The Changes of Phyllosphere Fungal Communities among Three Different Populus spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Experimental Field Site
2.2. Collection and Processing of the Samples
2.3. DNA Extraction and Fungal ITS rRNA Gene Amplification
2.4. Determination of Leaves Chemical Properties
2.5. Statistical Analysis
3. Results
3.1. Changes in the Chemical Traits of the Leaves in Three Different Populus spp.
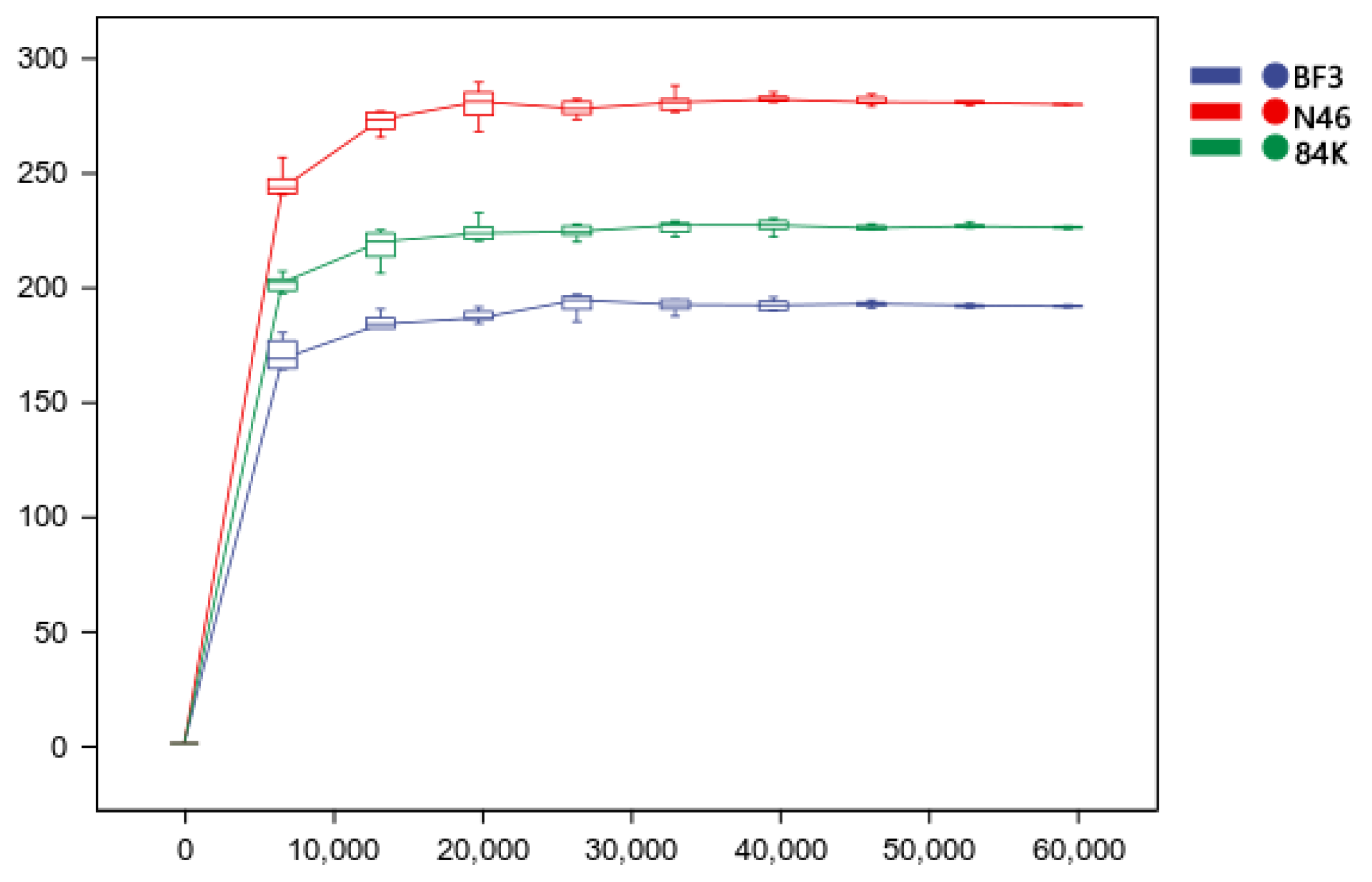
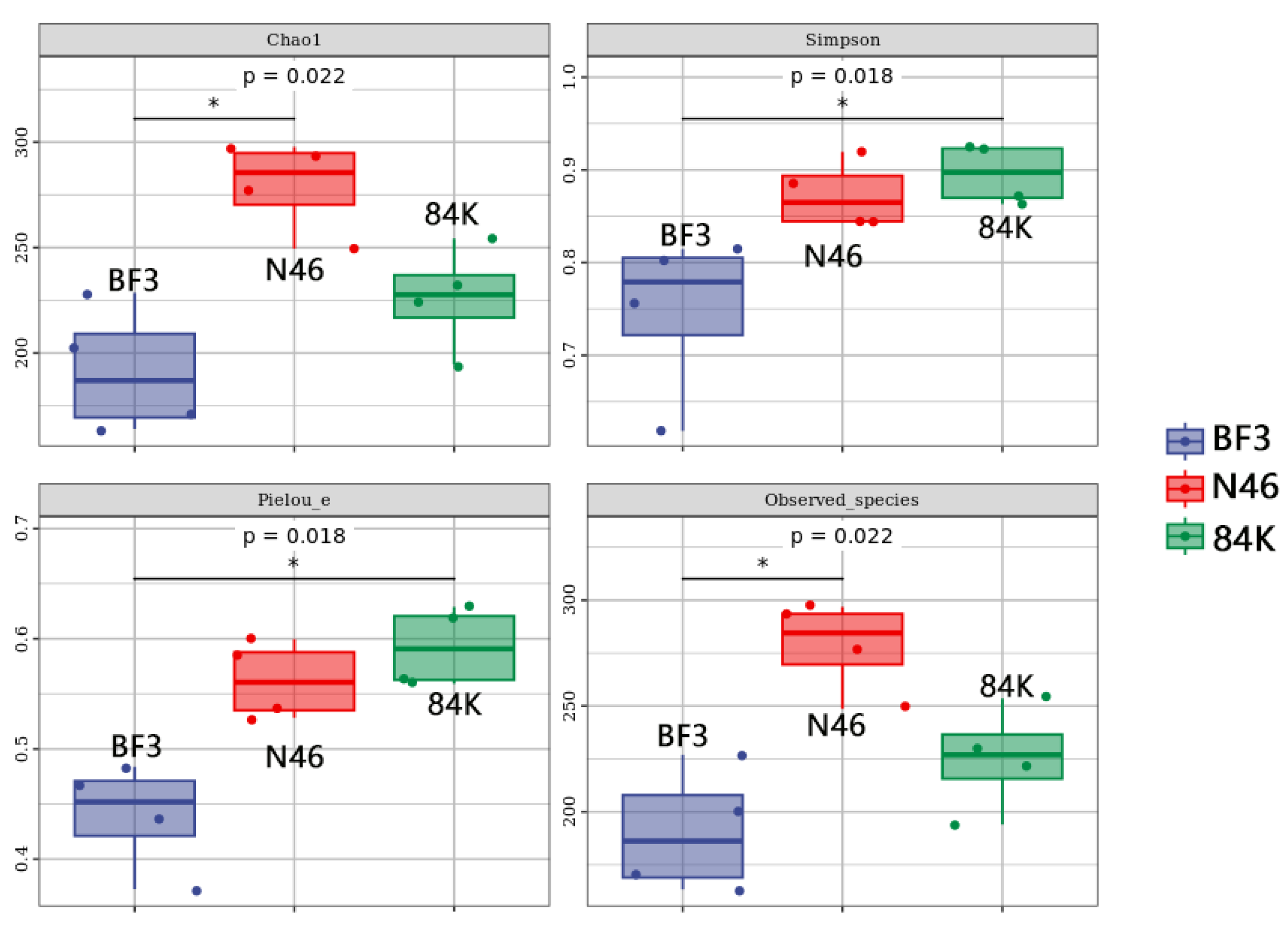
3.2. Diversity of Phyllosphere Fungal Communities in Three Different Populus spp.
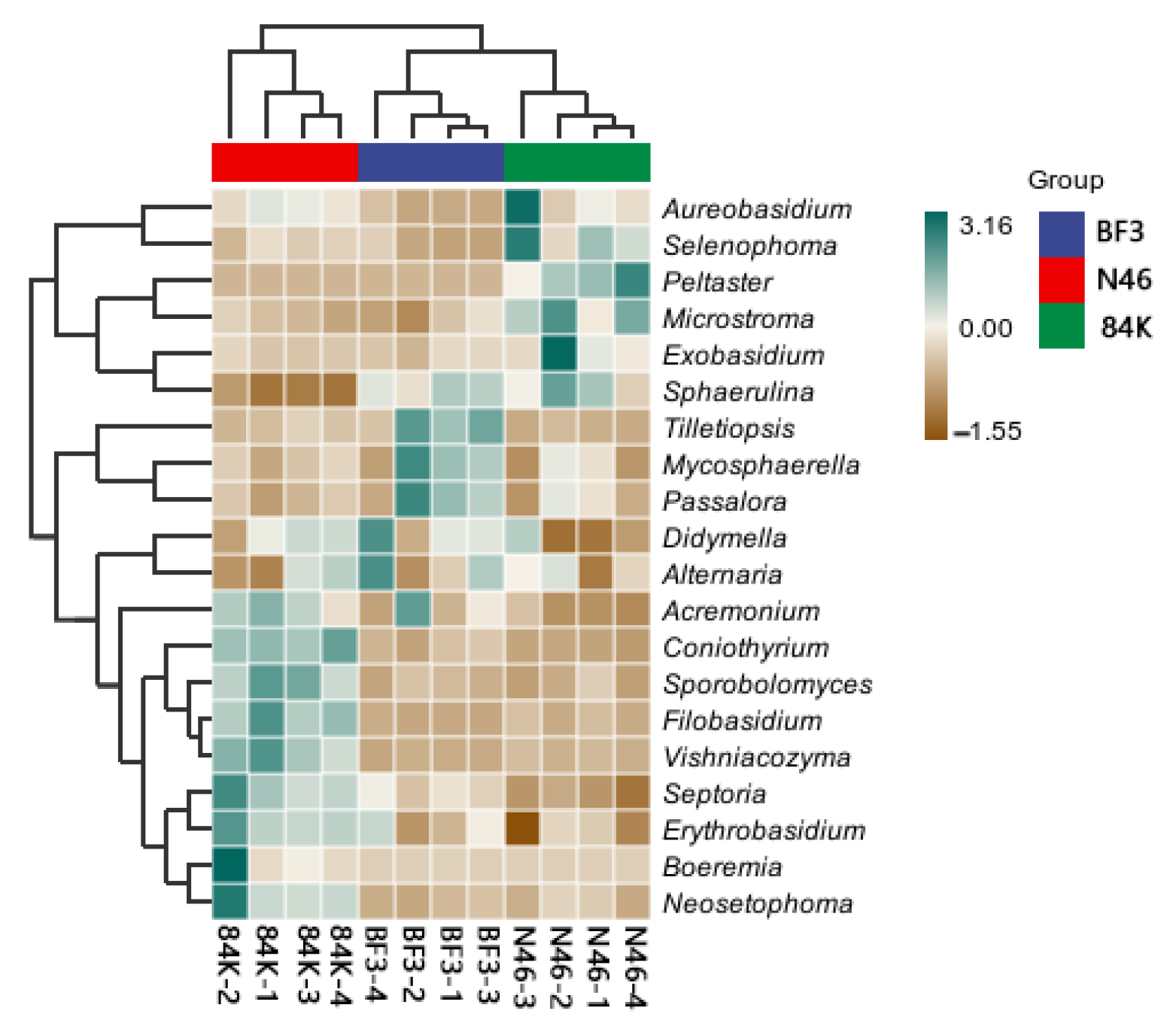
3.3. Composition of Phyllosphere Fungal Communities in Three Different Populus spp.
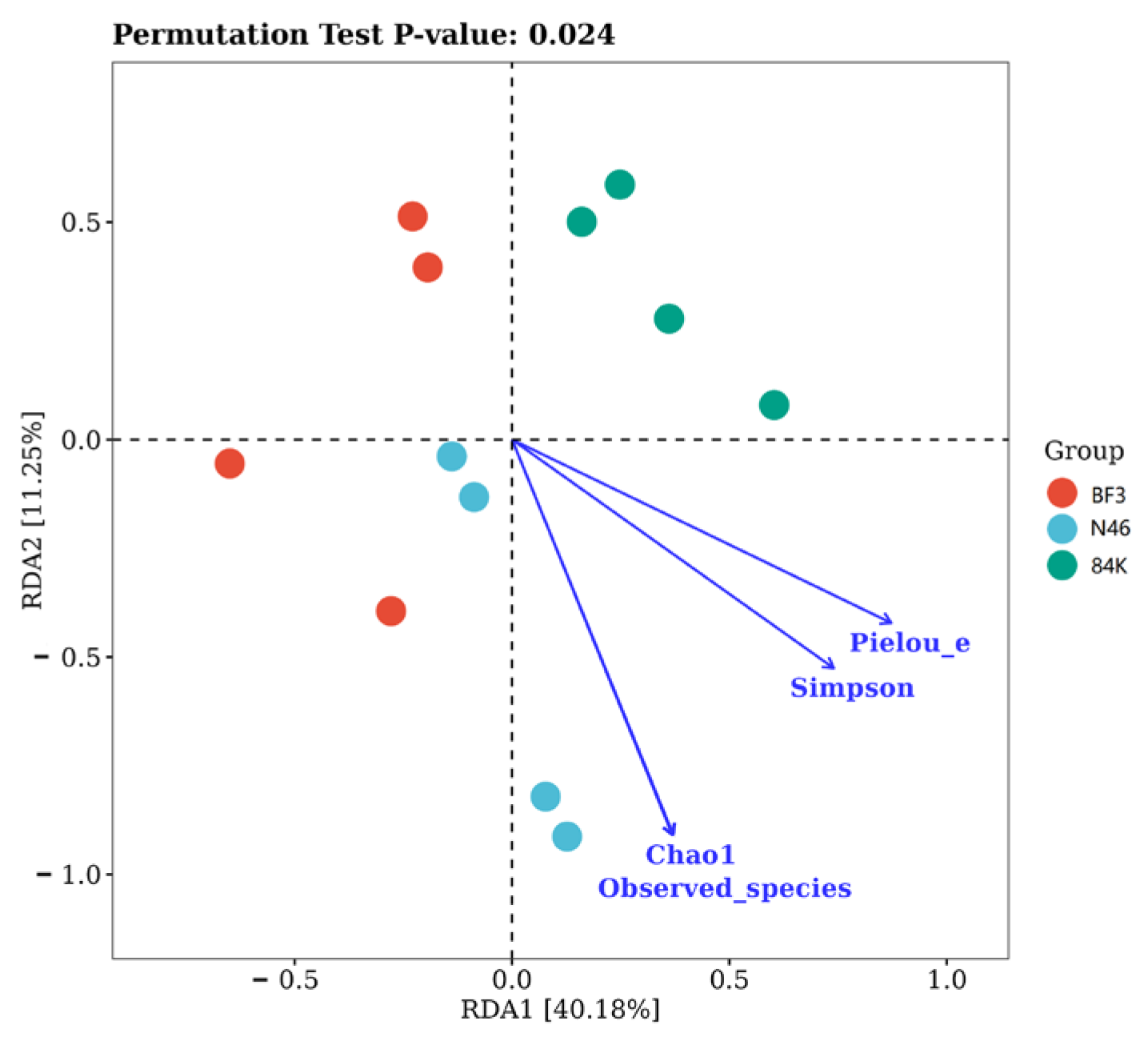
3.4. The Relationships between the Chemical Traits of the Leaves and Phyllosphere Fungal Community Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [PubMed]
- Vacher, C.; Hampe, A.; Porté, A.J. The Phyllosphere: Microbial jungle at the plant-climate interface. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Ursula, K.; Daniel, A.N.; Wilfried, S. Epiphytic microorganisms on strawberry plants (Fragaria ananassa cv. Elsanta): Identification of bacteria isolates and analysis of their interaction with leaf surfaces. FEMS Microbiol. Ecol. 2005, 53, 483–492. [Google Scholar] [CrossRef]
- Mallick, S.; Dutta, T.K. Kinetics of phenanthrene degradation by Staphylococcus sp.strain PN/Y involving 2-hydroxy-1-naphthoic acid in a novel metabolic pathway. Process Biochem. 2008, 9, 1004–1008. [Google Scholar] [CrossRef]
