Agar Contact Method as a Valuable Tool to Identify Slaughter Hygiene Deficiencies along the Slaughter Process by Longitudinally Sampling Pig Skin Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Slaughter Line
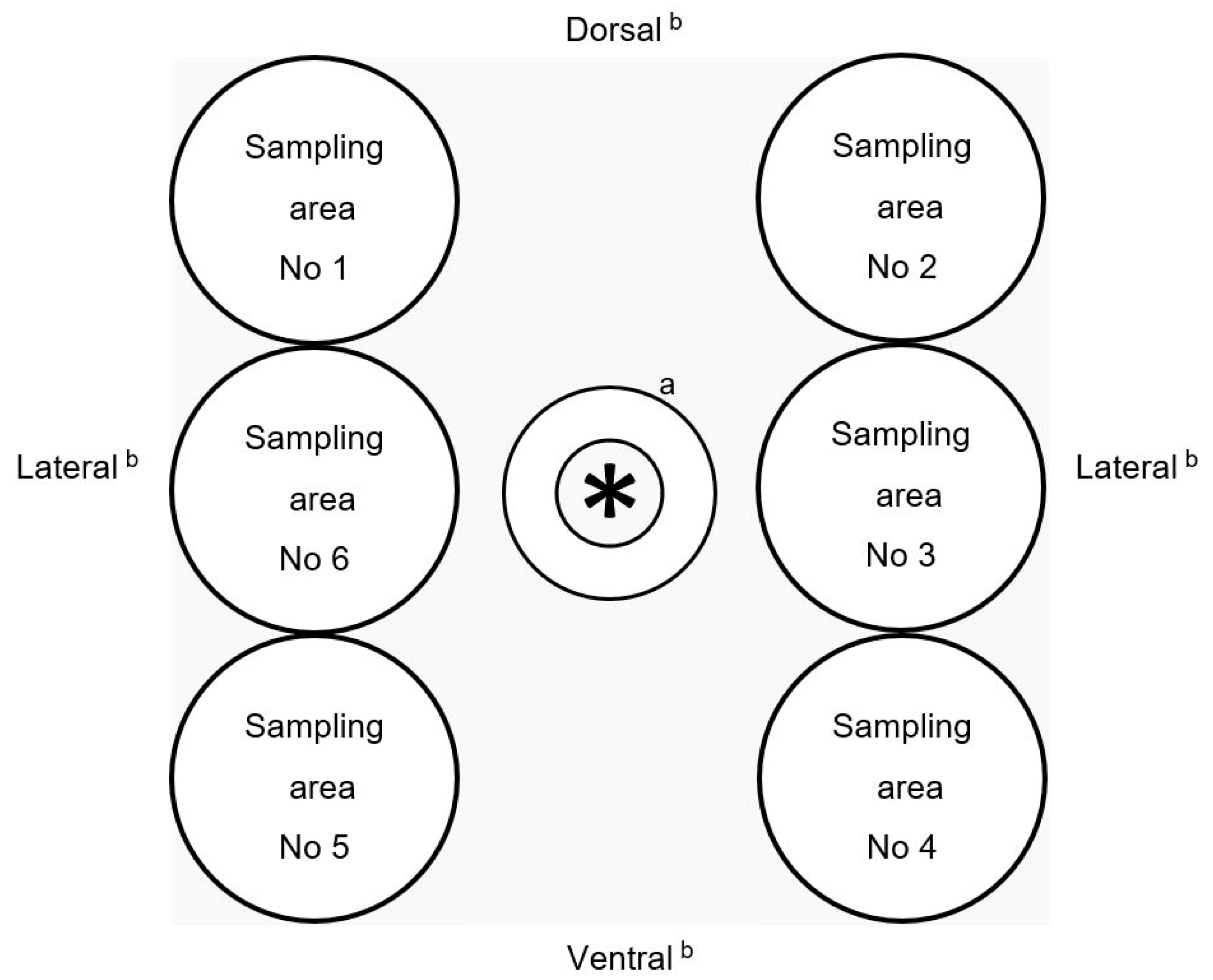
2.2. Sampling Procedure of Pig Skin and Carcass Surface
2.3. Microbiological Analysis
2.4. Isolation and Identification of Salmonella spp.
Minimal Inhibitory Concentration (MIC)
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analysis of Total Viable Count at Batch Level
3.2. ANOVA of Total Viable Count at Batch Level
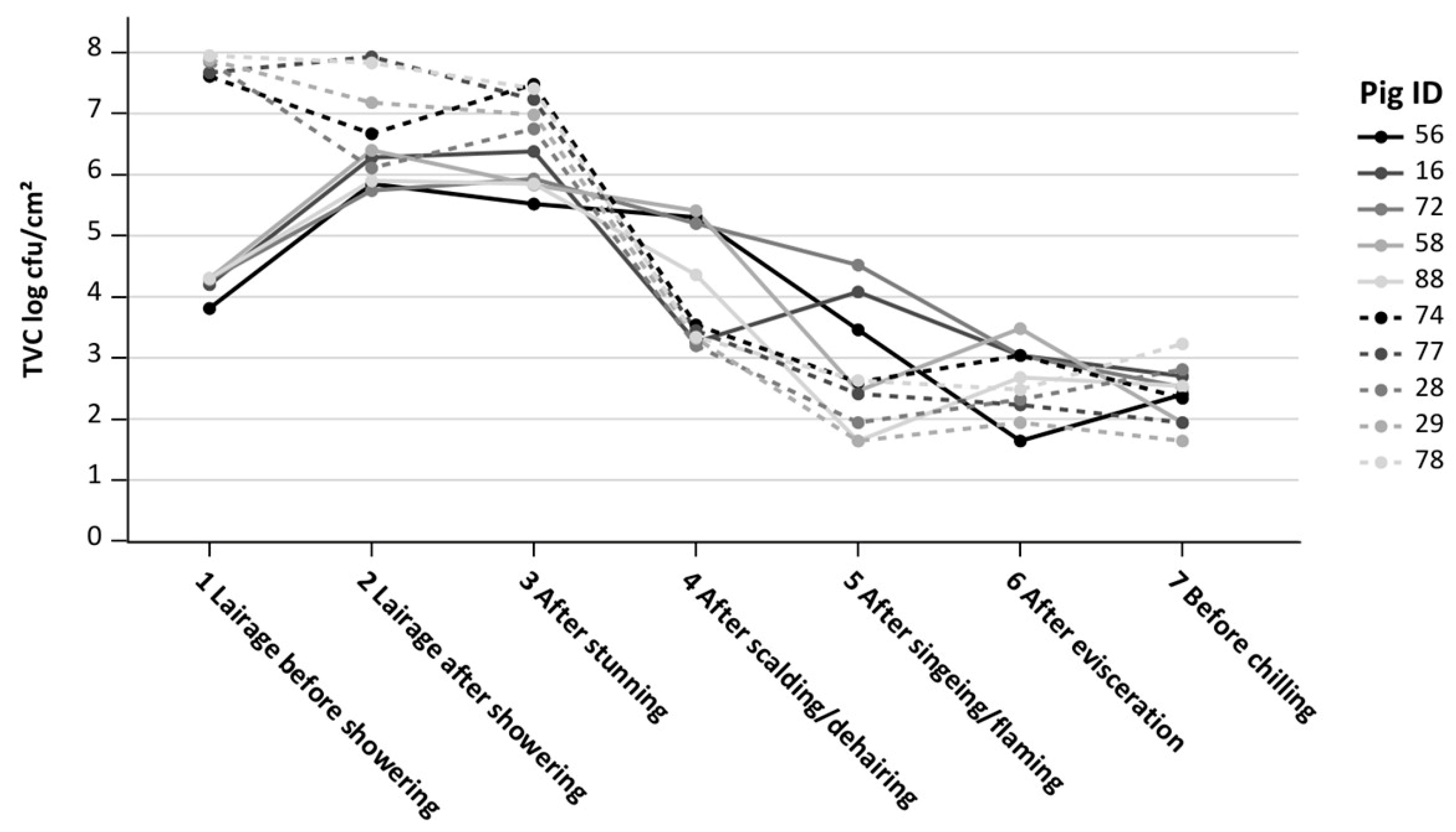
3.3. Total Viable Count at Individual Pig Level
3.4. Reduction Effect of Mechanical Stages
3.5. Salmonella spp. Occurrence
3.6. Phenotypic Characteristics of the Recovered Salmonella Spp. Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moura-Alves, M.; Carvalho, M.; Baggio Ribeiro, D.H.; Barbosa, J.; Silveira, L.; Pista, A.; Pinto, H.P.; Saraiva, C.; Teixeira, P.; Esteves, A. Hygiene indicators and salmonellae on surfaces of swine carcasses from two slaughterhouses in northern Portugal. J. Food Prot. 2022, 85, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Gallina, S.; Bianchi, D.M.; Ru, G.; Maurella, C.; Barzanti, P.; Baioni, E.; Virgilio, S.; Mioni, R.; Lanni, L.; Migliazzo, A.; et al. Microbiological recovery from bovine, swine, equine, and ovine carcasses: Comparison of excision, sponge and swab sampling methods. Food Control 2015, 50, 919–924. [Google Scholar] [CrossRef]
- EC. European Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission Regulation: Brussels, Belgium, 2005; pp. 1–26. [Google Scholar]
- Lindblad, M.; Berking, C. A meat control system achieving significant reduction of visible faecal and ingesta contamination of cattle, lamb and swine carcasses at Swedish slaughterhouses. Food Control 2013, 30, 101–105. [Google Scholar] [CrossRef]
- Piras, F.; Fois, F.; Mazza, R.; Putzolu, M.; Delogu, M.L.; Lochi, P.G.; Pani, S.P.; Mazzette, R. Salmonella Prevalence and Microbiological Contamination of Pig Carcasses and Slaughterhouse Environment. Ital. J. Food Saf. 2014, 3, 4581. [Google Scholar] [CrossRef] [PubMed]
- Betic, N.; Baltic, T.; Ciric, J.; Bajcic, A.; Raseta, M.; Mrdovic, B.; Karabasil, N. Process hygiene of pig carcasses in one large-scale slaughterhouse in the west of Serbia, during 48 months. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kopaonik, Serbia, 22–25 September 2019; p. 012046. [Google Scholar]
