Review of the Impact of Biofilm Formation on Recurrent Clostridioides difficile Infection
Abstract
1. Introduction
| Risk Factor | Odds Ratio | p | Ref. |
|---|---|---|---|
| Primary CDI a | |||
| Age ≥ 65 years | 2.4 (1.6–3.5) c | <0.001 | [6] |
| Recent hospitalization | 2.1 (1.5–3.1) b | <0.001 | [6] |
| Enteral feeding | 2.9 (2.0–4.1) | <0.001 | [7] |
| Vascular surgery | 2.4 (1.3–4.5) | 0.003 | [6] |
| Surgery in the preceding 12 weeks | 1.7 (1.3–2.4) c | <0.001 | [6] |
| Surgery and gastrointestinal interventions | 1.9 (1.2–3.0) b | 0.003 | [6] |
| Myocardial infarction | 1.7 (1.2–2.6) | 0.003 | [6] |
| Gastrointestinal intervention | 2.9 (1.9–4.6) | <0.001 | [6] |
| Congestive heart failure | 1.9 (1.4–2.5) | <0.001 | [6] |
| Chronic kidney disease | 1.9 (1.4–2.6) | <0.001 | [7] |
| Peripheral vascular disease | 1.5 (1.1–2.2) | 0.011 | [6] |
| Diabetes with organ damage | 2.1 (1.3–3.4) | 0.001 | [6] |
| Cerebrovascular disease | 2.0 (1.2–3.5) | 0.008 | [6] |
| Dementia | 4.0 (2.4–6.8) | <0.001 | [7] |
| Connective tissue disease | 3.3 (1.6–6.7) | <0.001 | [7] |
| Inflammatory bowel disease | 2.2 (1.2–4.1) | 0.006 | [7] |
| Urinary tract infection | 2.2 (1.3–4.1) | 0.004 | [6] |
| Dementia | 2.5 (1.3–4.8) | 0.003 | [6] |
| Chronic obstructive pulmonary disease | 2.0 (1.1–3.8) | 0.021 | [6] |
| Leukemia | 2.3 (1.2–4.1) | 0.004 | [7] |
| Charlson Comorbidity Index score 3 ≥ 3 vs. <3 | 1.5 (1.2–1.9) | <0.001 | [7] |
| Duration of acid-suppressive therapy (≥15 days) | 3.8 (2.9–4.8) | <0.001 | [7] |
| Chemotherapy | 1.8 (1.1–2.8) | 0.006 | [7] |
| Corticosteroids | 1.6 (1.2–2.1) | <0.001 | [7] |
| Immunosuppressant agent use | 1.6 (1.22.14) | <0.001 | [7] |
| Proton pump inhibitor use | 1.7 (1.3–2.4) c | 0.001 | [6] |
| At least one antibiotic (any class) | 1.3 (1.1–1.4) b | <0.001 | [6] |
| Cephalosporins | 2.2 (1.3–3.8) b | 0.003 | [6] |
| 2.1 (1.7–2.7) | <0.001 | [7] | |
| Third generation | 5.4 (3.0–9.8) | <0.001 | [7] |
| Fourth generation | 2.0 (1.4–2.9) | <0.001 | [7] |
| Glycopeptides | 3.2 (2.2–4.6) | <0.001 | [6] |
| Fluoroquinolones | 1.4 (1.1–2.1) | 0.022 | [6] |
| 1.6 (1.2–2.1) | <0.001 | [7] | |
| Meropenem | 1.7 (1.2–2.6) | 0.003 | [6] |
| Carbapenem | 4.8 (3.6–6.5) | <0.001 | [7] |
| Clindamycin | 2.0 (1.4–2.9) | <0.001 | [7] |
| Aminoglycoside | 2.7 (1.7–4.1) | <0.001 | [7] |
| Tetracycline | 2.9 (1.3–6.3) | 0.005 | [7] |
| Linezolid | 2.3 (1.3–4.2) | 0.003 | [7] |
| Rifampicin | 3.4 (1.2–9.4) | 0.013 | [7] |
| Total duration of antibiotic therapy (≥15 days) | 3.7 (2.9–4.8) | <0.001 | [7] |
| R-CDI a | |||
| Age ≥ 65 years | 1.6 (1.1–2.3) | 0.0012 | [14] |
| Additional non-CDI antibiotics during follow-up | 4.2 (2.1–8.5) | 0.001 | [14] |
| Proton pump inhibitors during follow-up | 2.14 (1.1–4.0) 1.6 (1.4–1.9) | 0.019 NR | [14,15] |
| Nasogastric tube insertion | 8.7 (1.2–59.1) | 0.026 | [16] |
| Cardiovascular disease | 3.0 (1.2–7.3) | 0.015 | [17] |
| Immunosuppressive comorbidities | 3.8 (1.3–11.2) | 0.012 | [17] |
| Dementia | 3.2 (1.2–8.4) | 0.014 | [17] |
2. Pathophysiology of C. difficile Infection
3. Virulence Factors: Toxins and Spores
4. Biofilm
4.1. Biofilm Composition
4.2. Biofilm Formation
4.3. Systems and Structures Associated with Adhesion and Biofilm Formation
4.3.1. Cell-Wall Proteins
4.3.2. Quorum Sensing
Lux S
Agr
4.3.3. Cyclic di-GMP
4.3.4. Type IV pili
4.3.5. Flagella
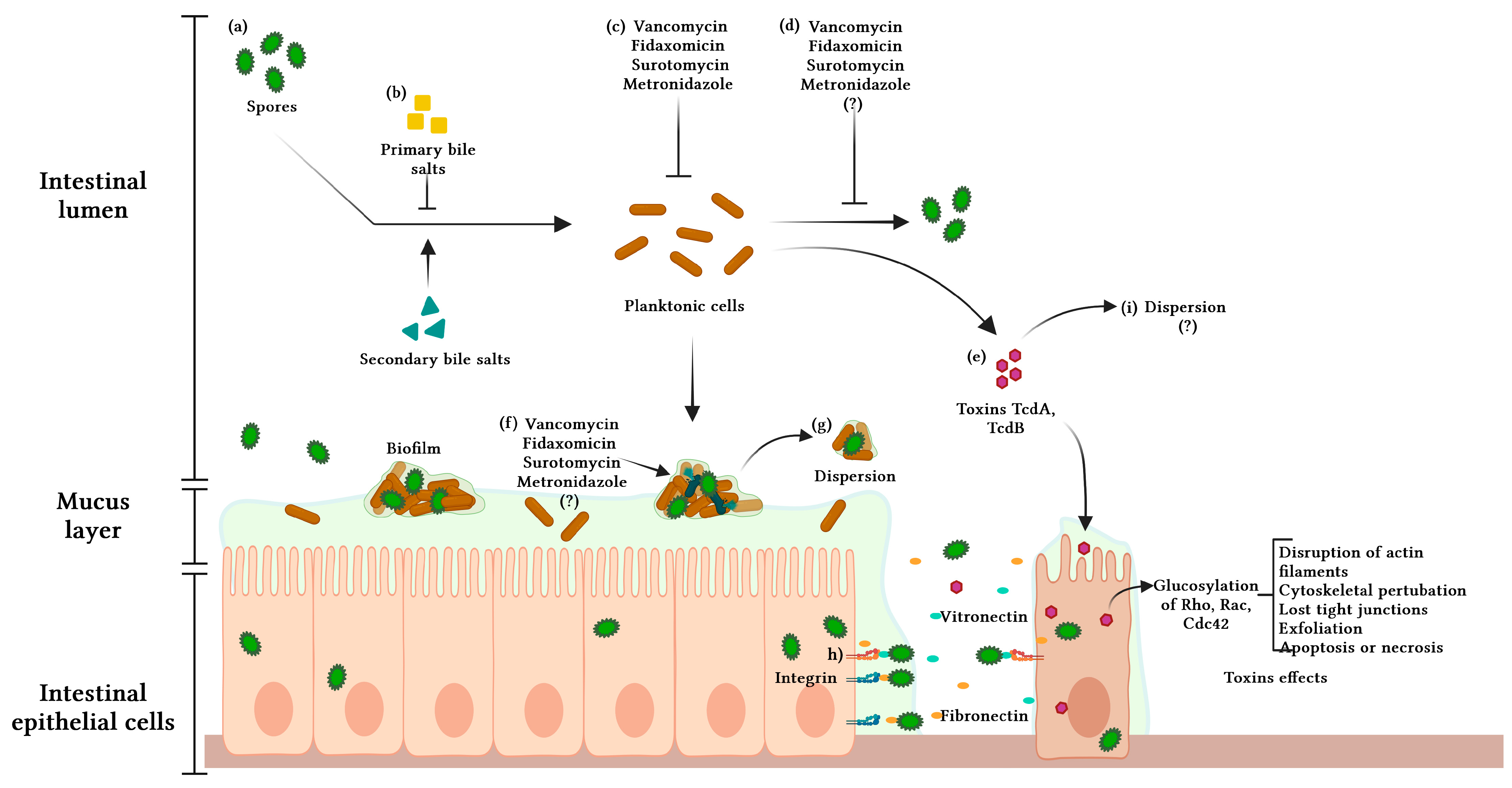
5. The Role of Spores and Biofilms in CDI and R-CDI
6. Biofilm and Antibiotic Resistance
6.1. Fidaxomicin
6.2. Vancomycin
6.3. Metronidazole
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium difficile and One Health. Clin. Microbiol. Infect. 2020, 26, 857–863. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Abreu, Y.; Abreu, A.T.; Velarde-Ruiz Velasco, J.A.; Zavala-Solares, M.R.; Remes-Troche, J.M.; Carmona-Sánchez, R.I.; Aldana-Ledesma, J.M.; Camacho-Ortiz, A.; Contreras-Omaña, R.; Díaz-Seoane, R.; et al. Consensus on the prevention, diagnosis, and treatment of Clostridium difficile infection. Rev. Gastroenterol. Mex. 2019, 84, 204–219. [Google Scholar] [CrossRef]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef] [PubMed]
