Abstract
Campylobacteriosis cases in humans are of global concern, with high prevalence rates in the poultry reservoir considered the most important source of infection. Research findings show Campylobacters’ ability to enter a viable but non-culturable (VBNC) state, remaining “viable” but unable to grow on culture media. We explored the persistence of VBNC states in specific environments, particularly at broiler farms, as this state may lead to an underestimation of the present Campylobacter prevalence. For VBNC detection, a propidium monoazide PMA-dye viability qPCR (v-qPCR) was used in combination with cultivation methods. We examined samples collected from broiler farm barns and their surroundings, as well as chicken manure from experimental pens. In addition, the tenacity of culturable and VBNC-Campylobacter was studied in vitro in soil and water. In a total of three visits, Campylobacter was not detected either culturally or by v-qPCR (no Campylobacter DNA) in the environment of the broiler farms. In four visits, however, VBNC-Campylobacter were detected both inside and outside the barns. The overall prevalence in environmental samples was 15.9% for VBNC-Campylobacter, 62.2% for Campylobacter DNA, and 1.2% for culturable C. jejuni. In the experimental pens, no cultivable C. jejuni was detected in chicken manure after 24 h. Strikingly, “VBNC-Campylobacter” persisted even after 72 h. “VBNC-Campylobacter” were confirmed in barn surroundings and naturally contaminated chicken manure. Laboratory studies revealed that VBNC-Campylobacter can remain intact in soil for up to 28 days and in water for at least 63 days, depending on environmental conditions.
1. Introduction
Poultry meat is a significant source of Campylobacter infections, with 127,840 recorded cases in the EU in 2021 [1]. The colonization of poultry at the farm level plays a crucial role in how Campylobacter enters the food chain. Most cases (20–30%) of human campylobacteriosis in the EU are attributed to the consumption of poultry meat. A significant proportion (50–80%) is thereby causally linked to the high prevalence of Campylobacter (C) C. jejuni and C. coli in the poultry reservoir. Campylobacter occurrence in poultry production is currently associated with emerging antimicrobial resistances to antibiotics. [2,3,4,5,6]. Despite extensive research at broiler farms, the knowledge to understand in which ways Campylobacter manages to colonize new flocks despite proper interventions remains incomplete. Some studies have provided valuable insights into the epidemiological situation using cultivation and molecular epidemiology. Notably, the environment is frequently mentioned as a reservoir for Campylobacter at chicken farms. However, the results of different studies suggest that cultivating Campylobacter in broiler farm environments remains challenging [4,7,8,9,10]. This could be attributed to the limited persistence of Campylobacter when exposed to various environmental conditions. Exposure to various environmental stressors, including oxidative stress, starvation, osmotic stress, temperature, pH, and UV light, has been discussed to induce a viable but non-culturable (VBNC) state in Campylobacter [11,12,13,14,15]. The VBNC state of Campylobacter was first described by Rollins and Colwell for its survival in natural aquatic environments [16]. Subsequent studies of food safety and primary production examined conditions within the poultry processing chain, with a particular focus on chicken carcasses, meat rinses, and raw milk [17,18,19,20]. The persistence of Campylobacter in the environment of broiler farms and subsequent colonization of broilers may also be related to the VBNC state. Previous studies have demonstrated that VBNC-Campylobacter (C. jejuni) can resuscitate in vivo or under laboratory conditions, as well as express pathogenicity [11,20,21]. Furthermore, recent research has underscored the importance of VBNC Campylobacter in food processing conditions. [22]. As culture-based methods cannot detect VBNC-Campylobacter, polymerase chain reaction (PCR) methods are used; however, they only amplify the total DNA (from both viable and dead cells). PMA pre-treatment combined with qPCR has been used in various investigations to confirm viable Campylobacter [17,19,23,24,25]. However, PMA treatment may fail to fully inactivate the remaining signal of dead cells in qPCR [19]. To address this, an internal sample process control (ISPC) was developed, which monitors dead cell signal reduction and DNA loss during extraction that allows accurate quantification [26,27]. In the current study, this sophisticated PMA dye-supported viability (v)-qPCR approach that was recently validated for meat rinses in line with ISO 16140-2:2016 [26] was used to examine various environmental matrices. The investigation focused on a one-year sampling campaign aimed at sampling the environment of three broiler farms in Germany. Additionally, naturally contaminated chicken manure obtained from experimental pens was analyzed. To detect and quantify VBNC-Campylobacter in poultry and environmental samples, a pretreatment step involving PMA and ISPC was employed. Subsequently, following the photoactivation of the dye, the samples were analyzed using qPCR. To further expand the understanding of VBNC states in the environment, (i) the tenacity of culturable Campylobacter, (ii) the stability of VBNC Campylobacter induced in raw milk, and (iii) the transition of culturable C. jejuni into the VBNC state in vitro was investigated.
2. Materials and Methods
2.1. Study Design
For the first part of the study (field trial), seven visits to broiler farms (A-C) were conducted between November 2019 and September 2020 (Table 1). All farms followed an all-in/all-out system and provided broilers ad libitum access to feed and water via drinker nipples with trays. Ross 308 broilers (farm A and C) were reared at a stocking density of 39 kg/m2 for 36–42 days, while Hubbard broilers (farm B) were stocked at 25 kg/m2 for 60 days. The chickens received a three-phase feeding diet matching the commercial standards. Access to outdoor areas was not provided. Thinning procedure was carried out approximately one week before the entire flocks were removed. The three rural farms are surrounded by fields, forests, and small artificial waterways with adjacent lakes present at distances of 0.5 to 1.5 km. The farms followed several biosecurity measures, including personal hygiene practices, disinfectant footbaths or mats and cleaning and disinfection of the broiler houses as specified in guidelines by the German Agricultural Society (DLG) and the German Veterinary Society (DVG).

Table 1.
Broiler farm visits and weather conditions at the day of sampling, Germany 2019–2020.
The second part of the study (experimental trial 1) was conducted at the experimental facilities of the Centre for Infection Medicine within the Department of Veterinary Medicine at Freie Universität Berlin. The investigation was carried out after the removal of the flocks. The natural Campylobacter-contaminated chicken manure (harboring the strain BfR-CA-14430) [28] from four separate animal rooms was investigated over a period of 72 h. Chicken manure was stored under stable environmental conditions: a temperature of approximately 20 °C, a relative humidity (RH) of about ~50%, an air exchange rate of 15 times per hour in the room, and artificial daylight of 400 lux.
The third part of the study was a laboratory-based in vitro study (experimental trial 2), which was split into three different trials. The first trial aimed at investigating the tenacity of cultivable Campylobacter. In the second trial, the stability of VBNC C. jejuni induced in raw milk was observed and determined. In the last part, a combination of both trials and a further determination of the transition of cultivable C. jejuni into their VBNC state was investigated. Soil and water were used as experimental matrices, and all trials were carried out in three different microhabitats, each characterized by unique features as outlined in Table 2. The soil originating from the Berlin region displays a light texture, characterized by a substantial presence (over 80%) of sand particles, a minor content (less than 10%) of clay particles, a moderate amount (10–40%) of silt particles, and a neutral pH 7. The water (drinking water with drinking water quality, pH 7.0) used for the experiment was obtained from the drinking water system of the experimental animal husbandry. The matrices were stored in sterile 120 mL specimen containers (VWR, Radnor, Pennsylvania).

Table 2.
Microhabitats of the laboratory-based trials.
2.2. Sampling and Pre-Treatment
2.2.1. Field Trial
Sampling was conducted at each farm after the broiler thinning procedure was carried out. Environmental samples (air, boot swabs, gauze swabs, and water) were collected outside the barns, as described previously [4]. After sample collection, the samples were transported in a cooling box (~4 °C) to the laboratory and analyzed within 2 h. One pair of boot swabs were homogenized in filtered blender bags by shaking for 120 s in 100 mL of peptone water (PW) using the “fast” (120 rounds per minute (rpm)) program of a laboratory stomacher. Gauze swabs were similarly homogenized in 50 mL PW. Chicken manure was initially homogenized in a 120 mL specimen tank using a sterile spatula. Subsequently, 5 g of the homogenized sample was diluted at a ratio of 1:10 in PW and further homogenized as described above using the stomacher. Water was homogenized by gently vortexing. After its initial homogenization, 50 mL of each pretreated sample was filtered through folded filters 5–13 µm (Rotilabo®-Faltenfilter, Typ 601P Carl Roth, Karlsruhe, Germany) and sterile glass funnels. Filtered samples were centrifuged at 4 °C (11,000× g) for 15 min, the supernatant discarded, and the pellet resuspended in 3 mL PW. The final sample was then divided into aliquots as follows: 1 mL reserved for cultivation and 2 mL for differentiation of live and dead cells using quantitative polymerase chain reaction (v-qPCR). Air samples (total volume of 1000 L/1 m3) were collected as described previously [4] using Coriolis® µ cones were transferred to a 15 mL sterile screw cap tube (Sarstedt, Nümbrecht, Germany), and the sample was then centrifuged at 4 °C (11,000× g) for 15 min. The supernatant was discarded, and the pellet was resuspended in 3 mL PW and aliquoted. From each water sample, 50 mL was either filtered and centrifuged or directly centrifuged, depending on the level of visible pollution.
2.2.2. Experimental Trial 1
Chicken manure was collected at 24 h intervals from 0 to 72 h after flock removal from four separate animal rooms. Per room, chicken manure was divided into four different areas (four biological replicates), which were distinguished in terms of moisture and dryness. Approximately 30–50 g of manure was collected with sterile spatulas and transferred into sterile 120 mL specimen containers. Manure samples were then prepared and handled as described above.