- Barco, L.; Belluco, S.; Roccato, A.; Ricci, A. Escherichia coli and Enterobacteriaceae counts on pig and ruminant carcasses along the slaughterline, factors influencing the counts and relationship between visual faecal contamination of carcasses and counts: A review. EFSA Support. Publ. 2014, 11, 634E. [Google Scholar] [CrossRef]
- Delhalle, L.; De Sadeleer, L.; Bollaerts, K.; Farnir, F.; Saegerman, C.; Korsak, N.; Dewulf, J.; De Zutter, L.; Daube, G. Risk factors for Salmonella and hygiene indicators in the 10 largest Belgian pig slaughterhouses. J. Food Prot. 2008, 71, 1320–1329. [Google Scholar] [CrossRef]
- EFSA. The assessment of the comparison of the Australian monitoring programme for carcasses to requirements in Regulation (EC) No 2073/2005 on microbiological criteria on foodstuffs. EFSA J. 2010, 8, 1452. [Google Scholar] [CrossRef]
- Martinez-Aviles, M.; Garrido-Estepa, M.; Alvarez, J.; de la Torre, A. Salmonella Surveillance Systems in Swine and Humans in Spain: A Review. Vet. Sci. 2019, 6, 20. [Google Scholar] [CrossRef]
- Chalias, A.; Grispoldi, L.; Cenci Goga, B. A risk assessment model for Salmonella spp. in swine carcasses. EFSA J 2022, 20, e200405. [Google Scholar] [CrossRef]
- EFSA. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Berends, B.R.; Urlings, H.A.P.; Snijders, J.M.A.; Van Knapen, F. Identification and quantification of risk factors in animal management and transport regarding Salmonella spp. in pigs. Int. J. Food Microbiol. 1996, 30, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Arguello, H.; Alvarez-Ordonez, A.; Carvajal, A.; Rubio, P.; Prieto, M. Role of slaughtering in Salmonella spreading and control in pork production. J. Food Prot. 2013, 76, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.; Hetta, H.F.; Mabrok, M.; Behzadi, P. Editorial: Emerging multidrug-resistant bacterial pathogens “superbugs”: A rising public health threat. Front. Microbiol. 2023, 14, 1135614. [Google Scholar] [CrossRef]
- Algammal, A.M.; Abo Hashem, M.E.; Alfifi, K.J.; Al-Otaibi, A.S.; Alatawy, M.; ElTarabili, R.M.; Abd El-Ghany, W.A.; Hetta, H.F.; Hamouda, A.M.; Elewa, A.A.; et al. Sequence Analysis, Antibiogram Profile, Virulence and Antibiotic Resistance Genes of XDR and MDR Gallibacterium anatis Isolated from Layer Chickens in Egypt. Infect Drug Resist. 2022, 15, 4321–4334. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Ibrahim, R.A.; Alfifi, K.J.; Ghabban, H.; Alghamdi, S.; Kabrah, A.; Khafagy, A.R.; Abou-Elela, G.M.; Abu-Elala, N.M.; Donadu, M.G.; et al. A First Report of Molecular Typing, Virulence Traits, and Phenotypic and Genotypic Resistance Patterns of Newly Emerging XDR and MDR Aeromonas veronii in Mugil seheli. Pathogens 2022, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Zeng, M.; Permana, B.; Bilal, H.; Huang, J.; Yao, F.; Algammal, A.M.; Li, X.; Yuan, Y.; Jiao, X. Coexistence of bla (NDM-5) and tet(X4) in international high-risk Escherichia coli clone ST648 of human origin in China. Front. Microbiol. 2022, 13, 1031688. [Google Scholar] [CrossRef]
- ISO 17604:2015-12; Microbiology of the food chain-Carcass sampling for microbiological analysis. International Organization for Standardization: Geneva, Switzerland, 2015. [CrossRef]
- Fliss, I.; Simard, R.; Ettriki, A. Comparison of three sampling techniques for microbiological analysis of meat surfaces. J. Food Sci. 1991, 56, 249–250. [Google Scholar] [CrossRef]
- Lindblad, M. Microbiological sampling of swine carcasses: A comparison of data obtained by swabbing with medical gauze and data collected routinely by excision at Swedish abattoirs. Int. J. Food Microbiol. 2007, 118, 180–185. [Google Scholar] [CrossRef]
- Tenhagen, B.A.; Arth, O.; Bandick, N.; Fetsch, A. Comparison of three sampling methods for the quantification of methicillin-resistant Staphylococcus aureus on the surface of pig carcases. Food Control 2011, 22, 643–645. [Google Scholar] [CrossRef]
- Milios, K.T.; Drosinos, E.H.; Zoiopoulos, P.E. Food Safety Management System validation and verification in meat industry: Carcass sampling methods for microbiological hygiene criteria—A review. Food Control 2014, 43, 74–81. [Google Scholar] [CrossRef]
- Pepperell, R.; Reid, C.A.; Solano, S.N.; Hutchison, M.L.; Walters, L.D.; Johnston, A.M.; Buncic, S. Experimental comparison of excision and swabbing microbiological sampling methods for carcasses. J. Food Prot. 2005, 68, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Louwers, J.; Klein, G. Suitability of sampling methods for the investigation of the environment in EC-licensed meat rendering and processing plants. Berl. Muench Tieraerztl. Wochenschr. 1994, 107, 367–373. [Google Scholar]
- Baumgart, J.; Kussmann, H. A spray method for determining the surface bacterial content of animals for slaughter. Fleischwirtschaft 1975, 55, 1113–1114. [Google Scholar]
