Exploring Microeukaryote Community Characteristics and Niche Differentiation in Arid Farmland Soil at the Northeastern Edge of the Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Areas and Site Descriptions
2.2. Soil Sample Collection and Storage
2.3. DNA Extraction and PCR Amplifications
2.4. High-Throughput Sequencing
2.5. Data Processing
2.6. Statistical Analysis
3. Results
3.1. Taxonomic Compositions of the Soil Microeukaryote Communities in Different Sampling Areas
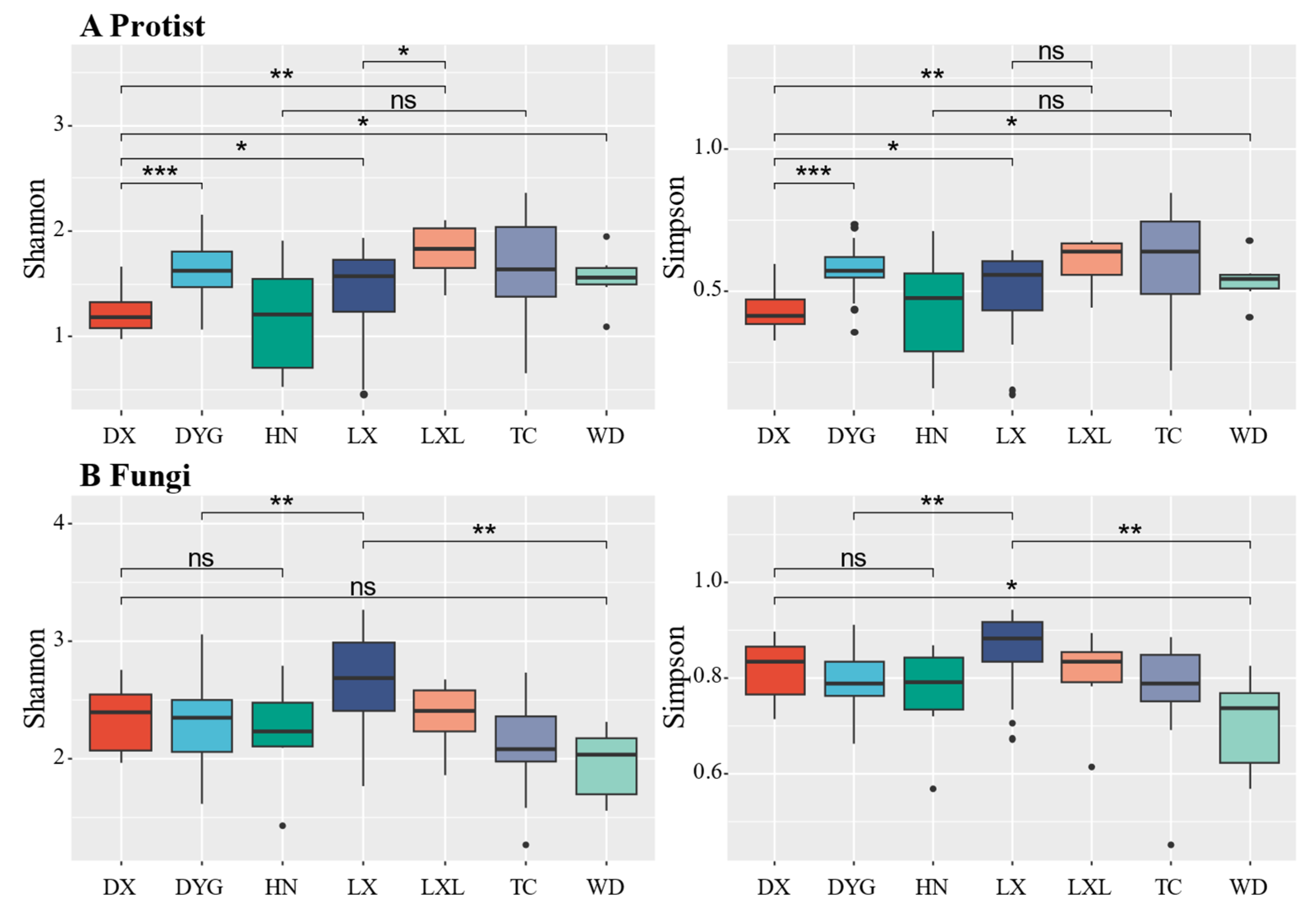
3.2. Alpha Diversity of the Soil Microeukaryote Communities
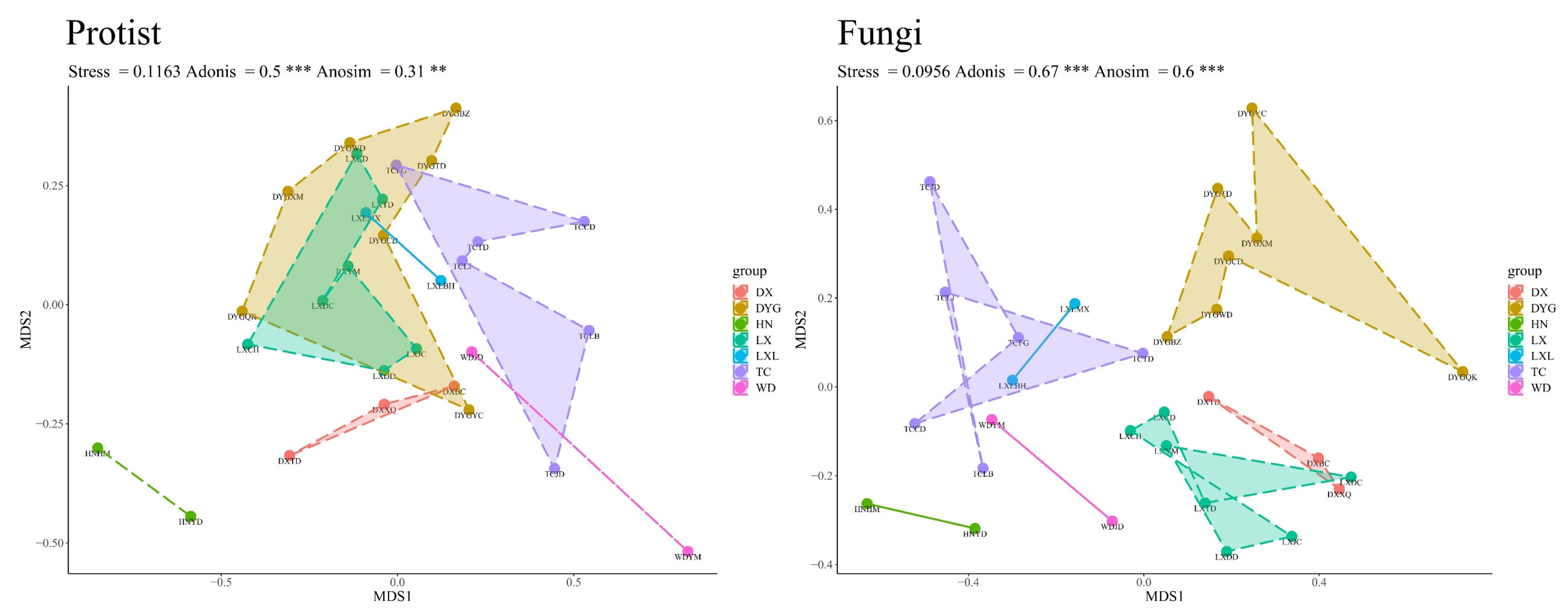
3.3. Beta Diversity of the Soil Microeukaryote Communities
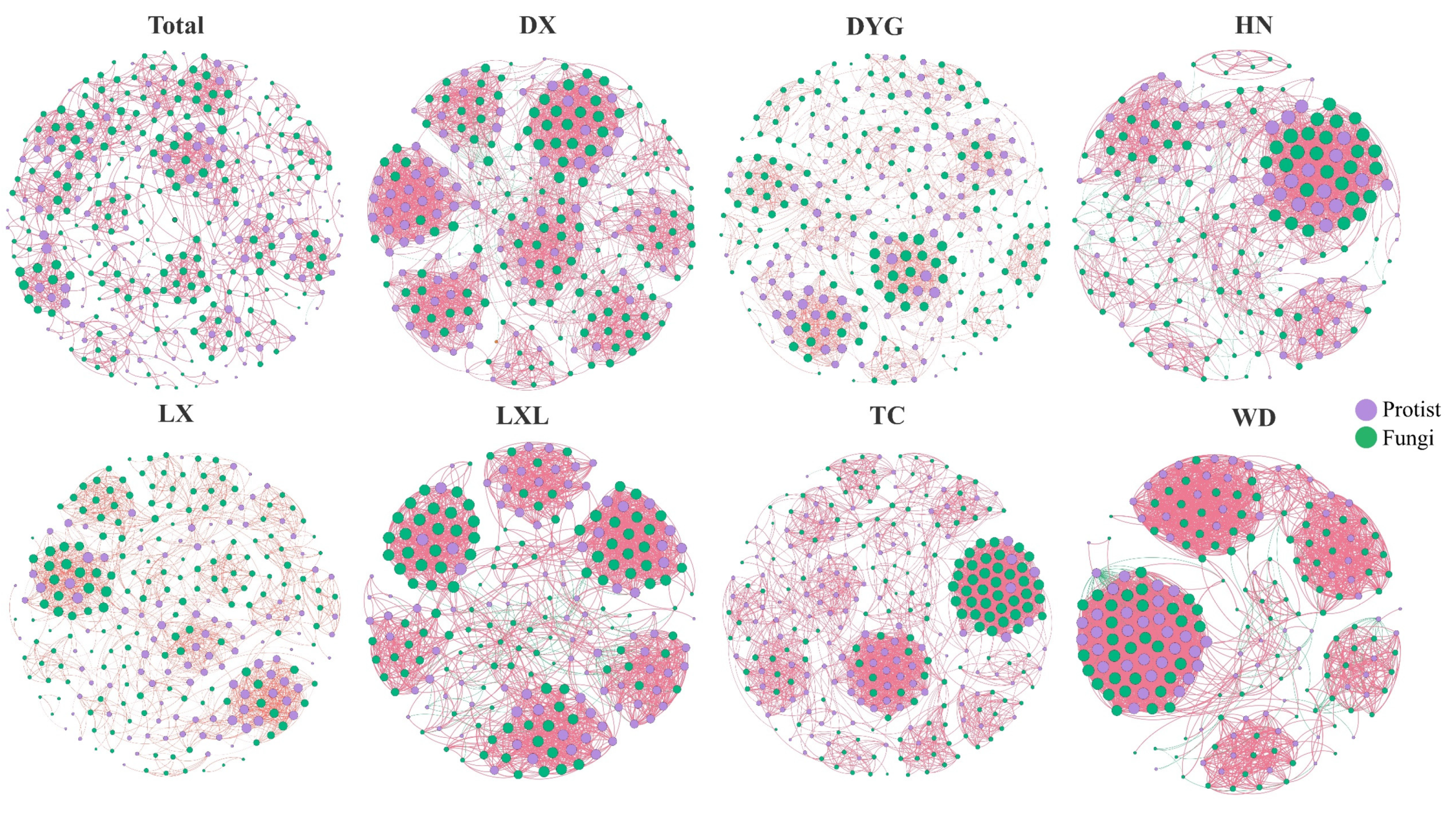
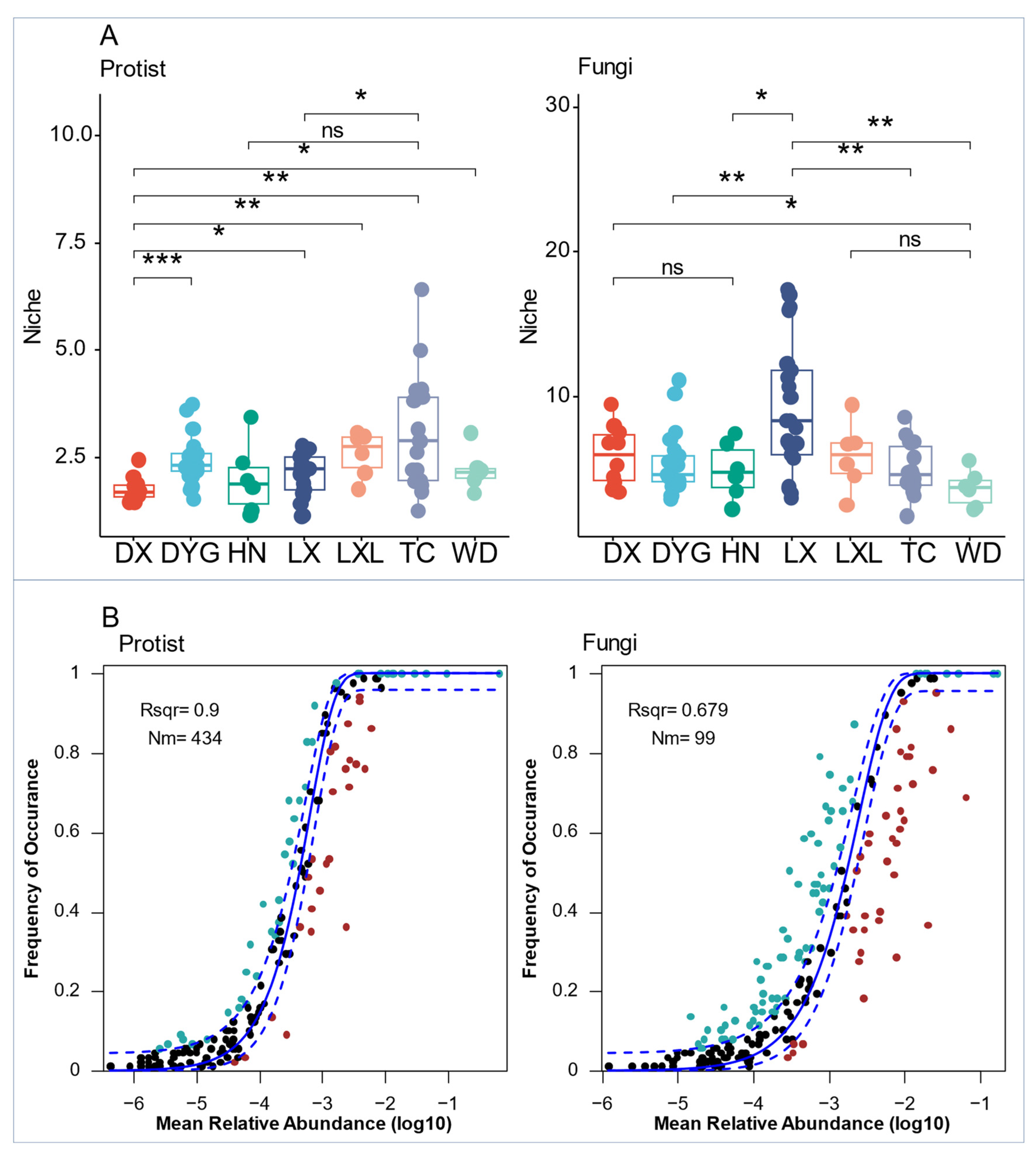