2.2.3. Experimental Trial 2
Water from the experimental trial 2 was vortexed, and 3 mL of the homogenized mixture was transferred to a sterile 15 mL tube, gently vortexed again, and aliquoted as described previously. Soil samples were collected using sterile spatulas and placed into sterile screw cap tubes (50 mL) (Sarstedt, Nümbrecht, Germany). Afterwards, samples were diluted 1:2 in 3 mL of PW and homogenized by vortexing. For the determination of colony-forming units (CFU) (cultivation), 1 mL of the suspension was used. For PMA-v-qPCR analysis, 2 mL of the sample mixture was centrifuged at 600× g for 1 min to eliminate soil particles, sand grains, and other organic matter that could not be readily filtered and may have a detrimental impact on DNA extraction and v-qPCR. Subsequently, the supernatant was transferred to a sterile tube and centrifuged again at 600× g for 1 min. Finally, the supernatant was homogenized and aliquoted as described above.
2.3. Inoculation Strain and Growth Conditions
For the laboratory-based study in experimental trial 2, the C. jejuni strain (BfR-CA-14430), preserved at −80 °C, was cultivated on Columbia agar supplemented with 5% sheep blood (ColBA, Oxoid, Thermo Fisher Scientific Inc., Waltham, MA, USA) for 24 h at 42 °C under microaerobic conditions (5% O2, 10% CO2 and 85% N2) in a tri-gas incubator (CB 160; Binder, Germany). After sub-culturing in BHI with twice the amount of Growth Supplement (SR0232; Oxoid, Wesel, Germany) for 18 ± 2 h, cells were suspended in BHI and adjusted to an optical density of 0.2 at a wavelength of 600 nm (OD600), equivalent to approximately 9 log10 cell counts per ml as reported earlier [25]. This suspension was spiked into soil or water to achieve a final concentration of ~8 log10 CFU/g or ml. The VBNC-C. jejuni cells (BfR-CA-14430) in raw milk (~7 log10 viable cells/mL) were provided by the German National Reference Laboratory (NRL) for Campylobacter [20] and spiked in soil and water at a final concentration of ~5 log10/g or ml.
2.4. Cultivation Methods for the Studies
Quantification was evaluated via colony forming units (CFU) following ISO 10272-2:2017 [29]. Pre-treated samples were diluted 10-fold in BHI and plated in duplicate on modified cefoperazone deoxycholate agar (mCCDA) (Oxoid, Thermo Fisher Scientific, Waltham, MA, USA). Samples that contained low levels of Campylobacter in experimental trial 1 were subjected to quantitative assessment using enrichment, following the ISO 10272-1:2017 [30] procedure B. In brief, samples were diluted at a 1:10 ratio in Preston Broth, (PB) supplemented with Preston Campylobacter selective Supplement (SR0117; Oxoid, Wesel, Germany), Growth Supplement (SR0232; Oxoid, Wesel, Germany), and defibrinated horse blood (SR0050; Oxoid, Wesel, Germany), then incubated in a microaerobic atmosphere at 41.5 °C for 24 h. Enriched cultures were inoculated onto selective mCCDA plates using a sterile 10-μL loop, followed by incubation at 41.5 °C under microaerobic conditions for another 48 ± 2 h. Putative colonies were isolated and streaked on Columbia blood agar with 5% sheep blood and then incubated as described above. Colonies were analyzed using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS, Bruker Microflex® system). When Campylobacter growth in experimental trial 2 was not achievable following ISO 10272-2:2017, putative VBNC cell suspensions (100 µL) from 1 mL reserved suspension from pre-treatment were transferred to supplemented PB and incubated under similar conditions for up to 72 h to confirm the absence of viable C. jejuni.
2.5. Determination of VBNC Campylobacter with qPCR
The method followed a previously established protocol [26,27]. In brief, for each pretreated sample, two “working” samples of each 1 mL were prepared. One sample was stained with 2.5 µL of a 50 μM PMA solution (viable cells) while the other sample served as a control to monitor total DNA of all cells (viable and dead cells). To monitor the reduction of the dead cell signal by PMA, an internal sample process control (ISPC) at high concentration was included in the PMA-treated samples [27]. The mixture was briefly vortexed and incubated in a laboratory thermomixer at 700 rpm for 15 min at 30 °C without light exposure (darkened room). Following incubation, samples were cross-linked for 15 min using a PMA-Lite™ LED Photolysis Device (Biotium Inc., Landing Parkway, Fremont, CA, USA). Subsequently, ISPC at low concentration was added to both PMA-treated and untreated samples, the samples were gently vortexed and centrifuged for 5 min at 16,000× g at 4 °C. The latter addition of low concentration of ISPC guarantees that putative DNA losses during extraction are additionally detected in individual samples [26]. The supernatant was discarded, and cell pellets were stored until DNA extraction at −20 °C. Genomic DNA was extracted using the GeneJet Genomic DNA Purification Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA), according to the manufacturer’s instructions.
The targets of the qPCR assay have been previously described (Table 3). Each qPCR run employed genomic standards (C. jejuni NCTC 11,168 or C. sputorum DSM 5363 (ISPC). Standards were used in duplicates in a set of serial dilutions (5000, 500, 50, 20, and 10 genomic copies) per reaction to generate standard curves for quantification. The National Reference Laboratory for Campylobacter provided the genomic standards in dried stabilized DNA aliquots as described previously [27].

Table 3.
Triplex qPCR primers and probes.
The triplex v-qPCR method was employed using the fluorophore combination Jos-P-FAM, Csput-P-Cy5 and IPC-ntb2-P-HEX [26]. The triplex-master mix was prepared as described by the standard operating procedure (SOP) (Suppl. Information 1–2) [26]: 1× Platinum Taq buffer, 2.5 mM MgCl2, 0.2 mM of each dNTP (Thermo Fischer Scientific, USA), 0.06 × ROX (Life Technologies, USA), 500 nM of each Jos-F1 and Jos-R1 primer, 500 nM of each Csput-F and Csput-R primer, 300 nM of each IPC-ntb2-F and IPC-ntb2-R primer, and 100 nM of each dark quenched (IPC-ntb2-P-HEX, Jos-P-FAM, and -Csput-P-Cy5) (Biomers GmbH, Ulm, Germany) (refer to Table 3) and 2U PlatinumTM Taq DNA Polymerase (Invitrogen, Thermo Fisher Scientific Inc.). The v-qPCR program started with a 15 min incubation at 95 °C, followed by 45 cycles of 30 s at 95 °C and 1 min at 60 °C (measure fluorescence) and 30 s at 72 °C for PlatinumTM Taq DNA Polymerase.
In cases where amplification was putatively hindered by elevated levels of humic acid, the PerfeCTa® qPCR ToughMix® (Quantabio, Beverly, MA, USA) was employed as recommended. The triplex qPCR method is effective for most samples, but in cases where Campylobacter spp. exceed the maximum limit of quantification (4.7 log10 genome equivalents per ml), it can hinder the ISPC signal and render it unsuitable for quantitative analysis [26]. In those cases, two duplex qPCR assays were used instead [27].
2.6. Statistical Analysis
All quantitative data were compiled in a Microsoft Excel spreadsheet. Statistical data analysis and graphs were created using GraphPad Prism 9.1.0 (221) (2020, GraphPad Software, 2365 Northside Dr. Suite 560, San Diego, CA 92108, USA). Mann-Whitney U-test (two-tailed) and Kruskal–Wallis test with Dunn’s multiple comparison test were employed. Differences were statistically different when p < 0.05 (**** p < 0.0001).Values are calculated as the mean (M) with a standard deviation (SD).
3. Results
3.1. Field Trial
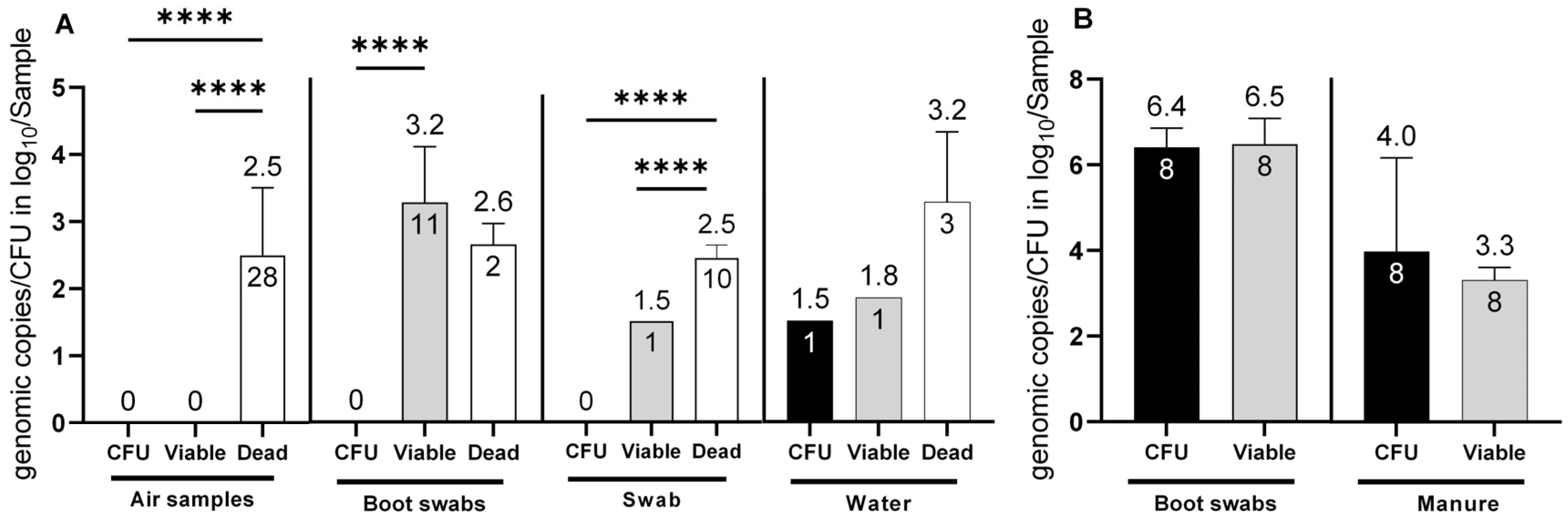
In total, seven sampling time points were analyzed for the presence of Campylobacter spp. Of these, Campylobacter spp. was detected only at four time points (1, 2, 6 and 7), while at visits 3–5, all samples (n = 12) from the barns tested negative for Campylobacter independent of the applied detection methods. Furthermore, no cultivable C. jejuni (CFU) or total C. jejuni DNA (e.g., from dead cells) was found in the environment (n = 72) at visits 3–5. Overall, 15.9% (13/86) of the environmental samples, were confirmed to be positive for viable C. jejuni cells when the barns were positive for C. jejuni, which corresponded to log10 viable C. jejuni (Cj)/sample after PMA treatment. Cultivable C. jejuni were confirmed in only one out of 86 environmental samples (water) (1.2%). Viable C. jejuni was not detected in any of the air samples (n = 28). However, when air samples were tested for C. jejuni DNA without PMA, all 28 samples (100%) were positive with a concentration of 2.5 ± 1 log10 dead Cj/m3 (p <0.0001). In boot swabs from the environment (n = 24), viable C. jejuni was determined in 11 samples (45.8%) at a concentration of 3.2 ± 0.8 log10 viable Cj/boot swab sample (p <0.0001). In environmental gauze swabs, C. jejuni DNA from dead cells was identified in 10 samples (n = 18), with a concentration of 2.5 ± 0.2 log10 dead Cj/gauze swab sample (p < 0.0001).