- Kusch, D. Ein Beitrag zur Hygienekontrolle in fleischverarbeitenden Betrieben. J. Food Saf. Food Qual. 1977, 28, 68–71. [Google Scholar]
- Globisch, H.; Wilkens, S.; Jacob, A.; Thien, J. Anwendbarkeit von Abklatschverfahren für die Untersuchung von Oberflächenkeimgehalten bei Schlachttierkörpern: Vergleichende Bestimmung der aeroben mesophilen Gesamtkeimzahl mittels Abklatschtechnik und destruktiver Probenahmetechnik. Fleischwirtschaft 1996, 76, 1116–1118. [Google Scholar]
- Kleiner, U.; Hilgert, S. Conversion of the decision 2001/471/EC: Comparison of the destructive and non-destructive sampling techniques for microbiological control of meat surfaces–2. Sections of swine. Fleischwirtschaft 2004, 84, 146–149. [Google Scholar]
- Kleiner, U.; Hilgert, S. Conversion of the decision 2001/471/EC: Comparison of the destructive and non-destructive sampling techniques for microbiological control of meat surfaces–1. Swine carcase. Fleischwirtschaft 2004, 84, 101–104. [Google Scholar]
- Hübner, P.; Gautsch, S.; Jemmi, T. In house validierung (single laboratory validation) of microbiological methods. Mitteilungen Aus. Lebensm. Und. Hyg. 2002, 93, 118–139. [Google Scholar]
- Gill, C.O.; McGinnis, J.C. Improvement of the hygienic performance of the hindquarters skinning operations at a beef packing plant. Int. J. Food Microbiol. 1999, 51, 123–132. [Google Scholar] [CrossRef]
- Fürstenberg, R.; Meemken, D.; Langforth, S.; Grosse-Kleimann, J.; Kreienbrock, L.; Langkabel, N. Comparison of the agar contact method and the wet-dry double swabbing method for determining the total viable bacterial count on pig carcass surfaces. J. Consum. Prot. Food Saf. 2023. submitted, revised, processing of minor revisions. [Google Scholar]
- Wheatley, P.; Giotis, E.S.; McKevitt, A.I. Effects of slaughtering operations on carcass contamination in an Irish pork production plant. Ir. Vet. J. 2014, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Spescha, C.; Stephan, R. Process stages in pig slaughter: Influence on the microbiological contamination of carcasses in two abattoirs. Arch. Lebensm. Hyg. 2007, 58, 7–12. [Google Scholar] [CrossRef]
- DIN 10161:2016-12; Microbiological analysis of meat and meat products-Aerobic count at 30 °C-Drop plating method. Deutsches Institut für Normung e.V., Germany: Berlin, Germany, 2016. [CrossRef]