3.4. Co-Occurrence Networks and Assembly Mechanisms of Soil Microeukaryote Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- George, R.J.; Saurav, D.; Salini, S.; Nagendra, T. Editorial: Soil biology for sustainable agriculture and environment. Front. Soil Sci. 2022, 2, 947619. [Google Scholar]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [PubMed]
- Zhu, Y.; Chen, B.; Fu, W. Research frontiers in soil ecology. Sci. Technol. Rev. 2022, 40, 25–31. [Google Scholar]
- Shen, Z.F.; Zhang, W.X.; Shao, Y.H.; Leilei, S.; Shengjie, L.; Nannan, S.; Shenglei, F. Soil biota and sustainable agriculture: A review. Acta Ecol. Sin. 2020, 10, 3183–3206. [Google Scholar]
- Santos, S.S.; Scholer, A.; Nielsen, T.K.; Hansen, L.H.; Schloter, M.; Winding, A. Land use as a driver for protist community structure in soils under agricultural use across Europe. Sci. Total Environ. 2020, 717, 137228. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar]
- Lehmann, J.; Bossio, D.A.; Kogel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Reviews. Earth Environ. 2020, 1, 544–553. [Google Scholar]
- Chertov, O.; Kuzyakov, Y.; Priputina, I.; Frolov, P.; Shanin, V.; Grabarnik, P. Modelling the rhizosphere priming effect in combination with soil food webs to quantify interaction between living plant, soil biota and soil organic matter. Plants 2022, 11, 2605. [Google Scholar] [CrossRef]
- Remke, M.J.; Johnson, N.C.; Bowker, M.A. Sympatric soil biota mitigate a warmer-drier climate for Bouteloua gracilis. Glob. Change Biol. 2022, 28, 6280–6292. [Google Scholar] [CrossRef]
- Zhao, Z.B.; He, J.Z.; Quan, Z.; Wu, C.F.; Sheng, R.; Zhang, L.M.; Geisen, S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020, 148, 107863. [Google Scholar]