- Abou Chakra, C.N.; Pepin, J.; Sirard, S.; Valiquette, L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: A systematic review. PLoS ONE 2014, 9, e98400. [Google Scholar] [CrossRef]
- Davies, K.; Lawrence, J.; Berry, C.; Davis, G.; Yu, H.; Cai, B.; Gonzalez, E.; Prantner, I.; Kurcz, A.; Macovei, I.; et al. Risk Factors for Primary. Front. Public. Health 2020, 8, 293. [Google Scholar] [CrossRef]
- Yu, H.; Flaster, N.; Casanello, A.L.; Curcio, D. Assessing risk factors, mortality, and healthcare utilization associated with Clostridioides difficile infection in four Latin American countries. Braz. J. Infect. Dis. 2021, 25, 101040. [Google Scholar] [CrossRef]
- Wang, R. infection: Microbe-microbe interactions and live biotherapeutics. Front. Microbiol. 2023, 14, 1182612. [Google Scholar] [CrossRef]
- Cho, J.M.; Pardi, D.S.; Khanna, S. Update on Treatment of Clostridioides difficile Infection. Mayo Clin. Proc. 2020, 95, 758–769. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Terveer, E.M.; Dahlerup, J.F.; Erikstrup, C.; Arkkila, P.; Vehreschild, M.J.; Ianiro, G.; Gasbarrini, A.; Sokol, H.; Kump, P.K.; et al. The use of Faecal Microbiota Transplantation (FMT) in Europe: A Europe-wide survey. Lancet Reg. Health Eur. 2021, 9, 100181. [Google Scholar] [CrossRef]
- Rounds, J.; Strain, J. Bezlotoxumab for Preventing Recurrent Clostridium difficile Infections. S D Med. 2017, 70, 422–423. [Google Scholar] [PubMed]
- Shakov, R.; Salazar, R.S.; Kagunye, S.K.; Baddoura, W.J.; DeBari, V.A. Diabetes mellitus as a risk factor for recurrence of Clostridium difficile infection in the acute care hospital setting. Am. J. Infect. Control 2011, 39, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Yang, J.; Huang, H. The role of single and mixed biofilms in The role of single and mixed biofilms in Clostridioides difficile infection and strategies for prevention and inhibition. Crit. Rev. Microbiol. Crit. Rev. Microbiol. 2023, 9, e1003356. [Google Scholar] [CrossRef]
- Garey, K.W.; Sethi, S.; Yadav, Y.; DuPont, H.L. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J. Hosp. Infect. 2008, 70, 298–304. [Google Scholar] [CrossRef]
- D’Silva, K.M.; Mehta, R.; Mitchell, M.; Lee, T.C.; Singhal, V.; Wilson, M.G.; McDonald, E.G. Proton pump inhibitor use and risk for recurrent Clostridioides difficile infection: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 697–703. [Google Scholar] [CrossRef]
- Doh, Y.S.; Kim, Y.S.; Jung, H.J.; Park, Y.I.; Mo, J.W.; Sung, H.; Lee, K.J.; Seo, Y.K.; Moon, J.S.; Hong, S.W. Long-Term Clinical Outcome of Clostridium difficile Infection in Hospitalized Patients: A Single Center Study. Intest. Res. 2014, 12, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Negrut, N.; Bungau, S.; Behl, T.; Khan, S.A.; Vesa, C.M.; Bustea, C.; Nistor-Cseppento, D.C.; Rus, M.; Pavel, F.M.; Tit, D.M. Risk Factors Associated with Recurrent. Healthcare 2020, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.B.; Allen, C.A.; Shrestha, R.; Sorg, J.A. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog. 2013, 9, e1003356. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Ascenzi, P.; Siarakas, S.; Petrosillo, N.; di Masi, A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins 2016, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Riegler, M.; Sedivy, R.; Pothoulakis, C.; Hamilton, G.; Zacherl, J.; Bischof, G.; Cosentini, E.; Feil, W.; Schiessel, R.; LaMont, J.T. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J. Clin. Invest. 1995, 95, 2004–2011. [Google Scholar] [CrossRef]
- Sun, X.; Savidge, T.; Feng, H. The enterotoxicity of Clostridium difficile toxins. Toxins 2010, 2, 1848–1880. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.F.; Maia, M.E.; Bezerra, L.R.; Lyerly, D.M.; Guerrant, R.L.; Ribeiro, R.A.; Lima, A.A. Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: Role of interleukin-1beta, tumor necrosis factor alpha, and leukotrienes. Infect. Immun. 1997, 65, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Hill, C. Virulence or niche factors: What’s in a name? J. Bacteriol. 2012, 194, 5725–5727. [Google Scholar] [CrossRef]
