Effects of Luteolin-7-O-Glucoside on Intestinal Microbiota Dysbiosis and Drug Resistance Transmission Caused by Raoultella ornithinolytica B1645-1: Modulating the Composition of Intestinal Microbiota and Promoting the Transfer of blaNDM-1 Gene from Genus Enterococcus to Lactobacillus in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Reagents
2.2. Animals and Sample Preparation
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Microbial Diversity Analysis
2.5. Screenation and Identification of Strains Carrying BlaNDM-1 Gene
2.6. Statistical Analysis
3. Results
3.1. The Diversity of Intestinal Microbiota
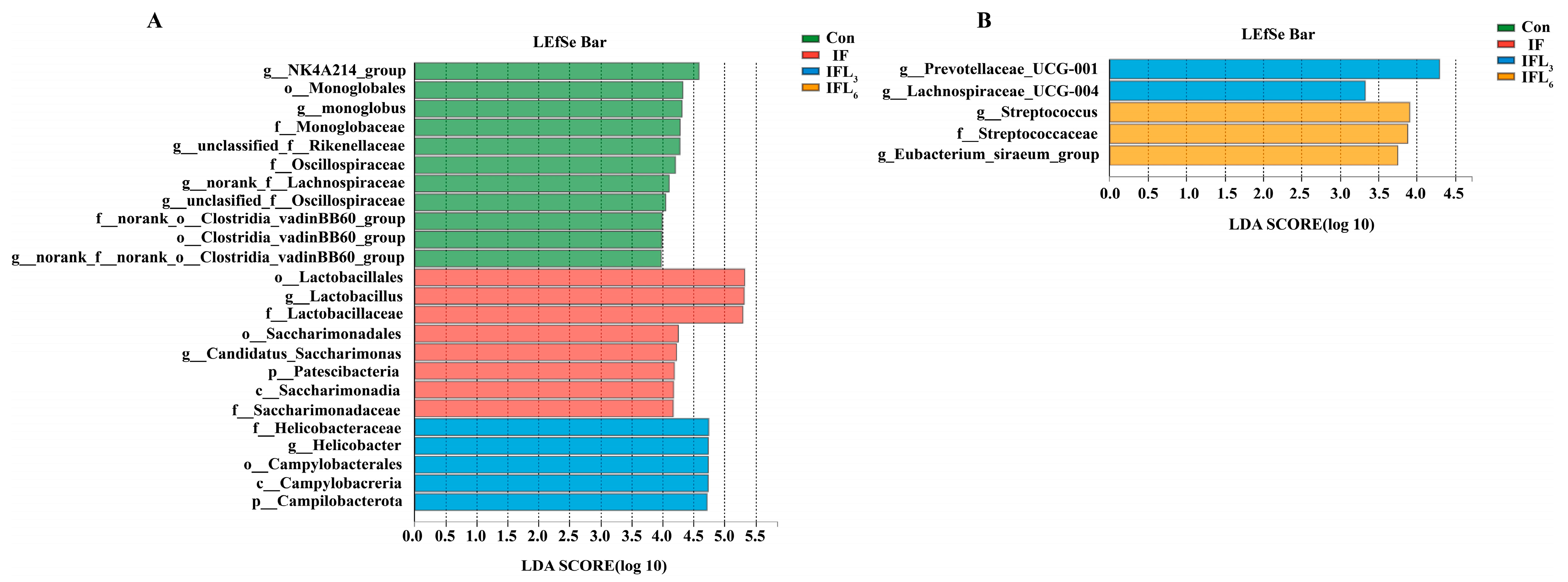
3.2. Taxonomic Comparison of Intestinal Microbiota
3.3. Transmission of BlaNDM-1 Gene in Intestinal Microbiota
3.3.1. Screening of Imipenem-Resistant Strains
3.3.2. Identification of Strains Carrying BlaNDM-1 Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Gu, S.; Guo, L.; Xia, X.; Zhang, H.; Wang, J. Preparative separation of five flavones from flowers of Polygonum cuspidatum by high-speed countercurrent chromatography. J. Sep. Sci. 2014, 37, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kitts, D.D. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol. Cell Biochem. 2004, 265, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, S.; Wang, W.; Hong, Y.; Tang, K.; Luo, Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem. 2013, 138, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Demachi, A.; Takenaka, A. Protective Effects of Hot Water Extract of Safflower Leaves and Its Component Luteolin-7-O-Glucoside on Paraquat-Induced Oxidative Stress in Rats. Food Sci. Technol. Res. 2001, 7, 224–230. [Google Scholar] [CrossRef]
- Yasuda, M.T.; Fujita, K.; Hosoya, T.; Imai, S.; Shimoi, K. Absorption and Metabolism of Luteolin and Its Glycosides from the Extract of Chrysanthemum morifolium Flowers in Rats and Caco-2 Cells. J. Agric. Food Chem. 2015, 63, 7693–7699. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Seker Karatoprak, G.; Ilgun, S.; Kosar, M. Phenolic Composition, Anti-Inflammatory, Antioxidant, and Antimicrobial Activities of Alchemilla mollis (Buser) Rothm. Chem. Biodivers. 2017, 14, e1700150. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Chen, L.; Sun, G. Hypolipidemic Effects and Preliminary Mechanism of Chrysanthemum Flavonoids, Its Main Components Luteolin and Luteoloside in Hyperlipidemia Rats. Antioxidants 2021, 10, 1309. [Google Scholar] [CrossRef]