- Shu, X.Y.; He, J.; Zhou, Z.H.; Xia, L.L.; Hu, Y.F.; Zhang, Y.L.; Zhang, Y.Y.; Luo, Y.Q.; Chu, H.Y.; Liu, W.J.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [PubMed]
- Jing, J.Y.; Cong, W.F.; Bezemer, T.M. Legacies at work: Plant-soil-microbiome interactions underpinning agricultural sustainability. Trends Plant Sci. 2022, 27, 781–792. [Google Scholar]
- Mackay, J.E.; Bernhardt, L.T.; Smith, R.G.; Ernakovich, J.G. Tillage and pesticide seed treatments have distinct effects on soil microbial diversity and function. Soil Biol. Biochem. 2023, 176, 108860. [Google Scholar]
- Xiong, W.; Song, Y.Q.; Yang, K.M.; Gu, Y.; Wei, Z.; Kowalchuk, G.A.; Xu, Y.C.; Jousset, A.; Shen, Q.R.; Geisen, S. Rhizosphere protists are key determinants of plant health. Microbiome 2020, 8, 27. [Google Scholar] [CrossRef]
- Bano, S.; Wu, X.G.; Zhang, X.J. Towards sustainable agriculture: Rhizosphere microbiome engineering. Appl. Microbiol. Biotechnol. 2021, 105, 7141–7160. [Google Scholar]
- Pulleman, M.M.; de Boer, W.; Giller, K.E.; Kuyper, T.W. Soil biodiversity and nature-mimicry in agriculture; the power of metaphor? Outlook Agric. 2022, 51, 75–90. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Bloem, J.; de Vries, F.T.; Kalbitz, K.; Wagg, C. Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sustain. 2012, 4, 523–528. [Google Scholar]
- National Catalogue Service For Geographic Information. Available online: https://www.webmap.cn/ (accessed on 11 July 2023).
- Geospatial Data Cloud. Available online: http://www.gscloud.cn (accessed on 11 July 2023).