- Gupta, V.; Ye, G.; Olesky, M.; Lawrence, K.; Murray, J.; Yu, K. National prevalence estimates for resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States. Int. J. Infect. Dis. 2019, 85, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile toxins: Mechanisms of action and antitoxin therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Martin-Verstraete, I.; Peltier, J.; Dupuy, B. The Regulatory Networks That Control Clostridium difficile Toxin Synthesis. Toxins 2016, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile colitis: Pathogenesis and host defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.A.; Tatge, H.; Schneider, C.; Ruschig, M.; Hust, M.; Barton, J.; Thiemann, S.; Fühner, V.; Russo, G.; Gerhard, R. The Binary Toxin CDT of. Toxins 2018, 10, 225. [Google Scholar] [CrossRef]
- Rupnik, M.; Avesani, V.; Janc, M.; von Eichel-Streiber, C.; Delmée, M. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J. Clin. Microbiol. 1998, 36, 2240–2247. [Google Scholar] [CrossRef]
- Rupnik, M.; Janezic, S. An Update on Clostridium difficile Toxinotyping. J. Clin. Microbiol. 2016, 54, 13–18. [Google Scholar] [CrossRef]
- Knight, D.R.; Riley, T.V. Genomic Delineation of Zoonotic Origins of. Front. Public. Health 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.R.; Elliott, B.; Chang, B.J.; Perkins, T.T.; Riley, T.V. Diversity and Evolution in the Genome of Clostridium difficile. Clin. Microbiol. Rev. 2015, 28, 721–741. [Google Scholar] [CrossRef] [PubMed]
- Kyne, L.; Warny, M.; Qamar, A.; Kelly, C.P. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001, 357, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Drudy, D.; Harnedy, N.; Fanning, S.; O’Mahony, R.; Kyne, L. Isolation and characterisation of toxin A-negative, toxin B-positive Clostridium difficile in Dublin, Ireland. Clin. Microbiol. Infect. 2007, 13, 298–304. [Google Scholar] [CrossRef]
- Drudy, D.; Fanning, S.; Kyne, L. Toxin A-negative, toxin B-positive Clostridium difficile. Int. J. Infect. Dis. 2007, 11, 5–10. [Google Scholar] [CrossRef]
- Lawler, A.J.; Lambert, P.A.; Worthington, T. A Revised Understanding of Clostridioides difficile Spore Germination. Trends Microbiol. 2020, 28, 744–752. [Google Scholar] [CrossRef]
- Setlow, P. Spore Resistance Properties. Microbiol. Spectr. 2014, 2, 201–215. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Cid-Rojas, F.; Pizarro-Guajardo, M. Assembly of the exosporium layer in Clostridioides difficile spores. Curr. Opin. Microbiol. 2022, 67, 102137. [Google Scholar] [CrossRef]
- Lee, C.D.; Rizvi, A.; Edwards, A.N.; DiCandia, M.A.; Vargas Cuebas, G.G.; Monteiro, M.P.; McBride, S.M. Genetic mechanisms governing sporulation initiation in Clostridioides difficile. Curr. Opin. Microbiol. 2022, 66, 32–38. [Google Scholar] [CrossRef]
- Normington, C.; Moura, I.B.; Bryant, J.A.; Ewin, D.J.; Clark, E.V.; Kettle, M.J.; Harris, H.C.; Spittal, W.; Davis, G.; Henn, M.R.; et al. Biofilms harbour Clostridioides difficile, serving as a reservoir for recurrent infection. NPJ Biofilms Microbiomes 2021, 7, 16. [Google Scholar] [CrossRef]
- Rossi, E.; La Rosa, R.; Bartell, J.A.; Marvig, R.L.; Haagensen, J.A.J.; Sommer, L.M.; Molin, S.; Johansen, H.K. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 2021, 19, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Dapa, T.; Unnikrishnan, M. Biofilm formation by Clostridium difficile. Gut Microbes 2013, 4, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Pantaléon, V.; Bouttier, S.; Soavelomandroso, A.P.; Janoir, C.; Candela, T. Biofilms of Clostridium species. Anaerobe 2014, 30, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Maldarelli, G.A.; Piepenbrink, K.H.; Scott, A.J.; Freiberg, J.A.; Song, Y.; Achermann, Y.; Ernst, R.K.; Shirtliff, M.E.; Sundberg, E.J.; Donnenberg, M.S.; et al. Type IV pili promote early biofilm formation by Clostridium difficile. Pathog. Dis. 2016, 74, ftw061. [Google Scholar] [CrossRef]