Using PMA-dye, viable C. jejuni was identified in one gauze swab at a concentration of log 1.5 log10 viable Cj/gauze swab. However, this result, along with a positive finding in one water sample of 1.8 log10 viable Cj/water sample (n = 16), was below the validated limit of quantification (LOQ of 2 log10) of the method and, thus, interpreted as semi-quantitative values. Nevertheless, equal quantities were cultivated in the water sample (1.5 log10 CFU/water sample). Regarding barn matrices, (Figure 1B) chicken manure samples from the inside of the barns revealed high levels of cultivable C. jejuni, with 4.5 ± 1.6 log10 CFU/mL. Moreover, v-qPCR determined 3.3 ± 0.3 log10 viable Cj/mL. This showed a high correlation between viable cells and CFU counts (no significant difference (p = 0.44), providing an accurate estimation by PMA despite potential negative matrix effects. Strikingly, Campylobacter was not cultivated from one chicken manure sample while simultaneously v-qPCR determined 3.5 log10 viable Cj/5 g manure. The corresponding boot swab sample, probably soiled with fresh fecal or cecal droppings, contained ~6.5 log10 viable C. jejuni with v-qPCR and CFU per boot swab (refer to Figure 1B).
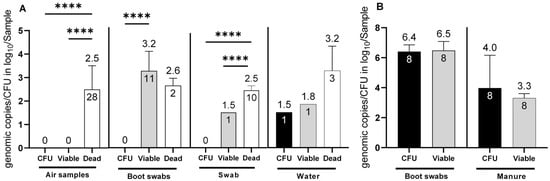
Figure 1.
Determination of C. jejuni in (A) environmental matrices (air samples, boot swabs, swabs and water) and (B) barn matrices (boot swabs and manure). Determination of C. jejuni: viable cell counts with v-qPCR (log10 viable Cj/sample (air (1 m3), boot swabs, gauze swabs, water (50 mL) and manure (5 g)) (grey bars), CFU (log10 CFU/Sample) (black bars) and exclusively dead cells (log10 total Cj/Sample) (white bars). The error bars depict the standard deviation of the mean counts (shown on top of the bars) (**** p < 0.0001). The number of positive samples is indicated within each bar. In total 86 environmental samples (28 air samples, 24 boot swab samples, 18, gauze swabs and 16 water samples from visits 1, 2, 6 and 7) were investigated. Simultaneously, 8 boot swabs and 8 manure samples were investigated from the inside of the barns.
3.2. Experimental Trial 1
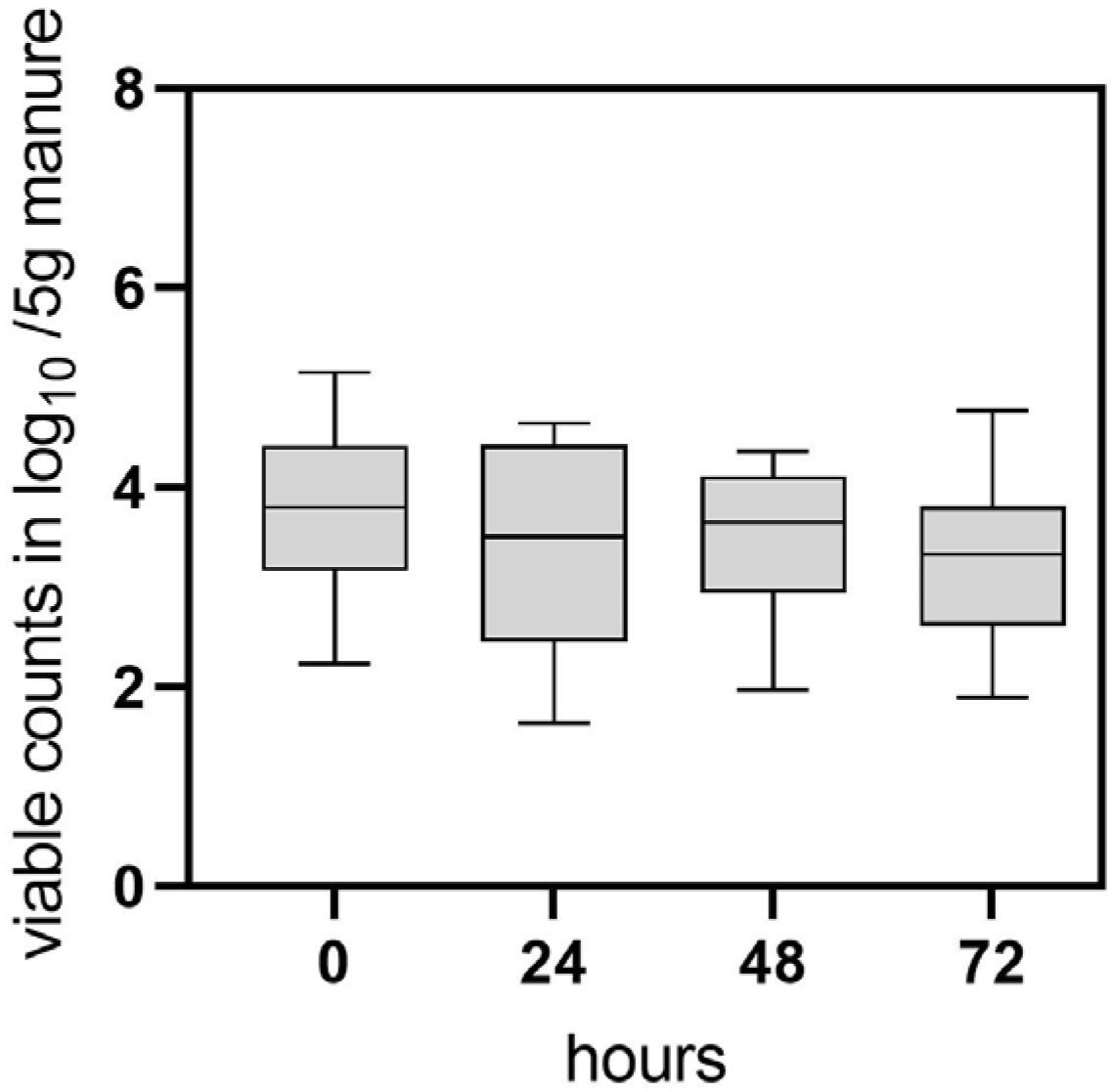
Based on the results of the field trial, an experimental investigation was conducted to further analyze C. jejuni persistence and possible transition to the VBNC state in naturally contaminated chicken manure. In this study, manure samples (four biological replicates) were collected after removal of the flocks from separate experimental animal rooms and analyzed using CFU and v-qPCR. The CFU method yielded 0 CFU/g (n = 16) of C. jejuni in all examined manure samples immediately after the removal of the flocks (sampling point 0). In contrast, the qualitative detection of C. jejuni using enrichment culture yielded positive results in the samples. Cultivable C. jejuni was detected for up to 24 h by qualitative detection methods. Interestingly, v-qPCR revealed that viable C. jejuni was quantifiable in most of the pre-treated samples at time point 0, with concentrations of 3.7 log10 ± 0.8 viable Cj/5 g manure (n = 16) (Figure 2). Throughout the 0–48 h investigation, viable counts remained relatively constant (Figure 2). After 72 h 3.2 ± 0.9 log10 viable Cj/5 g manure (n = 9) were still determined.