- ISO 6579-1:2020-08; Microbiology of the food chain-Horizontal method for the detection, enumeration and serotyping of Salmonella-Part 1: Detection of Salmonella spp. (ISO 6579-1:2017 + Amd.1:2020). International Organization for Standardization: Geneva, Switzerland, 2020. [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard—Tenth Edition. CLSI Document M07-A10; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020; ISBN 978-1-68440-066-9 (Print); ISBN 978-1-68440-067-6 (Electronic). [Google Scholar]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Reid, C.A.; Wilson, D.; Howell, M.; Johnston, A.M.; Buncic, S. A comparison of wet-dry swabbing and excision sampling methods for microbiological testing of bovine, porcine, and ovine carcasses at red meat slaughterhouses. J. Food Prot. 2005, 68, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Ghafir, Y.; China, B.; Dierick, K.; De Zutter, L.; Daube, G. Hygiene indicator microorganisms for selected pathogens on beef, pork, and poultry meats in Belgium. J. Food Prot. 2008, 71, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Schertenleib, T.I.; Stephan, R.; Scheeder, M.; Zweifel, C. Visual and microbiological process analysis of pig slaughtering in a small-scale abattoir. J. Food Saf. Food Qual. 2011, 62, 52–57. [Google Scholar] [CrossRef]
- Nastasijevic, I.; Lakicevic, B.; Raseta, M.; Djordjevic, V.; Jankovic, V.; Mrdovic, B.; Brankovic-Lazic, I. Evaluation of Pig Welfare in Lairage and Process Hygiene in a Single Abattoir. Meat. Technol. 2018, 59, 8–22. [Google Scholar] [CrossRef]
- Walia, K.; Lynch, H.; Grant, J.; Duffy, G.; Leonard, F.C.; Lawlor, P.G.; Gardiner, G.E. The efficacy of disinfectant misting in the lairage of a pig abattoir to reduce Salmonella and Enterobacteriaceae on pigs prior to slaughter. Food Control 2017, 75, 55–61. [Google Scholar] [CrossRef]
- Van Ba, H.; Seo, H.W.; Seong, P.N.; Kang, S.M.; Cho, S.H.; Kim, Y.S.; Park, B.Y.; Moon, S.S.; Kang, S.J.; Choi, Y.M.; et al. The fates of microbial populations on pig carcasses during slaughtering process, on retail cuts after slaughter, and intervention efficiency of lactic acid spraying. Int. J. Food Microbiol. 2019, 294, 10–17. [Google Scholar] [CrossRef]
- Swart, A.N.; Evers, E.G.; Simons, R.L.; Swanenburg, M. Modeling of Salmonella Contamination in the Pig Slaughterhouse. Risk Anal. 2016, 36, 498–515. [Google Scholar] [CrossRef]