- Beitelshees, M.; Hill, A.; Jones, C.H.; Pfeifer, B.A. Phenotypic Variation during Biofilm Formation: Implications for Anti-Biofilm Therapeutic Design. Materials 2018, 11, 1086. [Google Scholar] [CrossRef]
- Poquet, I.; Saujet, L.; Canette, A.; Monot, M.; Mihajlovic, J.; Ghigo, J.M.; Soutourina, O.; Briandet, R.; Martin-Verstraete, I.; Dupuy, B. Biofilm: Remodeling Metabolism and Cell Surface to Build a Sparse and Heterogeneously Aggregated Architecture. Front. Microbiol. 2018, 9, 2084. [Google Scholar] [CrossRef]
- Martínez-Meléndez, A.; Morfin-Otero, R.; Villarreal-Treviño, L.; Baines, S.D.; Camacho-Ortíz, A.; Garza-González, E. Analysis of biofilm production and expression of adhesion structures of circulating Clostridioides difficile strains from Mexico. Enferm Infecc. Microbiol. Clin. 2021, 40, 445–448. [Google Scholar] [CrossRef]
- Pantaléon, V.; Soavelomandroso, A.P.; Bouttier, S.; Briandet, R.; Roxas, B.; Chu, M.; Collignon, A.; Janoir, C.; Vedantam, G.; Candela, T. The Clostridium difficile Protease CwpModulates both Biofilm Formation and Cell-Surface Properties. PLoS ONE 2015, 10, e0124971. [Google Scholar] [CrossRef]
- Ðapa, T.; Leuzzi, R.; Ng, Y.K.; Baban, S.T.; Adamo, R.; Kuehne, S.A.; Scarselli, M.; Minton, N.P.; Serruto, D.; Unnikrishnan, M. Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. J. Bacteriol. 2013, 195, 545–555. [Google Scholar] [CrossRef]
- Frost, L.R.; Cheng, J.K.J.; Unnikrishnan, M. Clostridioides difficile biofilms: A mechanism of persistence in the gut? PLoS Pathog. 2021, 17, e1009348. [Google Scholar] [CrossRef]
- Dawson, L.F.; Peltier, J.; Hall, C.L.; Harrison, M.A.; Derakhshan, M.; Shaw, H.A.; Fairweather, N.F.; Wren, B.W. Extracellular DNA, cell surface proteins and c-di-GMP promote biofilm formation in Clostridioides difficile. Sci. Rep. 2021, 11, 3244. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Mallozzi, M.J.; Roxas, B.P.; Bertolo, L.; Monteiro, M.A.; Agellon, A.; Viswanathan, V.K.; Vedantam, G. A Clostridium difficile Cell Wall Glycopolymer Locus Influences Bacterial Shape, Polysaccharide Production and Virulence. PLoS Pathog. 2016, 12, e1005946. [Google Scholar] [CrossRef] [PubMed]
- Soutourina, O.A.; Monot, M.; Boudry, P.; Saujet, L.; Pichon, C.; Sismeiro, O.; Semenova, E.; Severinov, K.; Le Bouguenec, C.; Coppée, J.Y.; et al. Genome-wide identification of regulatory RNAs in the human pathogen Clostridium difficile. PLoS Genet. 2013, 9, e1003493. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Rathod, J.; Chiu, Y.C.; Chen, J.W.; Tsai, P.J.; Huang, I.H. The Transcriptional Regulator Lrp Contributes to Toxin Expression, Sporulation, and Swimming Motility in. Front. Cell Infect. Microbiol. 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.M.; Cartman, S.T.; Minton, N.P.; Butala, M.; Rupnik, M. The SOS Response Master Regulator LexA Is Associated with Sporulation, Motility and Biofilm Formation in Clostridium difficile. PLoS ONE 2015, 10, e0144763. [Google Scholar] [CrossRef] [PubMed]
- Slater, R.T.; Frost, L.R.; Jossi, S.E.; Millard, A.D.; Unnikrishnan, M. Clostridioides difficile LuxS mediates inter-bacterial interactions within biofilms. Sci. Rep. 2019, 9, 9903. [Google Scholar] [CrossRef]
- Jain, S.; Smyth, D.; O’Hagan, B.M.G.; Heap, J.T.; McMullan, G.; Minton, N.P.; Ternan, N.G. Inactivation of the dnaK gene in Clostridium difficile 630 Δerm yields a temperature-sensitive phenotype and increases biofilm-forming ability. Sci. Rep. 2017, 7, 17522. [Google Scholar] [CrossRef]
- Dubois, T.; Tremblay, Y.D.N.; Hamiot, A.; Martin-Verstraete, I.; Deschamps, J.; Monot, M.; Briandet, R.; Dupuy, B. A microbiota-generated bile salt induces biofilm formation in. NPJ Biofilms Microbiomes 2019, 5, 14. [Google Scholar] [CrossRef]