- Sun, W.; Xie, S.; Luo, C. Direct link between toluene degradation in contaminated-site microcosms and a Polaromonas strain. Appl. Environ. Microbiol. 2010, 76, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Kolvenbach, B.A.; Corvini, F.X. The degradation of alkylphenols by Sphingomonas sp. strain TTNP3—A review on seven years of research. New Biotechnol. 2012, 30, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Barrow, J.R.; Osuna, P.; Reyes, V.I. Fungal genomes that influence basic physiological processes that enhance survival of black gramaand fourwing saltbush in arid southwestern rangelands. In Shrubland Dynamics-Fire and Water, Fort Collins; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007; pp. 123–131. [Google Scholar]
- Bargabus, R.L.; Zidack, N.K.; Sherwood, J.E. Characterisation of systemic resistance in sugar beet elicited by a non-pathogenic, phyllosphere-colonizing Bacillusmycoides, biological control agent. Physiol. Mol. Plant Pathol. 2002, 61, 289–298. [Google Scholar] [CrossRef]
- Kinkel, L.L. Microbial population dynamics on leaves. Annu. Rev. Phytopathol. 1997, 35, 327–347. [Google Scholar] [CrossRef]
- Maignien, L.; Deforce, E.A.; Chafee, M.E.; Eren, A.M.; Simmons, S.L. Ecological succession and stochastic variation in the assembly of Arabidopsis thaliana phyllosphere communities. mBio 2014, 5, e00682-13. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Liu, J.X.; Jia, T.; Wang, Y.G.; Chai, B.F. Environmental filtering drives bacterial community structure and function in a subalpine area of northern China. Basic Micro. 2019, 59, 337–347. [Google Scholar] [CrossRef]
- Fürnkranz, M. Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica. Isme J. 2008, 2, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Friesen, M.L. Microbially mediated plant functional traits. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 23–46. [Google Scholar] [CrossRef]