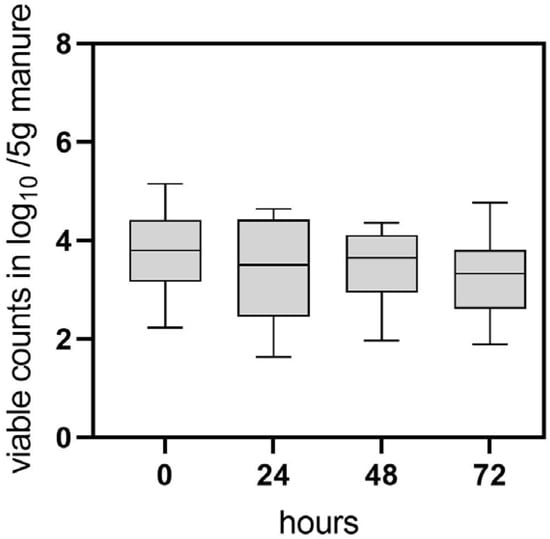
Figure 2.
Determination of viable C. jejuni BfR-CA-14430 counts from naturally contaminated chicken manure using v-qPCR. At each time point (hours), 16 manure samples were pretreated by homogenization and filtering and investigated. Grey bars depict the mean of positive v-qPCR detections in log10 viable Cj/5 g manure at 0 h (n = 16), 24 h (n = 15) 48 h (n = 16), 72 h (n = 9). The error bars represent the standard deviation for the mean (grey bar). No CFU was detected at sampling time point 0, while enrichment of C. jejuni was possible until 24 h.
3.3. Experimental Trial 2
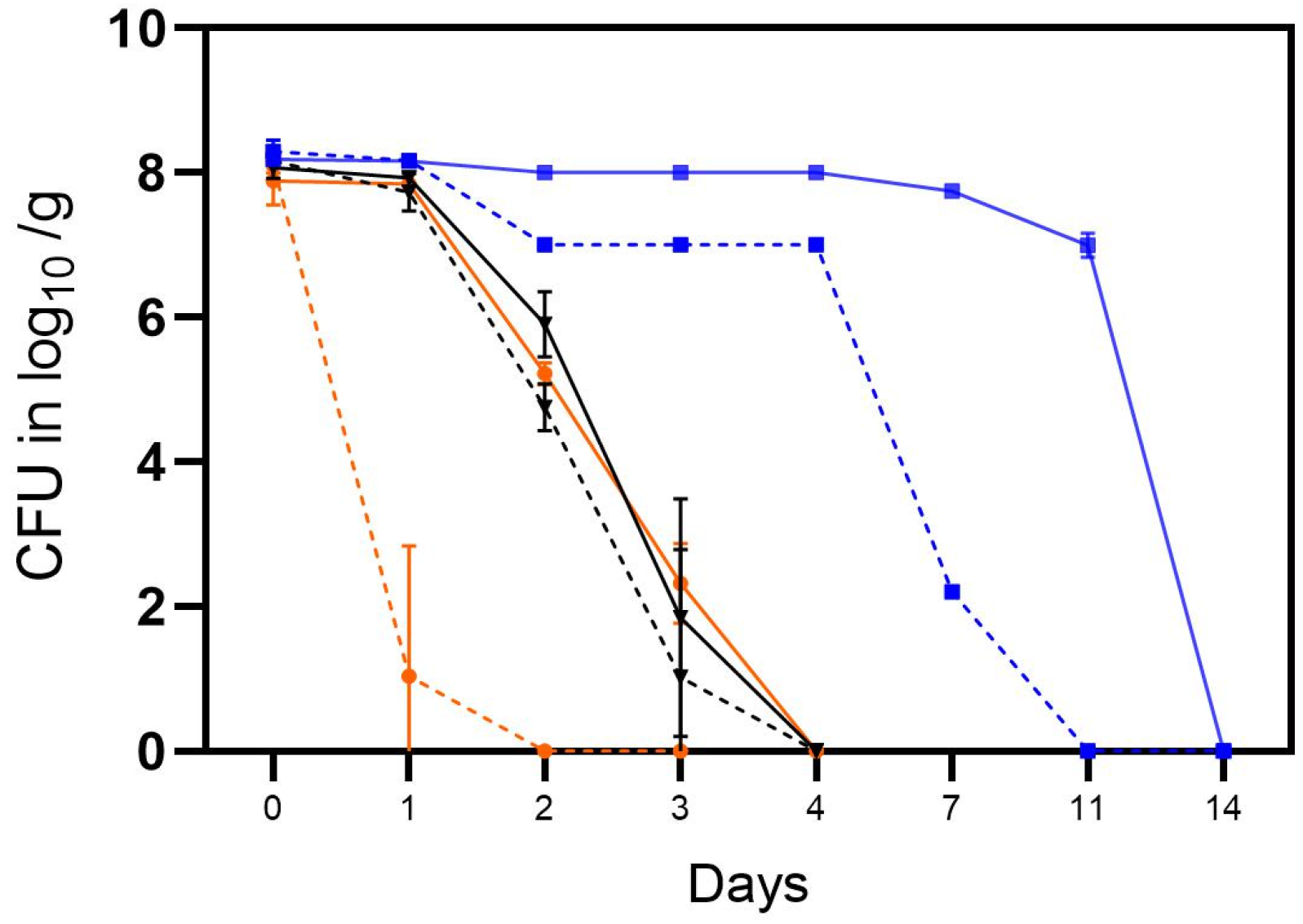
In the first trial, the tenacity of cultivable Campylobacter was determined in soil in open and closed containers in the laboratory at room temperature (RT) (A), in an incubator at high humidity at RT (B) or in the refrigerator at 4 °C at ambient humidity (Table 2). During this trial, the C. jejuni strain BfR-CA-14430 rapidly lost its cultivability in open containers under laboratory conditions at RT (A), thus being additionally exposed to daylight (Figure 3). From an initial concentration of ~8 log10 CFU/g, only 3.1 log10 CFU/g were observed in one of the three replicates after 24 h. Subsequently, Campylobacter was only qualitatively detected after enrichment procedures. In contrast, higher concentrations of 7.8 ± 0.11 log10 CFU/g C. jejuni (n = 3) were observed using containers with a closed lid after 24 h. The inactivation rate in soil using a closed container in habitat A was consistent with the inactivation rate in the incubator setting (B), regardless of whether it was stored in an open or closed container (Figure 3). C. jejuni demonstrated a notably longer cultivation period and higher quantities under refrigerator conditions (C) (Figure 3). Using an open container, quantitative detection was achievable in habitat C for up to 7 days (Figure 4) (2.2 ± 0.07 log log10 CFU/g (n = 3)), and qualitative detection for up to 11 days. Using a closed container, cultivation capacity was further extended in habitat C, with 6.9 ± 0.16 log10 CFU/g (n = 3) detected on day 11. Afterward, cultivation capacity rapidly declined in habitat C, as well and quantitative cultivability was achieved for the last time on day 14 (see Figure 3).
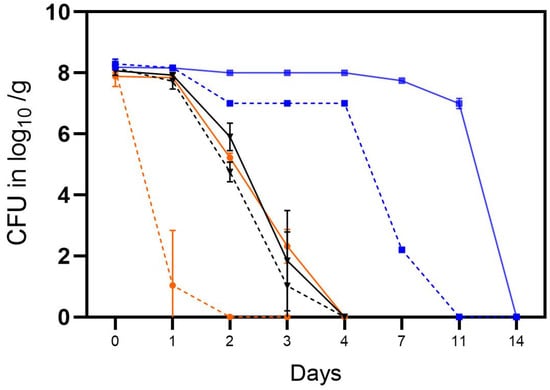
Figure 3.
The tenacity of cultivable C. jejuni BfR-CA-14443 in the soil in three different habitats: laboratory at RT, 35% RH, daylight (orange), incubator at RT, 9% RH, dark (black), and refrigerator at 4 °C, 64% RH, dark (blue). Counts were determined for closed containers (solid lines) and open containers (dotted lines) and are depicted in log10 CFU/g soil. Error bars indicate the standard deviation of the mean counts.
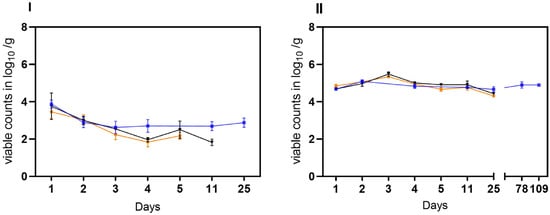
Figure 4.