- Rump, A.F.; Schüssler, M.; Acar, D.; Cordes, A.; Theisohn, M.; Rösen, R.; Klaus, W.; Fricke, U. Functional and antiischemic effects of luteolin-7-glucoside in isolated rabbit hearts. Gen Pharmacol. 1994, 25, 1137–1142. [Google Scholar] [CrossRef]
- de Oliveira, D.P.; de Almeida, L.; Marques, M.J.; de Carvalho, R.R.; Dias, A.L.T.; da Silva, G.A.; de Pádua, R.M.; Braga, F.C.; da Silva, M.A. Exploring the bioactivity potential of Leonotisnepetifolia: Phytochemical composition, antimicrobial and antileishmanial activities of extracts from different anatomical parts. Nat. Prod. Res. 2021, 35, 3120–3125. [Google Scholar] [CrossRef]
- Hawksworth, G.; Drasar, B.S.; Hill, M.J. Intestinal bacteria and the hydrolysis of glycosidic bonds. J. Med. Microbiol. 1971, 4, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, N.B.; Hemalatha, R.; Uppala, S.; Yathapu, S.R.; Mohammed, S.; Venkata Surekha, M.; Rajendran, A.; Bharadwaj, D.K. Ocimum sanctum, Zingiber officinale, and Piper nigrum extracts and their effects on gut microbiota modulations (prebiotic potential), basal inflammatory markers and lipid levels: Oral supplementation study in healthy rats. Pharm. Biol. 2022, 60, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Chiruvella, K.K.; Mohammed, A.; Dampuri, G.; Ghanta, R.G.; Raghavan, S.C. Phytochemical and Antimicrobial Studies of Methyl Angolensate and Luteolin-7-O-glucoside Isolated from Callus Cultures of Soymidafebrifuga. Int. J. Biomed. Sci. 2007, 3, 269–278. [Google Scholar]
- Kozyra, M.; Biernasiuk, A.; Malm, A.; Chowaniec, M. Chemical compositions and antibacterial activity of extracts obtained from the inflorescences of Cirsium canum (L.) all. Nat. Prod. Res. 2015, 29, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host. Microbe. 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Stecher, B.; Denzler, R.; Maier, L.; Bernet, F.; Sanders, M.J.; Pickard, D.J.; Barthel, M.; Westendorf, A.M.; Krogfelt, K.A.; Walker, A.W.; et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 2012, 109, 1269–1274. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Bakkeren, E.; Huisman, J.S.; Fattinger, S.A.; Hausmann, A.; Furter, M.; Egli, A.; Slack, E.; Sellin, M.E.; Bonhoeffer, S.; Regoes, R.R.; et al. Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature 2019, 573, 276–280. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q. Acquired metallo-β-lactamases and their genetic association with class 1 integrons and ISCR elements in Gram-negative bacteria. Future Microbiol. 2015, 10, 873–887. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Carmeli, Y. Carbapenem-resistant Enterobacteriaceae: A potential threat. JAMA 2008, 300, 2911–2913. [Google Scholar] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.; Ambaraghassi, G.; Sebajang, H.; Schwenter, F.; Su, S.H. Raoultellaornithinolytica: Emergence and Resistance. Infect Drug Resist. 2020, 13, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Levorova, J.; Machon, V.; Guha, A.; Foltan, R. Septic arthritis of the temporomandibular joint caused by rare bacteria Raoultellaornithinolytica. Int. J. Oral Maxillofac. Surg. 2017, 46, 111–115. [Google Scholar] [CrossRef]
- Seng, P.; Boushab, B.M.; Romain, F.; Gouriet, F.; Bruder, N.; Martin, C.; Paganelli, F.; Bernit, E.; Le Treut, Y.P.; Thomas, P.; et al. Emerging role of Raoultellaornithinolytica in human infections: A series of cases and review of the literature. Int. J. Infect. Dis. 2016, 45, 65–71. [Google Scholar] [CrossRef]
- Bhatt, P.; Tandel, K.; Das, N.K.; Rathi, K.R. New Delhi metallo-beta-lactamase producing extensively drug-resistant Raoultellaornithinolytica isolated from drain fluid following Whipple’s pancreaticoduodenectomy. Med. J. Armed Forces India. 2015, 71 (Suppl. 2), S609–S611. [Google Scholar] [CrossRef]
- Yu, C.; Wei, X.; Wang, Z.; Liu, L.; Liu, Z.; Liu, J.; Wu, L.; Guo, H.; Jin, Z. Occurrence of two NDM-1-producing Raoultellaornithinolytica and Enterobacter cloacae in a single patient in China: Probable a novel antimicrobial resistance plasmid transfer in vivo by conjugation. J. Glob. Antimicrob. Resist. 2020, 22, 835–841. [Google Scholar] [CrossRef]
- Lavergne, C.; Bovio-Winkler, P.; Etchebehere, C.; García-Gen, S. Towards centralized biogas plants: Co-digestion of sewage sludge and pig manure maintains process performance and active microbiome diversity. Bioresour Technol. 2020, 297, 122442. [Google Scholar] [CrossRef]
- Zhang, H.; Carnevale, G.; Polese, B.; Simard, M.; Thurairajah, B.; Khan, N.; Gentile, M.E.; Fontes, G.; Vinh, D.C.; Pouliot, R.; et al. CD109 Restrains Activation of Cutaneous IL-17-Producing γδ T Cells by Commensal Microbiota. Cell Rep. 2019, 29, 391–405.e5. [Google Scholar] [CrossRef]