- Han, Y.Y.; Guo, C.; Guan, X.R.; McMinn, A.; Liu, L.; Zheng, G.L.; Jiang, Y.; Liang, Y.T.; Shao, H.B.; Tian, J.W.; et al. Comparison of deep-sea picoeukaryotic composition estimated from the V4 and V9 regions of 18S rRNA gene with a focus on the Hadal Zone of the Mariana Trench. Microb. Ecol. 2022, 83, 34–47. [Google Scholar]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. Vsearch: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Tikhonenkov, D.V.; Strassert, J.F.H.; Janouškovec, J.; Mylnikov, A.P.; Aleoshin, V.V.; Burki, F.; Keeling, P.J. Predatory colponemids are the sister group to all other alveolates. Mol. Phylogenetics Evol. 2020, 149, 106839. [Google Scholar] [CrossRef]
- Dixon, P. Vegan, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Barberan, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research; Photographer: Evanston, IL, USA, 2022. [Google Scholar]
- Liu, Q.; Jiang, Y. Application of microbial network analysis to discriminate environmental heterogeneity in Fildes Peninsula, Antarctica. Mar. Pollut. Bull. 2020, 156, 111244. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the International Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar]
- Levins, R. Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Yu, X.W.; Li, X.R.; Liu, Q.; Yang, M.Y.; Wang, X.X.; Guan, Z.Y.; Yang, J.P.; Liu, M.J.; Yang, E.J.; Jiang, Y. Community assembly and co-occurrence network complexity of pelagic ciliates in response to environmental heterogeneity affected by sea ice melting in the Ross Sea, Antarctica. Sci. Total Environ. 2022, 836, 155695. [Google Scholar] [CrossRef]
- Mo, Y.Y.; Peng, F.; Gao, X.F.; Xiao, P.; Logares, R.; Jeppesen, E.; Ren, K.X.; Xue, Y.Y.; Yang, J. Low shifts in salinity determined assembly processes and network stability of microeukaryotic plankton communities in a subtropical urban reservoir. Microbiome 2021, 9, 128. [Google Scholar]
- Chen, W.D.; Ren, K.X.; Isabwe, A.; Chen, H.H.; Liu, M.; Yang, J. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 138. [Google Scholar]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [PubMed]
- Lucas-Borja, M.E.; Jing, X.; Van Stan, J.T.; Plaza-Alvarez, P.A.; Gonzalez-Romero, J.; Pena, E.; Moya, D.; Zema, D.A.; de Las Heras, J. Changes in soil functionality eight years after fire and post-fire hillslope stabilisation in Mediterranean forest ecosystems. Geoderma 2022, 409, 115603. [Google Scholar] [CrossRef]
- Lerch, T.Z.; Coucheney, E.; Herrmann, A.M. Sensitivity of soil microbial catabolic profiles to a gradient of carbon inputs: Does the soil organic matter? Soil Biol. Biochem. 2013, 57, 911–915. [Google Scholar] [CrossRef]
- Wei, Z.; Song, Y.; Xiong, W.; Xu, Y.; Shen, Q. Soil protozoa: Research methods and roles in the biocontrol of soil-borne diseases. Acta Pedol. Sin. 2021, 58, 14–22. [Google Scholar]
- Lehmann, A.; Zheng, W.S.; Rillig, M.C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 2017, 1, 1828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, B.G.; Liu, Y.; Guo, Y.Q.; Shi, P.; Wei, G.H. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.; Seppey, C.; Lentendu, G.; Dunthorn, M.; Bass, D.; Belbahri, L.; Blandenier, Q.; Debroas, D.; de Groot, G.A.; de Vargas, C.; et al. Protist taxonomic and functional diversity in soil, freshwater and marine ecosystems. Environ. Int. 2021, 146, 106262. [Google Scholar] [CrossRef]
- Luo, S.; Png, G.K.; Ostle, N.J.; Zhou, H.; Hou, X.; Luo, C.; Quinton, J.N.; Schaffner, U.; Sweeney, C.; Wang, D.; et al. Grassland degradation-induced declines in soil fungal complexity reduce fungal community stability and ecosystem multifunctionality. Soil Biol. Biochem. 2023, 176, 108865. [Google Scholar]