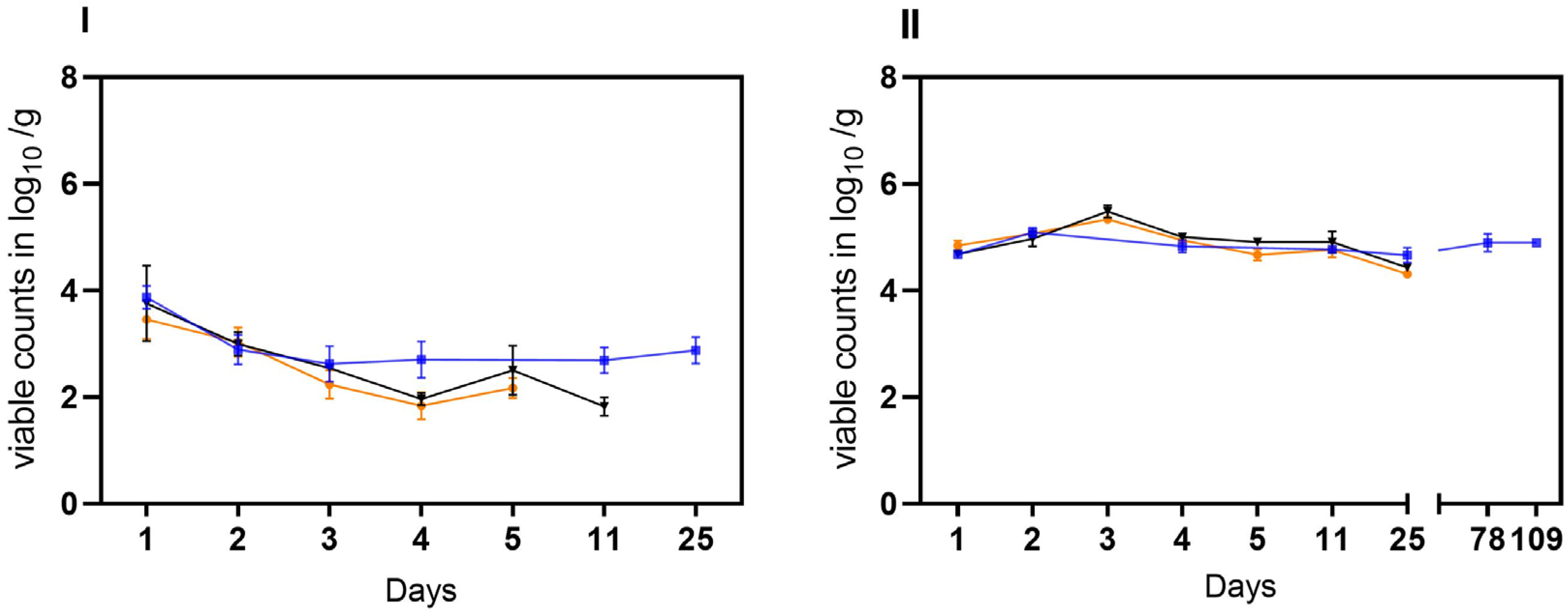
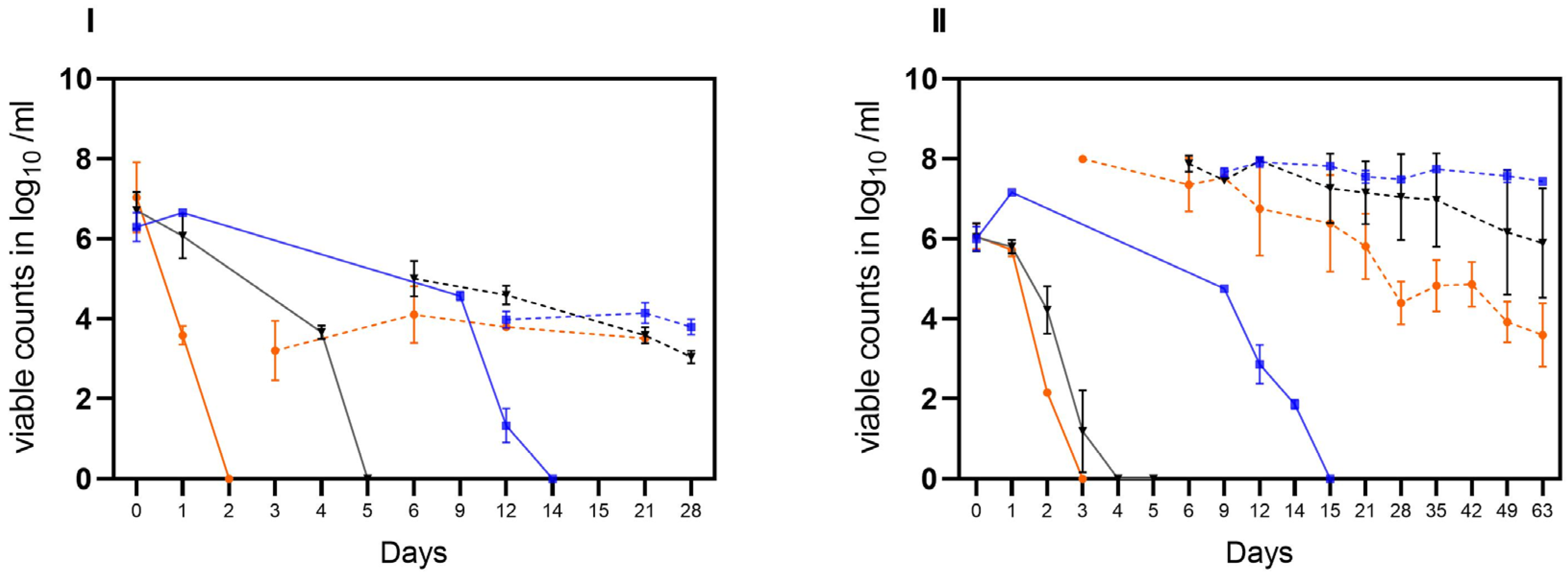
Stability of VBNC C. jejuni cells BfR-CA-14443 from raw milk in three habitats: laboratory at 21 °C (orange), incubator at 22 °C (black), and refrigerator at 4 °C (blue). Two different matrices were investigated: soil (I) and water (II). The sampling frequency is displayed in days. The counts, measured in log10 viable Cj/g soil or log10 viable Cj/mL water using v-qPCR, are depicted. The error bars indicate the standard deviation for the mean counts.
In the second trial, the stability of laboratory-induced VBNC Campylobacter generated in raw milk was investigated. Viable but not culturable cells of C. jejuni BfR-CA-14430 were introduced into two different matrices (water and soil) at an approximate initial concentration of ~5 log10 viable Cj/g as determined by v-qPCR. Under condition A, viable C. jejuni were recovered from soil at a concentration of 3.46 ± 0.37 log10 viable Cj/g (n = 3) after 24 h. Within five days, viable counts decreased gradually by an additional ~1.5 log level to 2.17 ± 0.18 log10 viable Cj/g (n = 3) (Figure 4I). Similarly, the viable counts of C. jejuni in soil decreased rapidly in habitat B. By day four, the determined viable counts of C. jejuni decreased from the initial 3.75 ± 0.7 log10 viable Cj/g (n = 3) to 1.96 ± 0.12 log10 Cj/g (n = 3). Moreover, condition B extended the stability in soil to day 11 (1.82 ± 0.17 viable Cj/g). In contrast, under condition C, an initial recovery of viable cells was detected after 24 h of 3.86 ± 0.2 log10 viable Cj/g (n = 3), while an average of 2.7 ± 0,3 log10 viable Cj/g was detectable until the end of the trial (day 25) in soil. In water, the introduced 5 log10 viable cells were detectable for up to 25 days in conditions A and B. In habitat C, on the other hand, there was no relevant decrease in viable cells even during prolonged storage over 109 days, when no viable Cj could be retrieved under conditions A and B (see Figure 5).
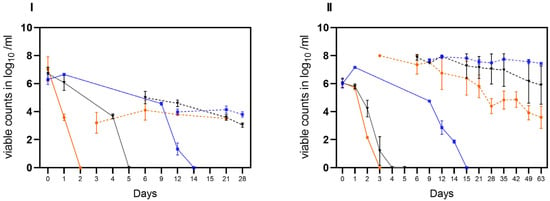
Figure 5.
Transition of cultivable C. jejuni (BfR-CA-14430) into viable but nonculturable (VBNC) state observed in three different habitats: laboratory at 21 °C (orange), incubator at 22 °C (black), and refrigerator at 4 °C (blue). Two different matrices were investigated: soil (I) and water (II). The sampling frequency is displayed in days. The counts, measured in log10 viable Cj/g soil or log10 viable Cj/mL water with v-qPCR (dotted lines) and CFU/g soil or ml water (solid lines) are depicted. The error bars indicate the standard deviation for the mean counts.
In the third trial, both matrices (water and soil) were spiked with cultivable C. jejuni BfR-CA-14430 to achieve an initial concentration of approximately ~8 log10 CFU/g soil or ml water. Then, the investigation aimed to explore the loss of cultivability and the possible transition into VBNC. As observed in the tenacity study, the initial recovered amount (d0) was 7.0 ± 0.8 log10 CFU/g (n = 3) in soil. Under conditions A, CFU decreased rapidly after one day (d1) to 3.58 ± 0.23 log10 CFU/g when compared to the other microhabitats (B, C). Thereafter, C. jejuni could only be qualitatively detected after 48 h (d2) in habitat A in soil (Figure 5I). After day three (d3), cultivable C. jejuni were not detected using qualitative detection, but with v-qPCR, 3.2 ± 0.7 log10 viable Cj/g (n = 3). Equivalent amounts could be detected until the end of the experiment on day 21 (Figure 5I). In contrast, cultivable Campylobacter were qualitatively detectable until day 6 in habitat B, when 5.0 ± 0.44 log10 (n = 3) viable counts were detected by v-qPCR. Viable counts were continuously detected until day 21. Furthermore, CFUs were determined in soil until day 12 in habitat C. With the loss of cultivability after day 15, viable (3.97 ± 0.2) log10 viable Cj/g (n = 9) were determined) counts in habitat C with v-qPCR, which were stable until the end of the experiment (d28) (Figure 5I).
Consistent with observations from soil samples, a rapid decline in the cultivability of C. jejuni in water (habitat A) was observed. By day two (d2), only one of the three replicates showed a detectable level of 2.2 log10 CFU/mL of cultivable C. jejuni. Subsequently, qualitative detection was last possible in one replicate on day 3. However, the v-qPCR analysis (Figure 5) revealed a substantial presence of viable C. jejuni cells (7.9 ± 0.05 log10 viable Cj/g (n = 3). The gradual decay of viable cells by ~4 log10 was observable using v-qPCR until day 63 (Figure 5II).
In comparison, in habitat B, the loss of cultivability of C. jejuni was delayed by two days (Figure 5II). After the loss of cultivability, viable cells were successfully detected using v-qPCR (Figure 5II). At the end of the experiment (d62), viable cells at a concentration of 5.8 ± 1.4 log10 viable Cj/mL (n = 3) were still determined. In habitat C, the conditions preserved viable cells until day 63. Remarkably, under these conditions, C. jejuni remained cultivable for approximately three times longer compared to the other habitats, as shown before. Specifically, culturable C. jejuni were observed until day 14, and viable cells were detected until day 63 in water under condition C at cooling temperature (Figure 5II).