- Garrett, E.M.; Sekulovic, O.; Wetzel, D.; Jones, J.B.; Edwards, A.N.; Vargas-Cuebas, G.; McBride, S.M.; Tamayo, R. Phase variation of a signal transduction system controls Clostridioides difficile colony morphology, motility, and virulence. PLoS Biol. 2019, 17, e3000379. [Google Scholar] [CrossRef]
- Cuenot, E.; Garcia-Garcia, T.; Douche, T.; Gorgette, O.; Courtin, P.; Denis-Quanquin, S.; Hoys, S.; Tremblay, Y.D.N.; Matondo, M.; Chapot-Chartier, M.P.; et al. The Ser/Thr Kinase PrkC Participates in Cell Wall Homeostasis and Antimicrobial Resistance in Clostridium difficile. Infect. Immun. 2019, 87, e00005-19. [Google Scholar] [CrossRef]
- Tremblay, Y.D.N.; Durand, B.A.R.; Hamiot, A.; Martin-Verstraete, I.; Oberkampf, M.; Monot, M.; Dupuy, B. Metabolic adaption to extracellular pyruvate triggers biofilm formation in Clostridioides difficile. ISME J. 2021, 15, 3623–3635. [Google Scholar] [CrossRef]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—Development, composition and regulation—Therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Vadyvaloo, V. Mechanisms of post-transcriptional gene regulation in bacterial biofilms. Front. Cell Infect. Microbiol. 2014, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.F.; Valiente, E.; Faulds-Pain, A.; Donahue, E.H.; Wren, B.W. Characterisation of Clostridium difficile biofilm formation, a role for Spo0A. PLoS ONE 2012, 7, e50527. [Google Scholar] [CrossRef]
- Semenyuk, E.G.; Laning, M.L.; Foley, J.; Johnston, P.F.; Knight, K.L.; Gerding, D.N.; Driks, A. Spore formation and toxin production in Clostridium difficile biofilms. PLoS ONE 2014, 9, e87757. [Google Scholar] [CrossRef]
- Soavelomandroso, A.P.; Gaudin, F.; Hoys, S.; Nicolas, V.; Vedantam, G.; Janoir, C.; Bouttier, S. Biofilm Structures in a Mono-Associated Mouse Model of. Front. Microbiol. 2017, 8, 2086. [Google Scholar] [CrossRef]
- Pantaléon, V.; Monot, M.; Eckert, C.; Hoys, S.; Collignon, A.; Janoir, C.; Candela, T. Clostridium difficile forms variable biofilms on abiotic surface. Anaerobe 2018, 53, 34–37. [Google Scholar] [CrossRef]
- Rubio-Mendoza, D.; Córdova-Fletes, C.; Martínez-Meléndez, A.; Morfín-Otero, R.; Maldonado-Garza, H.J.; Garza-González, E. Transcriptomic analysis of biofilm formation in strains of Clostridioides difficile associated with recurrent and non-recurrent infection reveals potential candidate markers for recurrence. PLoS ONE 2023, 18, e0289593. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Sarker, M.R. Adherence of Clostridium difficile spores to Caco-2 cells in culture. J. Med. Microbiol. 2012, 61, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Fagan, R.P.; Janoir, C.; Collignon, A.; Mastrantonio, P.; Poxton, I.R.; Fairweather, N.F. A proposed nomenclature for cell wall proteins of Clostridium difficile. J. Med. Microbiol. 2011, 60, 1225–1228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janoir, C.; Péchiné, S.; Grosdidier, C.; Collignon, A. Cwp84, a surface-associated protein of Clostridium difficile, is a cysteine protease with degrading activity on extracellular matrix proteins. J. Bacteriol. 2007, 189, 7174–7180. [Google Scholar] [CrossRef] [PubMed]
- Calabi, E.; Ward, S.; Wren, B.; Paxton, T.; Panico, M.; Morris, H.; Dell, A.; Dougan, G.; Fairweather, N. Molecular characterization of the surface layer proteins from Clostridium difficile. Mol. Microbiol. 2001, 40, 1187–1199. [Google Scholar] [CrossRef]
- Kirby, J.M.; Ahern, H.; Roberts, A.K.; Kumar, V.; Freeman, Z.; Acharya, K.R.; Shone, C.C. Cwp84, a surface-associated cysteine protease, plays a role in the maturation of the surface layer of Clostridium difficile. J. Biol. Chem. 2009, 284, 34666–34673. [Google Scholar] [CrossRef]
- Waligora, A.J.; Hennequin, C.; Mullany, P.; Bourlioux, P.; Collignon, A.; Karjalainen, T. Characterization of a cell surface protein of Clostridium difficile with adhesive properties. Infect. Immun. 2001, 69, 2144–2153. [Google Scholar] [CrossRef]