- Meyer, K.M.; Leveau, H. Microbiology of the phyllosphere: A playground for testing ecological concepts. Oecologia 2012, 168, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Cordier, T.; Robin, C.; Capdevielle, X.; Desprez-Loustau, M.L.; Vacher, C. Spatial variability of phyllosphere fungal assemblages: Genetic distance predominates over geographic distance in a European beech stand (Fagus sylvatica). Fungal Ecol. 2012, 5, 509–520. [Google Scholar] [CrossRef]
- Bálint, M.; Tiffin, P.; Hallström, B.; Olson, M.S.; Fankhauser, J.D. Host genotype shapes the foliar fungal microbiome of balsam poplar (Populus balsamifera). PLoS ONE 2013, 8, e53987. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Burch, A.Y.; Lindow, S.E. Geographical location determines the population structure in phyllosphere microbial communities of a salt-excreting desert tree. Apply Environ. Microbiol. 2011, 77, 7647–7655. [Google Scholar] [CrossRef]
- Rastogi, G.; Sbodio, A.; Tech, J.J. Leaf microbiota in an agroecosystem: Spatiotemporal variation in bacterial community composition on field-grown lettuce. Multidiscip. J. Microb. Ecol. 2012, 6, 1812. [Google Scholar] [CrossRef]
- Finkel, O.M.; Burch, A.Y.; Elad, T. Distance-decay relationships partially determine diversity patterns of phyllosphere bacteria on Tamarix trees across the Sonoran desert. Apply Environ. Microbiol. 2012, 78, 6187–6193. [Google Scholar] [CrossRef]
- Muller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systemslevel insights and perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Vogel, C.; Carlstrom, C.I.; Muller, D.B. Establishing