- Pearce, R.A.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A.; Blair, I.S.; Harrington, D. Studies to determine the critical control points in pork slaughter hazard analysis and critical control point systems. Int. J. Food Microbiol. 2004, 90, 331–339. [Google Scholar] [CrossRef]
- Spescha, C.; Stephan, R.; Zweifel, C. Microbiological contamination of pig carcasses at different stages of slaughter in two European Union-approved abattoirs. J. Food Prot. 2006, 69, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Fischer, R.; Stephan, R. Microbiological contamination of pig and cattle carcasses in different small-scale Swiss abattoirs. Meat Sci. 2008, 78, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bolton, D.J.; Pearce, R.A.; Sheridan, J.J.; Blair, I.S.; McDowell, D.A.; Harrington, D. Washing and chilling as critical control points in pork slaughter hazard analysis and critical control point (HACCP) systems. J. Appl. Microbiol. 2002, 92, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Rivas, T.; Vizcaino, J.A.; Herrera, F.J. Microbial contamination of carcasses and equipment from an Iberian pig slaughterhouse. J. Food Prot. 2000, 63, 1670–1675. [Google Scholar] [CrossRef]
- Biasino, W.; De Zutter, L.; Woollard, J.; Mattheus, W.; Bertrand, S.; Uyttendaele, M.; Van Damme, I. Reduced contamination of pig carcasses using an alternative pluck set removal procedure during slaughter. Meat Sci. 2018, 145, 23–30. [Google Scholar] [CrossRef]
- Petruzzelli, A.; Osimani, A.; Pasquini, M.; Clementi, F.; Vetrano, V.; Paolini, F.; Foglini, M.; Micci, E.; Paoloni, A.; Tonucci, F. Trends in the microbial contamination of bovine, ovine and swine carcasses in three small-scale abattoirs in central Italy: A four-year monitoring. Meat Sci. 2016, 111, 53–59. [Google Scholar] [CrossRef]
- Goverde, M.; Willrodt, J.; Staerk, A. Evaluation of the Recovery Rate of Different Swabs for Microbial Environmental Monitoring. PDA J. Pharm. Sci. Technol. 2017, 71, 33–42. [Google Scholar] [CrossRef]
- ISO 18593:2018; Microbiology of the food chain –Horizontal methods for surface sampling. International Organization for Standardization: Geneva, Switzerland, 2018. [CrossRef]
- Capita, R.; Prieto, M.; Alonso-Calleja, C. Sampling methods for microbiological analysis of red meat and poultry carcasses. J. Food Prot. 2004, 67, 1303–1308. [Google Scholar] [CrossRef]
- Bryant, J.; Brereton, D.A.; Gill, C.O. Implementation of a validated HACCP system for the control of microbiological contamination of pig carcasses at a small abattoir. Can. Vet. J. 2003, 44, 51–55. [Google Scholar]