- Gu, S.; Xiong, X.; Tan, L.; Deng, Y.; Du, X.; Yang, X.; Hu, Q. Soil microbial community assembly and stability are associated with potato (Solanum tuberosum L.) fitness under continuous cropping regime. Front. Plant Sci. 2022, 13, 1000045. [Google Scholar]
- Vera, A.; Bastida, F.; Patino-Garcia, M.; Moreno, J.L. The effects of boron-enriched water irrigation on soil microbial community are dependent on crop species. Appl. Soil Ecol. 2023, 181, 104677. [Google Scholar]
- Carrasco-Espinosa, K.; Avitia, M.; Barron-Sandoval, A.; Abbruzzini, T.F.; Cabrera, U.; Arroyo-Lambaer, D.; Uscanga, A.; Campo, J.; Benitez, M.; Wegier, A.; et al. Land-use change and management intensification is associated with shifts in composition of soil microbial communities and their functional diversity in coffee agroecosystems. Microorganism 2022, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ning, Y.; Yang, Y.; Huaiyin, Y.; Li, W.; Lingyun, C.; Yangcuo, W.; Xiaoya, S. Use of ciliate communities for monitoring ecological restoration of grain for the green in north-western China. Soil Ecol. Lett. 2021, 4, 264–275. [Google Scholar]
- Fox, A.; Widmer, F.; Luscher, A. Soil microbial community structures are shaped by agricultural systems revealing little temporal variation. Environ. Res. 2022, 214, 113915. [Google Scholar] [PubMed]
- Lammel, D.R.; Nusslein, K.; Cerri, C.; Veresoglou, S.D.; Rillig, M.C. Soil biota shift with land use change from pristine rainforest and Savannah (Cerrado) to agriculture in southern Amazonia. Mol. Ecol. 2021, 30, 4899–4912. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, K.; Zhang, N.; Lili, J. Responses of soil microbial community to different precipitation gradients in the alpine wetlands of Qinghai Lake Basin. Chin. J. Appl. Environ. Biol. 2022, 13, 290–299. [Google Scholar]
- Oliverio, A.M.; Geisen, S.; Delgado-Baquerizo, M.; Maestre, F.T.; Turner, B.L.; Fierer, N. The global-scale distributions of soil protists and their contributions to belowground systems. Sci. Adv. 2020, 6, eaax8787. [Google Scholar] [CrossRef]
- Stefana, G.; Cornelia, B.; Jorg, R.; Michael, B. Soil water availability strongly alters the community composition of soil protists. Pedobiologia 2014, 57, 205–213. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar]
- Guo, S.; Xiong, W.; Hang, X.N.; Gao, Z.L.; Jiao, Z.X.; Liu, H.J.; Mo, Y.N.; Zhang, N.; Kowalchuk, G.A.; Li, R.; et al. Protists as main indicators and determinants of plant performance. Microbiome 2021, 9, 64. [Google Scholar] [CrossRef]
- Wang, C.; Ning, P.; Li, J.Y.; Wei, X.M.; Ge, T.D.; Cui, Y.X.; Deng, X.P.; Jiang, Y.L.; Shen, W.J. Responses of soil microbial community composition and enzyme activities to long-term organic amendments in a continuous tobacco cropping system. Appl. Soil Ecol. 2022, 169, 172–181. [Google Scholar]
- Jia, M.; Huang, L.; Li, Q.; Zhao, J.; Zhang, Y.; Yang, D. Effects of tillage methods on physico-chemical and microbial characteristics of farmland soil and nutritional quality of wheat. J. Plant Nutr. Fertil. 2022, 26, 1964–1976. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [PubMed]
- Tatsumi, C.; Lin, J.; Ishiguro, M.; Uchida, Y. Natural farming diversifies resource-utilisation patterns and increases network complexity in soil microbial community of paddy fields. Agric. Ecosyst. Environ. 2023, 356, 108618. [Google Scholar] [CrossRef]