causality:opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.A.; Santos-Medellin, C.M.; Liechty, Z.S.; Nguyen, B.; Lurie, E.; Eason, S.; Phillips, G.; Sundaresan, V. Compositional shifts in root-associated bacterial and archaeal microbiota track the plant life cycle in field-grown rice. PLoS Biol. 2018, 16, e2003862. [Google Scholar] [CrossRef] [PubMed]
- Svetlana, F.; Yoram, G.; Malka, H.C. Variability of Bacterial Community Composition on Leaves Between and Within Plant Species Ido Izhaki. Curr. Microbiol. 2013, 66, 227–235. [Google Scholar] [CrossRef]
- Yadav, R.K.; Karamanoli, K.; Vokou, D. Bacterial colonization of the phyllosphere of mediterranean perennial species as influenced by leaf structural and chemical features. Microb. Ecol. 2005, 50, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.; Wright, S.J.; Green, G.L. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef]
- Kembel, S.W.; Mueller, R.C. Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities. Botany 2014, 92, 303–311. [Google Scholar] [CrossRef]
- Hunter, P.J.; Hand, P.; Pink, D.; Whipps, J.M.; Bending, G.D. Both leaf properties and microbe-microbe interactions influence within-species variation in bacterial population diversity and structure in the lettuce (lactuca species) phyllosphere. Appl. Environ. Microbiol. 2010, 76, 8117–8125. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhu, C.W.; Alam, M.S.; Tokida, T.; Sakai, H.; Nakamura, H.; Usui, Y.; Zhu, J.G.; Hasegawa, T.; Jia, Z.J. Response of soil, leaf endosphere and phyllosphere bacterial communities to elevated CO2 and soil temperature in a rice paddy. Plant Soil. 2015, 392, 27–44. [Google Scholar] [CrossRef]
