---|---|
| Vancomycin (μg/mL) | Telacebec (nM) | |
| Alone | >200/-/- | 5/-/- |
| +Telacebec (nM) | 5/<0.025/<0.275 | -/-/- |
| +Vancomycin (μg/mL) | -/-/- | 1.25/<0.25/<0.275 |
| Treatment | MIC/FIC/FICI | |
|---|---|---|
| Rifampicin (μg/mL) | Telacebec (nM) | |
| Alone | 0.5/-/- | 5/-/- |
| +Telacebec (nM) | 0.0625/0.125/0.375 | -/-/- |
| +Rifampicin (μg/mL) | -/-/- | 1.25/0.25/0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Wattiez, R.; Constant, P.; Marrakchi, H.; Soetaert, K.; Mathys, V.; Fontaine, V.; Zeng, S. Telacebec Interferes with Virulence Lipid Biosynthesis Protein Expression and Sensitizes to Other Antibiotics. Microorganisms 2023, 11, 2469. https://doi.org/10.3390/microorganisms11102469
Zhou Z, Wattiez R, Constant P, Marrakchi H, Soetaert K, Mathys V, Fontaine V, Zeng S. Telacebec Interferes with Virulence Lipid Biosynthesis Protein Expression and Sensitizes to Other Antibiotics. Microorganisms. 2023; 11(10):2469. https://doi.org/10.3390/microorganisms11102469
Chicago/Turabian StyleZhou, Zhiyu, Ruddy Wattiez, Patricia Constant, Hedia Marrakchi, Karine Soetaert, Vanessa Mathys, Véronique Fontaine, and Sheng Zeng. 2023. "Telacebec Interferes with Virulence Lipid Biosynthesis Protein Expression and Sensitizes to Other Antibiotics" Microorganisms 11, no. 10: 2469. https://doi.org/10.3390/microorganisms11102469
APA StyleZhou, Z., Wattiez, R., Constant, P., Marrakchi, H., Soetaert, K., Mathys, V., Fontaine, V., & Zeng, S. (2023). Telacebec Interferes with Virulence Lipid Biosynthesis Protein Expression and Sensitizes to Other Antibiotics. Microorganisms, 11(10), 2469. https://doi.org/10.3390/microorganisms11102469