- Cline, L.C.; Zak, D.R. Dispersal limitation structures fungal community assembly in a long-term glacial chronosequence. Environ. Microbiol. 2014, 16, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.S.; Furtak, K. Soil–Plant–Microbe interactions determine soil biological fertility by altering rhizospheric nutrient cycling and biocrust formation. Sustainability 2023, 15, 625. [Google Scholar]


| Area Number | Sampling Area Short Name | Climate Type | Crop Type | Longitude (E) | Latitude (N) | Average Soil Temperature (°C) |
|---|---|---|---|---|---|---|
| #1 | Longxi | Temperate monsoon | Bupleurum chinense | 104°41′20″ | 34°58′16″ | 29.67 |
| Phaseolus vulgaris | 104°41′20″ | 34°58′16″ | 24.33 | |||
| Solanum tuberosum | 104°41′20″ | 34°58′16″ | 27.33 | |||
| Allium.fistulosum | 104°41′21″ | 34°58′16″ | 28.67 | |||
| Zea mays | 104°41′21″ | 34°58′16″ | 28.33 | |||
| Glycine max | 104°41′19″ | 34°58′16″ | 35.00 | |||
| Allium tuberosum | 104°41′21″ | 34°58′15″ | 31.00 | |||
| #2 | Dingxi | Temperate continental | Solanum tuberosum | 104°30′32″ | 35°27′04″ | 30.00 |
| Brassica oleracea | 104°30′38″ | 35°27′01″ | 28.00 | |||
| Libanotis seseloides | 104°30′47″ | 35°26′59″ | 29.00 | |||
| #3 | Huining | Temperate monsoon | Zea mays | 105°02′35″ | 35°39′05″ | 34.33 |
| Sesamum indicum | 105°02′35″ | 35°39′05″ | 31.33 | |||
| #4 | Linxia | Temperate semi-arid and semi-humid | Medicago sativa | 103°16′01″ | 35°32′52″ | 23.33 |
| Lilium brownii | 103°16′19″ | 35°32′46″ | 30.83 | |||
| #5 | Tanchang | Temperate continental | Zea mays | 104°20′34″ | 34°02′02″ | 25.00 |
| Phaseolus vulgaris | 104°20′33″ | 34°01′59″ | 25.00 | |||
| Cucumis sativus | 104°20′32″ | 34°01′57″ | 23.33 | |||
| Cucurbita moschata | 104°20′32″ | 34°01′59″ | 24.00 | |||
| Vigna unguiculata | 104°20′32″ | 34°01′59″ | 23.67 | |||
| Raphanus sativus | 104°20′31″ | 34°01′59″ | 24.33 | |||
| Capsicum annuum | 104°20′31″ | 34°01′59″ | 25.00 | |||
| Solanum tuberosum | 104°20′33″ | 34°02′01″ | 27.67 | |||
| #6 | Wudu | Subtropical semi-humid | Vigna unguiculata | 105°04′46″ | 33°19′42″ | 30.33 |
| Zea mays | 105°04′46″ | 33°19′41″ | 30.33 | |||
| #7 | Dayugou | Plateau continental | Brassica napusL | 103°34′47″ | 34°25′52″ | 24.00 |
| Hordeum vulgare | 103°34′48″ | 34°25′53″ | 25.33 | |||
| Vicia faba | 103°34′49″ | 34°25′55″ | 22.33 | |||
| Pisum sativum | 103°34′48″ | 34°25′50″ | 21.33 | |||
| Angelica dahurica | 103°34′48″ | 34°25′55″ | 25.67 | |||
| Solanum tuberosum | 103°34′48″ | 34°25′56″ | 25.00 | |||
| Triticum aestivum | 103°34′4″ | 34°25′58″ | 26.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Han, H.; Wang, C.; Warren, A.; Ning, Y. Exploring Microeukaryote Community Characteristics and Niche Differentiation in Arid Farmland Soil at the Northeastern Edge of the Tibetan Plateau. Microorganisms 2023, 11, 2510. https://doi.org/10.3390/microorganisms11102510
Chen L, Han H, Wang C, Warren A, Ning Y. Exploring Microeukaryote Community Characteristics and Niche Differentiation in Arid Farmland Soil at the Northeastern Edge of the Tibetan Plateau. Microorganisms. 2023; 11(10):2510. https://doi.org/10.3390/microorganisms11102510
Chicago/Turabian StyleChen, Lingyun, Haifeng Han, Chunhui Wang, Alan Warren, and Yingzhi Ning. 2023. "Exploring Microeukaryote Community Characteristics and Niche Differentiation in Arid Farmland Soil at the Northeastern Edge of the Tibetan Plateau" Microorganisms 11, no. 10: 2510. https://doi.org/10.3390/microorganisms11102510
APA StyleChen, L., Han, H., Wang, C., Warren, A., & Ning, Y. (2023). Exploring Microeukaryote Community Characteristics and Niche Differentiation in Arid Farmland Soil at the Northeastern Edge of the Tibetan Plateau. Microorganisms, 11(10), 2510. https://doi.org/10.3390/microorganisms11102510