- Wiesmann, C.; Lehr, K.; Kupcinskas, J.; Vilchez-Vargas, R.; Link, A. Primers matter: Influence of the primer selection on human fungal detection using high throughput sequencing. Gut Microbes 2022, 14, 2110638. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Chang, S.; Peng, C.; Xiao, W.; Zhang, J. Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a moso bamboo forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Bioch. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. 2016, 174, 245–246. [Google Scholar]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Xu, S.; Dai, Z.; Guo, P.; Fu, X.; Liu, S.; Zhou, L.; Tang, W.; Feng, T.; Chen, M.; Zhan, L.; et al. GgtreeExtra: Compact Visualization of Richly Annotated Phylogenetic Data. Mol. Biol. Evol. 2021, 38, 4039–4042. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgei, S.N.; Brown, J.R.; Taylor, C.M. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Kato, S.; Fukasawa, Y.; Seiwa, K. Canopy tree species and openness affect foliar endophytic fungal communities of understory seedlings. Ecol. Res. 2017, 32, 157–162. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, S.; Krumbein, A.; Schreiner, M. Composition of the phyllospheric microbial populations on vegetable plants with different glucosinolate and carotenoid compositions. Microb. Ecol. 2008, 56, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Fiala, V.; Glad, C.; Martin, M. Occurrence of Soluble Carbohydrates on the Phylloplane of Maize (Zea mays L.): Variations in Relation to Leaf Heterogeneity and Position on the Plant. New Phytol. 1990, 115, 609–615. [Google Scholar] [CrossRef]
- Fokkema, N.J. Fungal leaf saprophytes, beneficial or detrimental? Microb. Ecol. Phylloplane 1981, 39, 433–439. [Google Scholar]
- Merrall, G.T. Physical factors that influence the behavior of chemicals on leaf surfaces. Microb. Ecol. Phylloplane 1981, 36, 265–281. [Google Scholar]