- Wydau-Dematteis, S.; El Meouche, I.; Courtin, P.; Hamiot, A.; Lai-Kuen, R.; Saubaméa, B.; Fenaille, F.; Butel, M.J.; Pons, J.L.; Dupuy, B.; et al. CwpIs a Novel Lytic Transglycosylase Involved in Stationary-Phase Autolysis Resulting in Toxin Release in. mBio 2018, 9, e00648-18. [Google Scholar] [CrossRef]
- Rothenbacher, F.P.; Suzuki, M.; Hurley, J.M.; Montville, T.J.; Kirn, T.J.; Ouyang, M.; Woychik, N.A. Clostridium difficile MazF toxin exhibits selective, not global, mRNA cleavage. J. Bacteriol. 2012, 194, 3464–3474. [Google Scholar] [CrossRef]
- Maikova, A.; Peltier, J.; Boudry, P.; Hajnsdorf, E.; Kint, N.; Monot, M.; Poquet, I.; Martin-Verstraete, I.; Dupuy, B.; Soutourina, O. Discovery of new type I toxin-antitoxin systems adjacent to CRISPR arrays in Clostridium difficile. Nucleic Acids Res. 2018, 46, 4733–4751. [Google Scholar] [CrossRef]
- Soutourina, O. Type I Toxin-Antitoxin Systems in Clostridia. Toxins 2019, 11, 253. [Google Scholar] [CrossRef]
- Peltier, J.; Hamiot, A.; Garneau, J.R.; Boudry, P.; Maikova, A.; Hajnsdorf, E.; Fortier, L.C.; Dupuy, B.; Soutourina, O. Type I toxin-antitoxin systems contribute to the maintenance of mobile genetic elements in Clostridioides difficile. Commun. Biol. 2020, 3, 718. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Greenberg, E.P. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: A signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 2000, 97, 8789–8793. [Google Scholar] [CrossRef] [PubMed]
- McBrayer, D.N.; Cameron, C.D.; Tal-Gan, Y. Development and utilization of peptide-based quorum sensing modulators in Gram-positive bacteria. Org. Biomol. Chem. 2020, 18, 7273–7290. [Google Scholar] [CrossRef] [PubMed]
- Sturme, M.H.; Kleerebezem, M.; Nakayama, J.; Akkermans, A.D.; Vaugha, E.E.; de Vos, W.M. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van. Leeuwenhoek 2002, 81, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.K.B.; Ballard, J.D. Autoinducing peptide-based quorum signaling systems in Clostridioides difficile. Curr. Opin. Microbiol. 2022, 65, 81–86. [Google Scholar] [CrossRef]
- Martin, M.J.; Clare, S.; Goulding, D.; Faulds-Pain, A.; Barquist, L.; Browne, H.P.; Pettit, L.; Dougan, G.; Lawley, T.D.; Wren, B.W. The agr locus regulates virulence and colonization genes in Clostridium difficile 027. J. Bacteriol. 2013, 195, 3672–3681. [Google Scholar] [CrossRef]
- Lee, A.S.; Song, K.P. LuxS/autoinducer-2 quorum sensing molecule regulates transcriptional virulence gene expression in Clostridium difficile. Biochem. Biophys. Res. Commun. 2005, 335, 659–666. [Google Scholar] [CrossRef]
- Zhu, D.; Sorg, J.A.; Sun, X. Biology: Sporulation, Germination, and Corresponding Therapies for. Front. Cell Infect. Microbiol. 2018, 8, 29. [Google Scholar] [CrossRef]
- Gunaratnam, S.; Millette, M.; McFarland, L.V.; DuPont, H.L.; Lacroix, M. Potential role of probiotics in reducing Clostridioides difficile virulence: Interference with quorum sensing systems. Microb. Pathog. 2021, 153, 104798. [Google Scholar] [CrossRef]
- Schauder, S.; Shokat, K.; Surette, M.G.; Bassler, B.L. The LuxS family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 2001, 41, 463–476. [Google Scholar] [CrossRef]
- Carter, G.P.; Purdy, D.; Williams, P.; Minton, N.P. Quorum sensing in Clostridium difficile: Analysis of a luxS-type signalling system. J. Med. Microbiol. 2005, 54, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.K.B.; Shadid, T.M.; Larabee, J.L.; Ballard, J.D. Combined and Distinct Roles of Agr Proteins in Clostridioides difficile Sporulation, Motility, and Toxin Production. mBio 2020, 11, e03190-20. [Google Scholar] [CrossRef] [PubMed]
- Sidote, D.J.; Barbieri, C.M.; Wu, T.; Stock, A.M. Structure of the Staphylococcus aureus AgrA LytTR domain bound to DNA reveals a beta fold with an unusual mode of binding. Structure 2008, 16, 727–735. [Google Scholar] [CrossRef]
- Hargreaves, K.R.; Kropinski, A.M.; Clokie, M.R. What does the talking?: Quorum sensing signalling genes discovered in a bacteriophage genome. PLoS ONE 2014, 9, e85131. [Google Scholar] [CrossRef] [PubMed]