- Kim, J.Y.; Whon, T.W.; Lim, M.Y.; Kim, Y.B.; Kim, N.; Kwon, M.S.; Kim, J.; Lee, S.H.; Choi, H.J.; Nam, I.H.; et al. The human gut archaeome: Identification of diverse haloarchaea in Korean subjects. Microbiome 2020, 8, 114. [Google Scholar] [CrossRef]
- Pi, X.; Yu, Z.; Yang, X.; Du, Z.; Liu, W. Effects of Zymosan on Short-Chain Fatty Acid and Gas Production in in vitro Fermentation Models of the Human Intestinal Microbiota. Front Nutr. 2022, 9, 921137. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Y.; Yan, L.; Sun, C.; Miao, Q.; Wang, Q.; Xiao, X.; Lian, M.; Li, B.; Chen, Y.; et al. Alterations of gut microbiome in autoimmune hepatitis. Gut 2020, 69, 569–577. [Google Scholar] [CrossRef]
- Foster, A.W.; Clough, S.E.; Aki, Z.; Young, T.R.; Clarke, A.R.; Robinson, N.J. Metalation calculators for E. coli strain JM109 (DE3): Aerobic, anaerobic, and hydrogen peroxide exposed cells cultured in LB media. Metallomics 2022, 14, mfac058. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.Y.; Da’na, D.A.; Al-Ghouti, M.A. Application of MALDI-TOF MS for identification of environmental bacteria: A review. J. Environ. Manag. 2022, 305, 114359. [Google Scholar] [CrossRef] [PubMed]
- Orhan, E.O.; Irmak, O.; Ertuğrul, I.F. Kinematics of a novel reciprocating endodontic handpiece. Int. Endod. J. 2019, 52, 1235–1243. [Google Scholar] [CrossRef]
- Sato, N.; Kakuta, M.; Hasegawa, T.; Yamaguchi, R.; Uchino, E.; Murashita, K.; Nakaji, S.; Imoto, S.; Yanagita, M.; Okuno, Y. Metagenomic profiling of gut microbiome in early chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 1675–1684. [Google Scholar] [CrossRef]
- Leon-Sampedro, R.; DelaFuente, J.; Diaz-Agero, C.; Crellen, T.; Musicha, P.; Rodriguez-Beltran, J.; de la Vega, C.; Hernandez-Garcia, M.; Group, R.G.W.S.; Lopez-Fresnena, N.; et al. Pervasive transmission of a carbapenem resistance plasmid in the gut microbiota of hospitalized patients. Nat. Microbiol. 2021, 6, 606–616. [Google Scholar] [CrossRef]
- Goldstein, E.J.; Tyrrell, K.L.; Citron, D.M. Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S98–S107. [Google Scholar] [CrossRef]





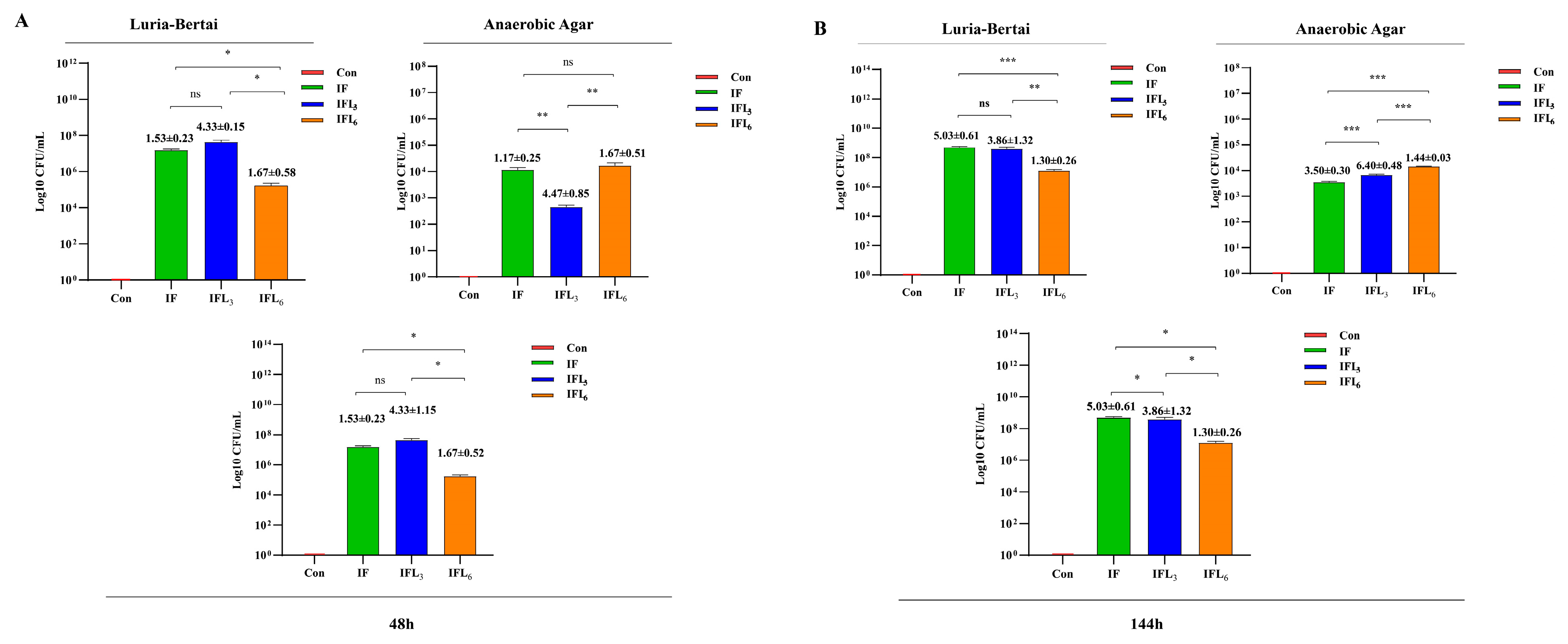
| Time | Groups | IPM | Species | |
|---|---|---|---|---|
| Luria–Bertani Agar | Anaerobic Agar | |||
| 48 h | Con | 4 μg/mL | ---- | ---- |