- Sivakumar, N.; Sathishkumar, R.; Selvakumar, G.; Shyamkumar, R.; Arjunekumar, K. Phyllospheric microbiomes: Diversity, ecological significance and biotechnological applications. Nat. Public Health Emerg. Collect. 2020, 25, 113–172. [Google Scholar] [CrossRef]
- Knoll, D.; Schreiber, L. Plant–microbe interactions: Wetting of ivy (Hedera helix L.) leaf surfaces in relation to colonization by epiphytic microorganisms. Microb. Ecol. 2000, 40, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E.; Brandl, M.T.; Lindow, S.E.; Brandl, M.T. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef]
- Whipps, J.M.; Hand, P.; Pink, D.; Bending, G.D. Phyllosphere microbiology with special reference to diversity and plant genotype. J. Appl. Microbiol. 2008, 105, 1744–1755. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.G.; Wang, J.T.; Singh, B.; Han, L.L.; Shen, J.P.; Li, P.P.; Wang, G.B.; Wu, C.F.; Ge, A.H. Host selection shapes crop microbiome assembly and network complexity. New Phytol. 2021, 229, 1091–1104. [Google Scholar] [CrossRef]
- Kim, M.; Singh, D.; Lai-Hoe, A.; Rahim, R.A.; Ainuddin, A.N.; Chun, J.; Adams, J.M. Distinctive Phyllosphere Bacterial Communities in Tropical Trees. Microb. Ecol. 2012, 63, 674–681. [Google Scholar] [CrossRef]



| Total Carbon/g kg−1 | Total Nitrogen/g kg−1 | C/N | Total Phosphorus/g kg−1 | Soluble Sugar/mg kg−1 | Starch/mg kg−1 | |
|---|---|---|---|---|---|---|
| BF3 | 439.26 ± 5.47 b | 25.19 ± 0.97 b | 17.51 ± 0.64 a | 2.09 ± 0.16 b | 15.72 ± 3.76 a | 57.52 ± 4.61 a |
| N46 | 443.82 ± 0.94 b | 31.37 ± 0.56 a | 14.16 ± 0.28 b | 6.40 ± 0.41 a | 24.08 ± 5.53 a | 74.13 ± 6.77 a |
| 84 K | 467.34 ± 1.75 a | 26.46 ± 1.33 b | 17.79 ± 0.87 a | 2.10 ± 0.06 b | 21.61 ± 2.89 a | 68.44 ± 4.50 a |