- Darkoh, C.; DuPont, H.L.; Norris, S.J.; Kaplan, H.B. Toxin synthesis by Clostridium difficile is regulated through quorum signaling. mBio 2015, 6, e02569. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Estrada, J.; Aceves-Diez, A.E.; Guarneros, G.; de la Torre, M. The RNPP family of quorum-sensing proteins in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2010, 87, 913–923. [Google Scholar] [CrossRef]
- Maldarelli, G.A.; De Masi, L.; von Rosenvinge, E.C.; Carter, M.; Donnenberg, M.S. Identification, immunogenicity, and cross-reactivity of type IV pilin and pilin-like proteins from Clostridium difficile. Pathog. Dis. 2014, 71, 302–314. [Google Scholar] [CrossRef]
- Siegel, S.D.; Amer, B.R.; Wu, C.; Sawaya, M.R.; Gosschalk, J.E.; Clubb, R.T.; Ton-That, H. Structure and Mechanism of LcpA, a Phosphotransferase That Mediates Glycosylation of a Gram-Positive Bacterial Cell Wall-Anchored Protein. mBio 2019, 10, e01580-18. [Google Scholar] [CrossRef]
- McKee, R.W.; Aleksanyan, N.; Garrett, E.M.; Tamayo, R. Type IV Pili Promote Clostridium difficile Adherence and Persistence in a Mouse Model of Infection. Infect. Immun. 2018, 86, e00943-17. [Google Scholar] [CrossRef]
- Vuotto, C.; Donelli, G.; Buckley, A.; Chilton, C. Clostridium difficile Biofilm. Adv. Exp. Med. Biol. 2018, 1050, 97–115. [Google Scholar] [CrossRef]
- Bordeleau, E.; Fortier, L.C.; Malouin, F.; Burrus, V. c-di-GMP turn-over in Clostridium difficile is controlled by a plethora of diguanylate cyclases and phosphodiesterases. PLoS Genet. 2011, 7, e1002039. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.B.; McKee, R.W.; McBride, S.M.; Waters, C.M.; Tamayo, R. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J. Bacteriol. 2012, 194, 3307–3316. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Pique, M.E.; Tainer, J.A. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2004, 2, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Kirn, T.J.; Lafferty, M.J.; Sandoe, C.M.; Taylor, R.K. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 2000, 35, 896–910. [Google Scholar] [CrossRef]
- Diepold, A.; Armitage, J.P. Type III secretion systems: The bacterial flagellum and the injectisome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20150020. [Google Scholar] [CrossRef]
- Stevenson, E.; Minton, N.P.; Kuehne, S.A. The role of flagella in Clostridium difficile pathogenicity. Trends Microbiol. 2015, 23, 275–282. [Google Scholar] [CrossRef]
- Duan, Q.; Zhou, M.; Zhu, L.; Zhu, G. Flagella and bacterial pathogenicity. J. Basic. Microbiol. 2013, 53, 1–8. [Google Scholar] [CrossRef]
- Castro-Córdova, P.; Mora-Uribe, P.; Reyes-Ramírez, R.; Cofré-Araneda, G.; Orozco-Aguilar, J.; Brito-Silva, C.; Mendoza-León, M.J.; Kuehne, S.A.; Minton, N.P.; Pizarro-Guajardo, M.; et al. Entry of spores into intestinal epithelial cells contributes to recurrence of Clostridioides difficile infection. Nat. Commun. 2021, 12, 1140. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral. Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef]
- Heuler, J.; Fortier, L.C.; Sun, X. Clostridioides difficile phage biology and application. FEMS Microbiol. Rev. 2021, 45, fuab012. [Google Scholar] [CrossRef] [PubMed]
- Tijerina-Rodríguez, L.; Villarreal-Treviño, L.; Baines, S.D.; Morfín-Otero, R.; Camacho-Ortíz, A.; Flores-Treviño, S.; Maldonado-Garza, H.; Rodríguez-Noriega, E.; Garza-González, E. High sporulation and overexpression of virulence factors in biofilms and reduced susceptibility to vancomycin and linezolid in recurrent Clostridium [Clostridioides] difficile infection isolates. PLoS ONE 2019, 14, e0220671. [Google Scholar] [CrossRef] [PubMed]
- Deakin, L.J.; Clare, S.; Fagan, R.P.; Dawson, L.F.; Pickard, D.J.; West, M.R.; Wren, B.W.; Fairweather, N.F.; Dougan, G.; Lawley, T.D. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 2012, 80, 2704–2711. [Google Scholar] [CrossRef] [PubMed]