4. Discussion
4.1. VBNC Campylobacter in the Environment of Broiler Farms (Field Trial)
The one-year investigation of seven broiler farms revealed that, in total, 15.9% of the environmental samples from Campylobacter-positive broiler flocks contained viable cells, while only one sample (1.2%) could be retrieved as CFU. In contrast, the absence of Campylobacter DNA (from dead and viable cells) in the environment of broiler farms correlated with the absence of cultivable Campylobacter in the barns. Campylobacter was primarily absent during the winter. This seasonal phenomenon was previously described [32]. Although the detection of potential VBNC Campylobacter was infrequent, these recent data show VBNC Campylobacter presence which suggest Campylobacter transmission into the environment. Specifically, the determination of viable C. jejuni cells with v-qPCR in one water sample with simultaneous observation of lower CFU loads indicated a potential transition into the VBNC state. Therefore, it is reasonable to hypothesize that water bodies favor prolonged persistence while supporting the gradual transition of cultivable cells to the VBNC state. In support, reduced environmental stressors, such as low levels of dissolved oxygen and UV-light exposure in water, could be promoting longevity and thus a gradual transition of Campylobacter in the VBNC state [33,34]. Moreover, it has been suggested that the availability of organic matter (manure remnants) in water bodies provides ample nutrients that allow Campylobacter persistence [35]. Yet, it can be contended that nutritional and oxidative stress also promote rapid VBNC induction, as observed in a recent study [21]. In this study, most viable C. jejuni were primarily detected by v-qPCR after treatment with PMA in sock swab samples (45.8%), collected by walking a predetermined route at Campylobacter-positive farms. This phenomenon could potentially be elucidated by the presence of broiler manure residues in the surroundings, along with boot swabs frequently being contaminated with chicken manure. It is conceivable that contaminated manure is spread by personnel and their vehicles after partial depopulation, as this practice has been recently connected with Campylobacter transmission at broiler farms [36]. This assumption is also consistent with the results of the gauze swabs, as the sampled surfaces were barely in direct contact with contaminated chicken manure. Thus, a high proportion of Campylobacter DNA from dead cells was determined in gauze swabs, although one gauze swab was semi-quantitatively positive for viable C. jejuni with v-qPCR. This could be due to the increasing deposition and accumulation of ventilated broiler sheds and manure in the environment [37]. In contrast, VBNC Campylobacter was not determined in air samples (only Campylobacter DNA from dead cells was determined). These findings show the discrepancy between viable and dead cells, providing a new insight as previous research only utilized PCR methods without PMA and the ISPC [38,39]. Viable C. jejuni (potentially in the VBNC state) were observed more frequently during periods of ‘mild weather’ characterized by overcast, cloudy, and rainy conditions (Table 1). These environmental weather conditions could potentially trigger Campylobacter persistence and transition into the VBNC state. This hypothesis is supported by the observations obtained from laboratory survival experiments conducted in water and soil at different temperatures, in which water at cooling temperatures led to maximally enhanced survival of C. jejuni (independent of the culturable or VBNC state (Figure 5)).
4.2. VBNC Campylobacter in Naturally Contaminated Chicken Manure (Experimental Trial 1)
During the field trial, chicken manure and water were found to be two conceivable reservoirs of VBNC Campylobacter in the environment. The investigation of natural contaminated chicken manure from experimental pens aimed at the detection of VBNC C. jejuni in a partially controlled environment where both, the inoculation strain and the conditions of the experimental animal rooms were known. Overall, using cultural quantitative analysis, cultivable C. jejuni were not determined, although cultural qualitative detection was possible through enrichment directly after the removal of the flocks (0-h mark). This observation is consistent with previous research suggesting that Campylobacter may rapidly lose its cultivability in manure [40]. More importantly, a continuous number of viable cells was detected between the 24- and 48-h mark. However, it is important to highlight that the presence of turbidity resulting from total suspended solids (TSS) and organic components in manure had adverse impacts on the effectiveness of PMA inactivation in the used protocol, as previously mentioned [41,42]. Consequently, the successful inactivation of ISPC was achieved after pretreating the manure samples, a process that involved centrifugation and filtration. However, pretreatment might have contributed to a loss of total viable cells before addition of ISPC. Nevertheless, the application of pretreatment methods under experimental conditions resulted in the detection of viable C. jejuni counts, comparable to those obtained in the field trials of barns (~3.5 log10 viable Cj/5 g manure), indicating that any potential bias introduced by physical pretreatment of the samples was at least reproduced. These results indicate that VBNC C. jejuni remained stable in chicken manure for several days. Indeed, these observations confirm the hypothesis from the field trial, and substantiate the assumption that VBNC Campylobacter originating from manure can be released directly from the animal barn into the environment. Nevertheless, it is important to reiterate that the storage of the manure was carried out under controlled and stable conditions, within an experimental animal room. These conditions encompassed factors like: the absence of UV light exposure, variations in air exchange rate, and relative humidity, which could differ from the conditions found in the natural environment.
4.3. Persistence and Transformation of Campylobacter (Experimental Trial 2)
First, the natural decay and loss of cultivability of the C. jejuni strain in soil (placed in open (with desiccation) and closed containers (without desiccation)) were determined under laboratory conditions (A). In open containers, a rapid loss of C. jejuni cultivability (within one day) was observed at 21 °C and low RH, which was anticipated due to desiccation stress caused by low humidity [43]. In contrast, when placed in closed containers, C. jejuni was detectable by cultural detection methods for up to three days under laboratory (A) and incubator (B) conditions when using high relative humidity (99%). Desiccation appeared to be an important environmental driver for C. jejuni survival as its capacity was reduced by a loss of moisture, which might additionally lead to increased oxygen tension. Similarly, shifts in temperature were shown to correlate with C. jejuni viability. In particular, low temperatures around 4 °C were observed to extend the survival capacity of C. jejuni in soil. This observation is substantiated by an extensive body of research that has noted prolonged survival in both water and food matrices at 4 °C [20,44,45,46].
Second, the stability of VBNC C. jejuni BfR-CA-14430 (induced in raw milk) was investigated in the same microhabitats. Despite observing cell losses during the recovery process from the soil matrix, as discussed above, VBNC C. jejuni were still detected for an extended period, depending on the utilized microhabitat. However, it should be noted that soil components themselves, such as humic acid, may interfere with qPCR and may have a negative impact on qPCR [47]. These effects were ascertained using the ISPC, as this standard behaved similarly to the C. jejuni target. Hence, it was manageable to address this issue by implementing a more resilient DNA polymerase for the soil samples. This adjustment allowed us to accurately assess inactivation through PMA monitored by ISPC. Remarkably, within the laboratory environment (A), desiccation and oxygen stress had a less severe impact on the integrity of C. jejuni cells that were already in the VBNC state. Despite the lack of desiccation and moisture loss (induced by inoculation) in habitat (B), maintaining a relative humidity of 99%, the initial higher levels of viable cells observed in comparison to habitat (A) experienced a subsequent 2-log reduction by the end of the 11-day trial period. This decrease could be linked to other biotic factors within the soil matrix at the temperature range of 21–22 °C. At refrigerator conditions (C), however, viable C. jejuni cell counts remained stable for prolonged periods, again indicating that low temperatures are favorable for VBNC C. jejuni. Interestingly, storing milk-induced VBNC C. jejuni in water resulted in maintenance of viable C. jejuni counts during the experimental period of 25 days in habitats A and B and even 109 days in habitat C. This may imply that stress conditions in water have less impact on VBNC stability than those encountered in soil [33,48]. These findings are consistent with prior research that has examined VBNC Campylobacter in both water- and food-related matrices under comparable conditions [20,21,22,33].
Finally, the gradual transition of culturable C. jejuni into VBNC C. jejuni in soil and water was observed. Under laboratory conditions (A), rapid loss of cultivability in soil was observed as previously described. Thereafter, viable C. jejuni was detected using v-qPCR until day 28. Notably, the inoculation strain also lost its cultivability very rapidly in water (within three days) at RT. Overall, a ~4 log reduction of viable C. jejuni cells was observed in water samples over time at any condition but slower at refrigerating temperature. However, cells remained viable until the end of the investigation on day 63. It might be assumed that the exposure to daylight under condition A in open containers, filtered by glass windows, might have harmed viable cells over time due to the sensitivity of bacteria to photo-oxidative damage [34]. Under incubator conditions (B) of 21 °C, cultivable C. jejuni were detectable in both water and soil for up to five days by qualitative detection, suggesting that the matrix itself was negligible at high humidity. Moreover, viable C. jejuni cells were detectable until the end of the trials (in soil until day 28 and in water until day d63). These results are in line with prior observations of in milk-induced VBNC C. jejuni, which corroborates the aforementioned studies.