| p value | <0.001 | 0.004 | 0.005 | <0.001 | 0.392 | 0.142 |
| F value | 20.12 | 10.58 | 9.86 | 94.07 | 1.04 | 2.45 |
| Fungi | TC | Nt | C/N | Pt | Souble Sugar | Starch |
|---|---|---|---|---|---|---|
| Chao1 | 0.903 | 0.035 * | 0.065 | 0.006 ** | 0.416 | 0.039 * |
| Observed_species | 0.898 | 0.035 * | 0.065 | 0.006 ** | 0.415 | 0.039 * |
| Pielou_e index | 0.054 | 0.228 | 0.531 | 0.411 | 0.553 | 0.009 ** |
| Simpson | 0.143 | 0.165 | 0.351 | 0.453 | 0.903 | 0.012 * |
| Fungi | TC | Nt | C/N | Pt | Souble Sugar | Starch |
|---|---|---|---|---|---|---|
| Ascomycota | 0.467 | 0.025 * | 0.034 * | 0.001 ** | 0.289 | 0.006 ** |
| Basidiomycota | 0.469 | 0.005 ** | 0.009 ** | 0 ** | 0.158 | 0.022 * |
| Rozellomycota | 0.114 | 0.326 | 0.497 | 0.882 | 0.540 | 0.830 |
| Glomeromycota | 0.903 | 0.227 | 0.253 | 0.068 | 0.768 | 0.342 |
| Chytridiomycota | 0.289 | 0.672 | 0.494 | 0.322 | 0.645 | 0.751 |
| Mortierellomycota | 0.376 | 0.835 | 0.641 | 0.780 | 0.676 | 0.406 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhang, W.; Liu, Y.; Ding, C.; Zhu, W. The Changes of Phyllosphere Fungal Communities among Three Different Populus spp. Microorganisms 2023, 11, 2479. https://doi.org/10.3390/microorganisms11102479
Sun Z, Zhang W, Liu Y, Ding C, Zhu W. The Changes of Phyllosphere Fungal Communities among Three Different Populus spp. Microorganisms. 2023; 11(10):2479. https://doi.org/10.3390/microorganisms11102479
Chicago/Turabian StyleSun, Zhuo, Weixi Zhang, Yuting Liu, Changjun Ding, and Wenxu Zhu. 2023. "The Changes of Phyllosphere Fungal Communities among Three Different Populus spp." Microorganisms 11, no. 10: 2479. https://doi.org/10.3390/microorganisms11102479