| IF | Enterococcus faecalis, E. gallinarum, Acinetobacter baumannii, Klebsiella pneumoniae | ---- | ||
| IFL3 | E. faecalis, E. gallinarum, Escherichia coli, Bacillus pumilus, Brevibacterium linens, K. pneumoniae | Lactobacillus johnsonii | ||
| IFL6 | E. faecalis, E. gallinarum E. coli, K. pneumoniae | ---- | ||
| 144 h | Con | 4 μg/mL | ---- | ---- |
| IF | E. faecalis, Chromobacterium violaceum | L. johnsonii, L. murinus, L. reuteri | ||
| IFL3 | Microbacterium sp., E. coli | L. johnsonii, L. gasseri, L. murinus, L. reuteri | ||
| IFL6 | E. faecalis | L. gasseri, L. johnsonii, L. reuteri | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Gou, R.; Sha, L.; Yu, C.; Meng, L.; Jin, Z. Effects of Luteolin-7-O-Glucoside on Intestinal Microbiota Dysbiosis and Drug Resistance Transmission Caused by Raoultella ornithinolytica B1645-1: Modulating the Composition of Intestinal Microbiota and Promoting the Transfer of blaNDM-1 Gene from Genus Enterococcus to Lactobacillus in Mice. Microorganisms 2023, 11, 2477. https://doi.org/10.3390/microorganisms11102477
Wu Z, Gou R, Sha L, Yu C, Meng L, Jin Z. Effects of Luteolin-7-O-Glucoside on Intestinal Microbiota Dysbiosis and Drug Resistance Transmission Caused by Raoultella ornithinolytica B1645-1: Modulating the Composition of Intestinal Microbiota and Promoting the Transfer of blaNDM-1 Gene from Genus Enterococcus to Lactobacillus in Mice. Microorganisms. 2023; 11(10):2477. https://doi.org/10.3390/microorganisms11102477
Chicago/Turabian StyleWu, Zhaomeng, Ronghui Gou, Longhua Sha, Chunfang Yu, Lixue Meng, and Zhixiong Jin. 2023. "Effects of Luteolin-7-O-Glucoside on Intestinal Microbiota Dysbiosis and Drug Resistance Transmission Caused by Raoultella ornithinolytica B1645-1: Modulating the Composition of Intestinal Microbiota and Promoting the Transfer of blaNDM-1 Gene from Genus Enterococcus to Lactobacillus in Mice" Microorganisms 11, no. 10: 2477. https://doi.org/10.3390/microorganisms11102477
APA StyleWu, Z., Gou, R., Sha, L., Yu, C., Meng, L., & Jin, Z. (2023). Effects of Luteolin-7-O-Glucoside on Intestinal Microbiota Dysbiosis and Drug Resistance Transmission Caused by Raoultella ornithinolytica B1645-1: Modulating the Composition of Intestinal Microbiota and Promoting the Transfer of blaNDM-1 Gene from Genus Enterococcus to Lactobacillus in Mice. Microorganisms, 11(10), 2477. https://doi.org/10.3390/microorganisms11102477