- Hurd, H.S.; McKean, J.D.; Griffith, R.W.; Wesley, I.V.; Rostagno, M.H. Salmonella enterica Infections in Market Swine with and without Transport and Holding. Appl. Environ. Microbiol. 2002, 68, 2376–2381. [Google Scholar] [CrossRef]
- González Santamarina, B. Salmonella Carried over by Pigs during Transport and Lairage. Doctoral Thesis, Freie Universität Berlin, Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Hurd, H.S.; Gailey, J.K.; McKean, J.D.; Rostagno, M.H. Rapid infection in market-weight swine following exposure to a Salmonella Typhimurium-contaminated environment. Am. J. Vet. Res. 2001, 62, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- van der Gaag, M.A.; Vos, F.; Saatkamp, H.W.; van Boven, M.; van Beek, P.; Huirne, R.B.M. A state-transition simulation model for the spread of Salmonella in the pork supply chain. Eur. J. Oper. Res. 2004, 156, 782–798. [Google Scholar] [CrossRef]
- De Busser, E.V.; Maes, D.; Houf, K.; Dewulf, J.; Imberechts, H.; Bertrand, S.; De Zutter, L. Detection and characterization of Salmonella in lairage, on pig carcasses and intestines in five slaughterhouses. Int. J. Food Microbiol. 2011, 145, 279–286. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Cohen, A.L.; Calfee, D.; Fridkin, S.K.; Huang, S.S.; Jernigan, J.A.; Lautenbach, E.; Oriola, S.; Ramsey, K.M.; Salgado, C.D.; Weinstein, R.A.; et al. Recommendations for metrics for multidrug-resistant organisms in healthcare settings: SHEA/HICPAC Position paper. Infect. Control Hosp. Epidemiol. 2008, 29, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Koletsi, P.K.; Bliziotis, I.A. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J. Med. Microbiol. 2006, 55, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K.; National Healthcare Safety Network, T.; Participating National Healthcare Safety Network, F. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef]
- Kallen, A.J.; Hidron, A.I.; Patel, J.; Srinivasan, A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp. Epidemiol. 2010, 31, 528–531. [Google Scholar] [CrossRef]
- The Federal Office of Consumer Protection and Food Safety (BVL). BVL-Report 16.1 Berichte zur Lebensmittelsicherheit, Zoonosen-Monitoring 2020, BVL. 2020. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/01_Lebensmittel/04_Zoonosen_Monitoring/Zoonosen_Monitoring_Bericht_2020.pdf?__blob=publicationFile&v=7 (accessed on 21 September 2023). (In German).