4.4. VBNC Campylobacter in Diverse Environmental Matrices
The results of the field study show that VBNC Campylobacter can persist in contaminated environmental matrices under favorable conditions. In the subsequent experimental trials, prolonged stability of viable C. jejuni in water was demonstrated by v-qPCR, especially at 4 °C. Therefore, it is of major interest to understand the potential risk of water-associated VBNC C. jejuni in the agricultural environment. Several studies assessed C. jejuni in different waters using qPCR [49,50,51,52]. However, these observations confirm and underline the hypothesis that microbiological enumeration considerably underestimates the fraction of viable C. jejuni. Thus, employing v-qPCR in conjunction with ISPC could offer additional insights into the presence of VBNC-Campylobacter in aquatic farm environments, as previously indicated [53]. The urgency for this is further supported by recent findings where the virulence of VBNC C. jejuni in primary chicken embryo cells (accordingly outgoing pathogenicity) was demonstrated [21]. As described earlier, at the farm level, VBNC C. jejuni may persist in manure residues or contaminations in the environment. Consequently, it is feasible that VBNC-Campylobacter are transmitted via contaminated manure that remains in the environment after flock removal and are introduced into subsequent flocks [10]. Furthermore, it was possible to detect and quantify VBNC C. jejuni in naturally contaminated manure from experimental pens for up to 72 h. It can be assumed that the transition into VBNC states within manure may pose the challenges encountered in cultivating Campylobacter from chicken manure [54]. Indeed, this could also limit the time frame for Campylobacter detection in manure by cultivation, as observed in this and previous studies [40]. This might be explained by the different properties manure provides. One driving factor could be the abundance of nutrients, as Yagi et al. [33] found that nutrient-rich conditions induced the VBNC state faster than nutrient-poor conditions. In terms of stability and VBNC formation in soil, a rapid decrease in cultivability and rapid subsequent formation of VBNC C. jejuni were found. It is possible that various abiotic factors in soil, such as desiccation, physical entrapment, fluctuating oxygen levels, and biotic factors like competition from soil microflora, may be potential drivers of rapid VBNC formation, which resulted in, on the one hand, faster formation of VBNC Campylobacter and, on the other hand, reduced stability compared to water [21].
5. Conclusions
This study aimed to investigate the transition and persistence of VBNC Campylobacter in the environment and under predefined conditions. Utilizing v-qPCR for the analysis of samples obtained under favorable environmental conditions (rainy, cloudy, moist, low temperature), we were able to display viable C. jejuni and, thus, potential VBNC Campylobacter in the environment of broiler farms. The results of the experimental trials showed that viable C. jejuni cells could be detected in manure for up to 48 h. In our laboratory experiments, we were able to demonstrate that VBNC Campylobacter can remain viable over extended periods in different evaluated settings. Specifically, VBNC Campylobacter was viable for prolonged periods in water. However, it is important to note that controlled experimental settings do not necessarily reflect and mimic the VBNC Campylobacter persistence in nature (the environment). While we found that temperature, desiccation, humidity, and UV light appear to be important environmental drivers for VBNC Campylobacter, it should be emphasized, however, that other factors such as strain-specific differences and nutrient availability may also influence the persistence of VBNC Campylobacter. It is important to note that PMA alone confirms cell membrane integrity but provides no data on metabolic activity, pathogenicity, or infectivity. To gain deeper insights into VBNC states, further concurrent studies with v-qPCR, colored staining, microscopy, and in vivo/in vitro assays using isolated VBNC Campylobacter are of particular interest. To conclude, this comprehensive approach enhances and elucidates the overall understanding of the role of VBNC Campylobacter in the poultry reservoir.
Author Contributions
Conceptualization, B.R., A.F. and U.R.; formal analysis, B.R. and V.S.; investigation, B.R. and V.S.; resources, A.F. and U.R.; original draft preparation, B.R.; writing review and editing, B.R., A.F., V.S., K.S. and U.R.; visualization, B.R.; supervision, A.F.; project administration, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Federal Ministry of Education and Research (BMBF) within the framework of the consortium “PAC-Campy” (IP1/01KI1725A).
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to express our gratitude to Kerstin Stingl and the NRL Campylobacter team for their technical support and provision of the v-qPCR method, standards and materials. Besides, we would like to thank our colleagues at the Institute for Animal Hygiene and Environmental Health for their continuous support. We acknowledge support by the Open Access Publication Initiative of Freie Universität Berlin.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Lin, J. Novel approaches for Campylobacter control in poultry. Foodborne Pathog. Dis. 2009, 6, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Skarp, C.P.A.; Hänninen, M.L.; Rautelin, H.I.K. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, B.; Szott, V.; Epping, L.; Semmler, T.; Merle, R.; Roesler, U.; Friese, A. Transmission pathways of Campylobacter spp. at broiler farms and their environment in Brandenburg, Germany. Front. Microbiol. 2022, 13, 982693. [Google Scholar] [CrossRef] [PubMed]
- Hazards, E.P.o.B. Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Popa, S.A.; Morar, A.; Ban-Cucerzan, A.; Tîrziu, E.; Herman, V.; Sallam, K.I.; Morar, D.; Acaroz, U.; Imre, M.; Florea, T.; et al. Occurrence of Campylobacter spp. and Phenotypic Antimicrobial Resistance Profiles of Campylobacter jejuni in Slaughtered Broiler Chickens in North-Western Romania. Antibiotics 2022, 11, 1713. [Google Scholar]
- Bull, S.A.; Allen, V.M.; Domingue, G.; Jorgensen, F.; Frost, J.A.; Ure, R.; Whyte, R.; Tinker, D.; Corry, J.E.; Gillard-King, J.; et al. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol. 2006, 72, 645–652. [Google Scholar] [CrossRef]
- Ridley, A.M.; Morris, V.K.; Cawthraw, S.A.; Ellis-Iversen, J.; Harris, J.A.; Kennedy, E.M.; Newell, D.G.; Allen, V.M. Longitudinal molecular epidemiological study of thermophilic campylobacters on one conventional broiler chicken farm. Appl. Environ. Microbiol. 2011, 77, 98–107. [Google Scholar] [CrossRef]
- Thakur, S.; Brake, J.; Keelara, S.; Zou, M.; Susick, E. Farm and environmental distribution of Campylobacter and Salmonella in broiler flocks. Res. Vet. Sci. 2013, 94, 33–42. [Google Scholar] [CrossRef]
- Hertogs, K.; Haegeman, A.; Schaumont, D.; Gelaude, P.; De Zutter, L.; Dewulf, J.; Heyndrickx, M.; Rasschaert, G. Contamination Sources and Transmission Routes for Campylobacter on (Mixed) Broiler Farms in Belgium, and Comparison of the Gut Microbiota of Flocks Colonized and Uncolonized with Campylobacter. Pathogens 2021, 10, 66. [Google Scholar] [CrossRef]
- Baffone, W.; Casaroli, A.; Citterio, B.; Pierfelici, L.; Campana, R.; Vittoria, E.; Guaglianone, E.; Donelli, G. Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model. Int. J. Food Microbiol. 2006, 107, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Klancnik, A.; Guzej, B.; Jamnik, P.; Vuckovic, D.; Abram, M.; Mozina, S.S. Stress response and pathogenic potential of Campylobacter jejuni cells exposed to starvation. Res. Microbiol. 2009, 160, 345–352. [Google Scholar] [CrossRef]
- Chaveerach, P.; ter Huurne, A.A.; Lipman, L.J.; van Knapen, F. Survival and resuscitation of ten strains of Campylobacter jejuni and Campylobacter coli under acid conditions. Appl. Environ. Microbiol. 2003, 69, 711–714. [Google Scholar] [CrossRef]
- Jackson, D.N.; Davis, B.; Tirado, S.M.; Duggal, M.; van Frankenhuyzen, J.K.; Deaville, D.; Wijesinghe, M.A.; Tessaro, M.; Trevors, J.T. Survival mechanisms and culturability of Campylobacter jejuni under stress conditions. Antonie Van Leeuwenhoek 2009, 96, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; McMullen, L.; Jeon, B. Impact of oxidative stress defense on bacterial survival and morphological change in Campylobacter jejuni under aerobic conditions. Front. Microbiol. 2015, 6, 295. [Google Scholar] [CrossRef]
- Rollins, D.M.; Colwell, R.R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 1986, 52, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, M.H.; Lofstrom, C.; Hansen, T.B.; Christensen, L.S.; Olsen, J.E.; Hoorfar, J. Rapid quantification of viable Campylobacter bacteria on chicken carcasses, using real-time PCR and propidium monoazide treatment, as a tool for quantitative risk assessment. Appl. Environ. Microbiol. 2010, 76, 5097–5104. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Botteldoorn, N.; Coucke, W.; Denayer, S.; Dierick, K.; Uyttendaele, M. Effect of exposure to stress conditions on propidium monoazide (PMA)-qPCR based Campylobacter enumeration in broiler carcass rinses. Food Microbiol. 2015, 48, 182–190. [Google Scholar] [CrossRef]
- Pacholewicz, E.; Swart, A.; Lipman, L.J.; Wagenaar, J.A.; Havelaar, A.H.; Duim, B. Propidium monoazide does not fully inhibit the detection of dead Campylobacter on broiler chicken carcasses by qPCR. J. Microbiol. Methods 2013, 95, 32–38. [Google Scholar] [CrossRef]