| Process Stage | n | Mean | Median | STD | CV | Min | Max | <LOD |
|---|---|---|---|---|---|---|---|---|
| 1 Lairage before showering | 86 | 5.70 | 5.71 | ±1.05 | 18.36 | 3.81 | 7.95 | 0 |
| 2 Lairage after showering | 86 | 6.27 | 6.11 | ±0.72 | 11.54 | 4.86 | 8.00 | 0 |
| 3 After stunning | 87 | 6.48 | 6.38 | ±0.70 | 10.85 | 4.97 | 8.15 | 0 |
| 4 After scalding/dehairing | 86 | 3.71 | 3.49 | ±0.78 | 21.10 | 2.23 | 6.15 | 0 |
| 5 After singeing/flaming | 86 | 2.70 | 2.46 | ±0.86 | 31.99 | 1.64 | 5.57 | 7 |
| 6 After evisceration | 87 | 2.44 | 2.40 | ±0.64 | 26.06 | 1.64 | 4.59 | 14 |
| 7 Before chilling | 85 | 2.33 | 2.20 | ±0.72 | 30.69 | 1.64 | 4.86 | 23 |
| Compared Process Stages | Mean Difference * [TVC log cfu/cm2] | p-Value |
|---|---|---|
| 1 Lairage before showering–2 Lairage after showering | 0.57 | <0.0001 |
| 2 Lairage after showering–3 After stunning | 0.21 | 0.0466 |
| 3 After stunning–4 After scalding/dehairing | −2.78 | <0.0001 |
| 4 After scalding/dehairing–5 After singeing/flaming | −1.00 | <0.0001 |
| 5 After singeing/flaming–6 After evisceration | −0.26 | 0.0139 |
| 6 After evisceration–7 Before chilling | −0.11 | 0.2891 |
| Process Stage | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Stage 6 | Stage 7 | |
|---|---|---|---|---|---|---|---|---|
| Pig Batch | ||||||||
| 1 | 0 | ONST (2 *) | ONST (6 *) | 0 | 0 | 0 | 0 | |
| 2 | ONST (12 *); ONST (14 *) | ONST (11 *); ONST (18 *) | ONST (17 *) | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | MST (51 *) | MST (49 *); MST (53 *); MST (54 *) | 0 | 0 | 0 | |
| 7 | 0 | 0 | SP (56 *) | 0 | 0 | 0 | 0 | |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Antimicrobial Agent(s) | Susceptible * | Intermediate * | Resistant * | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Amikacin | 12 | 100 | 0 | 0 | 0 | 0 |
| Ampicillin | 1 | 8 | 0 | 0 | 11 | 92 |
| Azithromycin | 12 | 100 | 0 | 0 | 0 | 0 |
| Cefotaxime | 12 | 100 | 0 | 0 | 0 | 0 |
| Ceftazidime | 12 | 100 | 0 | 0 | 0 | 0 |
| Chloramphenicol | 12 | 100 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 11 | 92 | 0 | 0 | 1 | 8 |
| Colistin | 12 | 100 | 0 | 0 | 0 | 0 |
| Gentamicin | 12 | 100 | 0 | 0 | 0 | 0 |
| Meropenem | 12 | 100 | 0 | 0 | 0 | 0 |
| Nalidixic acid | 11 | 92 | 0 | 0 | 1 | 8 |
| Sulfamethoxazole | 0 | 0 | 0 | 0 | 12 | 100 |
| Tetracycline | 5 | 42 | 0 | 0 | 7 | 58 |
| Tigecycline | 12 | 100 | 0 | 0 | 0 | 0 |
| Trimethoprim | 12 | 100 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fürstenberg, R.; Langkabel, N.; Grosse-Kleimann, J.; Kreienbrock, L.; Meemken, D. Agar Contact Method as a Valuable Tool to Identify Slaughter Hygiene Deficiencies along the Slaughter Process by Longitudinally Sampling Pig Skin Surfaces. Microorganisms 2023, 11, 2512. https://doi.org/10.3390/microorganisms11102512
Fürstenberg R, Langkabel N, Grosse-Kleimann J, Kreienbrock L, Meemken D. Agar Contact Method as a Valuable Tool to Identify Slaughter Hygiene Deficiencies along the Slaughter Process by Longitudinally Sampling Pig Skin Surfaces. Microorganisms. 2023; 11(10):2512. https://doi.org/10.3390/microorganisms11102512
Chicago/Turabian StyleFürstenberg, Roland, Nina Langkabel, Julia Grosse-Kleimann, Lothar Kreienbrock, and Diana Meemken. 2023. "Agar Contact Method as a Valuable Tool to Identify Slaughter Hygiene Deficiencies along the Slaughter Process by Longitudinally Sampling Pig Skin Surfaces" Microorganisms 11, no. 10: 2512. https://doi.org/10.3390/microorganisms11102512