- Wulsten, I.F.; Galeev, A.; Stingl, K. Underestimated Survival of Campylobacter in Raw Milk Highlighted by Viability Real-Time PCR and Growth Recovery. Front. Microbiol. 2020, 11, 1107. [Google Scholar] [CrossRef]
- Santos, L.S.; Rossi, D.A.; Braz, R.F.; Fonseca, B.B.; Guidotti-Takeuchi, M.; Alves, R.N.; Beletti, M.E.; Almeida-Souza, H.O.; Maia, L.P.; Santos, P.S.; et al. Roles of viable but non-culturable state in the survival of Campylobacter jejuni. Front. Cell Infect. Microbiol. 2023, 13, 1122450. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X. Susceptibility of Campylobacter jejuni to Stressors in Agrifood Systems and Induction of a Viable-but-Nonculturable State. Appl. Environ. Microbiol. 2023, 89, e0009623. [Google Scholar] [CrossRef] [PubMed]
- Magajna, B.; Schraft, H. Evaluation of Propidium Monoazide and Quantitative PCR To Quantify Viable Campylobacter jejuni Biofilm and Planktonic Cells in Log Phase and in a Viable but Nonculturable State. J. Food Prot. 2015, 78, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Wang, K.; Feng, J.; Heeney, D.D.; Liu, D.; Lu, X. Detection and Quantification of Viable but Non-culturable Campylobacter jejuni. Front. Microbiol. 2019, 10, 2920. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J.; Buhler, C.; Iwobi, A.N.; Huber, I.; Ellerbroek, L.; Appel, B.; Stingl, K. “Limits of control”—Crucial parameters for a reliable quantification of viable campylobacter by real-time PCR. PLoS ONE 2014, 9, e88108. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.; Heise, J.; Thieck, M.; Wulsten, I.F.; Pacholewicz, E.; Iwobi, A.N.; Govindaswamy, J.; Zeller-Peronnet, V.; Scheuring, S.; Luu, H.Q.; et al. Challenging the "gold standard" of colony-forming units—Validation of a multiplex real-time PCR for quantification of viable Campylobacter spp. in meat rinses. Int. J. Food Microbiol. 2021, 359, 109417. [Google Scholar] [CrossRef]
- Pacholewicz, E.; Buhler, C.; Wulsten, I.F.; Kraushaar, B.; Luu, H.Q.; Iwobi, A.N.; Huber, I.; Stingl, K. Internal sample process control improves cultivation-independent quantification of thermotolerant Campylobacter. Food Microbiol. 2019, 78, 53–61. [Google Scholar] [CrossRef]
- Epping, L.; Golz, J.C.; Knuver, M.T.; Huber, C.; Thurmer, A.; Wieler, L.H.; Stingl, K.; Semmler, T. Comparison of different technologies for the decipherment of the whole genome sequence of Campylobacter jejuni BfR-CA-14430. Gut Pathog. 2019, 11, 59. [Google Scholar] [CrossRef]
- Jacobs-Reitsma, W.F.; Jongenburger, I.; de Boer, E.; Biesta-Peters, E.G. Validation by interlaboratory trials of EN ISO 10272—Microbiology of the food chain—Horizontal method for detection and enumeration of Campylobacter spp. —Part 2: Colony-count technique. Int. J. Food Microbiol. 2019, 288, 32–38. [Google Scholar] [CrossRef]
- Biesta-Peters, E.G.; Jongenburger, I.; de Boer, E.; Jacobs-Reitsma, W.F. Validation by interlaboratory trials of EN ISO 10272-Microbiology of the food chain-Horizontal method for detection and enumeration of Campylobacter spp.—Part 1: Detection method. Int. J. Food Microbiol. 2019, 288, 39–46. [Google Scholar] [CrossRef]
- Anderson, A.; Pietsch, K.; Zucker, R.; Mayr, A.; Müller-Hohe, E.; Messelhäusser, U.; Sing, A.; Busch, U.; Huber, I. Validation of a Duplex Real-Time PCR for the Detection of Salmonella spp. in Different Food Products. Food Anal. Methods 2011, 4, 259–267. [Google Scholar] [CrossRef]
- Hansson, I.; Vagsholm, I.; Svensson, L.; Olsson Engvall, E. Correlations between Campylobacter spp. prevalence in the environment and broiler flocks. J. Appl. Microbiol. 2007, 103, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Okada, A.; Inoshima, Y. Role of temperature, nutrition, oxygen, osmolality, and bacterial strain in inducing a viable but non-culturable state in Campylobacter jejuni. J. Microbiol. Methods 2022, 195, 106456. [Google Scholar] [CrossRef] [PubMed]
- Sinton, L.; Hall, C.; Braithwaite, R. Sunlight inactivation of Campylobacter jejuni and Salmonella enterica, compared with Escherichia coli, in seawater and river water. J. Water Health 2007, 5, 357–365. [Google Scholar] [CrossRef]
- Thomas, C.; Hill, D.J.; Mabey, M. Evaluation of the effect of temperature and nutrients on the survival of Campylobacter spp. in water microcosms. J. Appl. Microbiol. 1999, 86, 1024–1032. [Google Scholar] [CrossRef]
- Hertogs, K.; Heyndrickx, M.; Gelaude, P.; De Zutter, L.; Dewulf, J.; Rasschaert, G. The effect of partial depopulation on Campylobacter introduction in broiler houses. Poult. Sci. 2021, 100, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Chinivasagam, H.N.; Tran, T.; Maddock, L.; Gale, A.; Blackall, P.J. Mechanically ventilated broiler sheds: A possible source of aerosolized Salmonella, Campylobacter, and Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 7417–7425. [Google Scholar] [CrossRef]
- Johannessen, G.S.; Garofolo, G.; Di Serafino, G.; Kolackova, I.; Karpiskova, R.; Wieczorek, K.; Osek, J.; Christensen, J.; Torp, M.; Hoorfar, J. Campylobacter in chicken—Critical parameters for international, multicentre evaluation of air sampling and detection methods. Food Microbiol. 2020, 90, 103455. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, M.S.; Josefsen, M.H.; Lofstrom, C.; Christensen, L.S.; Wieczorek, K.; Osek, J.; Hoorfar, J. Low-cost monitoring of Campylobacter in poultry houses by air sampling and quantitative PCR. J. Food Prot. 2014, 77, 325–330. [Google Scholar] [CrossRef]
- Smith, S.; Meade, J.; Gibbons, J.; McGill, K.; Bolton, D.; Whyte, P. The impact of environmental conditions on Campylobacter jejuni survival in broiler faeces and litter. Infect. Ecol. Epidemiol. 2016, 6, 31685. [Google Scholar] [CrossRef][Green Version]
- Desneux, J.; Chemaly, M.; Pourcher, A.M. Experimental design for the optimization of propidium monoazide treatment to quantify viable and non-viable bacteria in piggery effluents. BMC Microbiol. 2015, 15, 164. [Google Scholar] [CrossRef]
- Varma, M.; Field, R.; Stinson, M.; Rukovets, B.; Wymer, L.; Haugland, R. Quantitative real-time PCR analysis of total and propidium monoazide-resistant fecal indicator bacteria in wastewater. Water Res. 2009, 43, 4790–4801. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Roman, D.J. Sensitivity of Campylobacter jejuni to Drying. J. Food Prot. 1982, 45, 507–510. [Google Scholar] [CrossRef]
- Trigui, H.; Thibodeau, A.; Fravalo, P.; Letellier, A.; P Faucher, S. Survival in water of Campylobacter jejuni strains isolated from the slaughterhouse. Springerplus 2015, 4, 799. [Google Scholar] [CrossRef][Green Version]
- Haddad, N.; Burns, C.M.; Bolla, J.M.; Prevost, H.; Federighi, M.; Drider, D.; Cappelier, J.M. Long-term survival of Campylobacter jejuni at low temperatures is dependent on polynucleotide phosphorylase activity. Appl. Environ. Microbiol. 2009, 75, 7310–7318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bywater, A.; Alexander, K.; Eifert, J.; Strawn, L.K.; Ponder, M.A. Survival of Inoculated Campylobacter jejuni and Escherichia coli O157:H7 on Kale During Refrigerated Storage. J. Food Prot. 2023, 86, 100042. [Google Scholar] [CrossRef]
- Sidstedt, M.; Radstrom, P.; Hedman, J. PCR inhibition in qPCR, dPCR and MPS-mechanisms and solutions. Anal. Bioanal. Chem. 2020, 412, 2009–2023. [Google Scholar] [CrossRef]
- Pokhrel, D.; Thames, H.T.; Zhang, L.; Dinh, T.T.N.; Schilling, W.; White, S.B.; Ramachandran, R.; Theradiyil Sukumaran, A. Roles of Aerotolerance, Biofilm Formation, and Viable but Non-Culturable State in the Survival of Campylobacter jejuni in Poultry Processing Environments. Microorganisms 2022, 10, 2165. [Google Scholar] [CrossRef]
- Sun, A.; Mirzayans, P.M.; Piggott, A.M.; Stanton, J.L.; Sunna, A. Adapted method for rapid detection and quantification of pathogen Campylobacter jejuni from environmental water samples. FEMS Microbiol. Ecol. 2023, 99, fiad058. [Google Scholar] [CrossRef]
- Ahmed, W.; Sawant, S.; Huygens, F.; Goonetilleke, A.; Gardner, T. Prevalence and occurrence of zoonotic bacterial pathogens in surface waters determined by quantitative PCR. Water Res. 2009, 43, 4918–4928. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Y.; Huang, K.; Zhu, C.; Yin, Y. Application of real-time PCR for quantitative detection of Campylobacter jejuni in poultry, milk and environmental water. FEMS Immunol. Med. Microbiol. 2003, 38, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.H.; Murdock, A.; Mahmud, M.; Cloutier, M.; Benoit, T.; Bashar, S.; Patidar, R.; Mi, R.; Daneshfar, B.; Farenhorst, A.; et al. Quantitative Assessment of First Nations Drinking Water Distribution Systems for Detection and Prevalence of Thermophilic Campylobacter Species. Int. J. Environ. Res. Public Health 2022, 19, 10466. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.A.; Arsenault, J.; Kotchi, S.O.; Gosselin-Theberge, M.; Champagne, M.J.; Berthiaume, P. Campylobacter in recreational lake water in southern Quebec, Canada: Presence, concentration, and association with precipitation and ruminant farm proximity. J. Water Health 2018, 16, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Kassem, I.I.; Helmy, Y.A.; Kathayat, D.; Candelero-Rueda, R.A.; Kumar, A.; Deblais, L.; Huang, H.C.; Sahin, O.; Zhang, Q.; Rajashekara, G. Nonculturability Might Underestimate the Occurrence of Campylobacter in Broiler Litter. Foodborne Pathog. Dis. 2017, 14, 472–477. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).