- Mullane, K. Fidaxomicin in Clostridium difficile infection: Latest evidence and clinical guidance. Ther. Adv. Chronic Dis. 2014, 5, 69–84. [Google Scholar] [CrossRef]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.K.; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef]
- Freeman, J.; Baines, S.D.; Saxton, K.; Wilcox, M.H. Effect of metronidazole on growth and toxin production by epidemic Clostridium difficile PCR ribotypes 001 and 027 in a human gut model. J. Antimicrob. Chemother. 2007, 60, 83–91. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Rahmoun, L.A.; Azrad, M.; Peretz, A. Antibiotic Resistance and Biofilm Production Capacity in. Front. Cell Infect. Microbiol. 2021, 11, 683464. [Google Scholar] [CrossRef]
- James, G.A.; Chesnel, L.; Boegli, L.; deLancey Pulcini, E.; Fisher, S.; Stewart, P.S. Analysis of Clostridium difficile biofilms: Imaging and antimicrobial treatment. J. Antimicrob. Chemother. 2018, 73, 102–108. [Google Scholar] [CrossRef]
- Hamada, M.; Yamaguchi, T.; Ishii, Y.; Chono, K.; Tateda, K. Inhibitory effect of fidaxomicin on biofilm formation in Clostridioides difficile. J. Infect. Chemother. 2020, 26, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Babakhani, F.; Bouillaut, L.; Gomez, A.; Sears, P.; Nguyen, L.; Sonenshein, A.L. Fidaxomicin inhibits spore production in Clostridium difficile. Clin. Infect. Dis. 2012, 55, S162–S169. [Google Scholar] [CrossRef] [PubMed]
- Aldape, M.J.; Packham, A.E.; Heeney, D.D.; Rice, S.N.; Bryant, A.E.; Stevens, D.L. Fidaxomicin reduces early toxin A and B production and sporulation in Clostridium difficile in vitro. J. Med. Microbiol. 2017, 66, 1393–1399. [Google Scholar] [CrossRef]
- Garneau, J.R.; Valiquette, L.; Fortier, L.C. Prevention of Clostridium difficile spore formation by sub-inhibitory concentrations of tigecycline and piperacillin/tazobactam. BMC Infect. Dis. 2014, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.A.; Babakhani, F.; Sears, P.; Nguyen, L.; Sorg, J.A. Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Antimicrob. Agents Chemother. 2013, 57, 664–667. [Google Scholar] [CrossRef]
- Vuotto, C.; Moura, I.; Barbanti, F.; Donelli, G.; Spigaglia, P. Subinhibitory concentrations of metronidazole increase biofilm formation in Clostridium difficile strains. Pathog. Dis. 2016, 74, ftv114. [Google Scholar] [CrossRef]
- Bouillaut, L.; McBride, S.; Sorg, J.A.; Schmidt, D.J.; Suarez, J.M.; Tzipori, S.; Mascio, C.; Chesnel, L.; Sonenshein, A.L. Effects of surotomycin on Clostridium difficile viability and toxin production in vitro. Antimicrob. Agents Chemother. 2015, 59, 4199–4205. [Google Scholar] [CrossRef]
- Artsimovitch, I.; Seddon, J.; Sears, P. Fidaxomicin is an inhibitor of the initiation of bacterial RNA synthesis. Clin. Infect. Dis. 2012, 55, S127–S131. [Google Scholar] [CrossRef]
- Polivkova, S.; Krutova, M.; Capek, V.; Sykorova, B.; Benes, J. Fidaxomicin versus metronidazole, vancomycin and their combination for initial episode, first recurrence and severe Clostridioides difficile infection—An observational cohort study. Int. J. Infect. Dis. 2021, 103, 226–233. [Google Scholar] [CrossRef]
- Louie, T.J.; Miller, M.A.; Crook, D.W.; Lentnek, A.; Bernard, L.; High, K.P.; Shue, Y.K.; Gorbach, S.L. Effect of age on treatment outcomes in Clostridium difficile infection. J. Am. Geriatr. Soc. 2013, 61, 222–230. [Google Scholar] [CrossRef]
- Cornely, O.A.; Crook, D.W.; Esposito, R.; Poirier, A.; Somero, M.S.; Weiss, K.; Sears, P.; Gorbach, S.; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 2012, 12, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Keynan, Y. Vancomycin revisited—60 years later. Front. Public. Health 2014, 2, 217. [Google Scholar] [CrossRef] [PubMed]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef]
| Strain | Biofilm Model | Biofilm Effect | Ref. |
|---|---|---|---|
| R20291::fliC430s | 24-WPP; BHISB + 0.1 M glucose; 120 h | Decreased biofilm production on day 5 | [42] |
| R20291::luxS161a | 24-WPP; BHISB + 0.1 M glucose; 72 h | Decreased biofilm production. Unable to form even a bacterial monolayer | [42] |
| R20291::sleC128a | 24-WPP; BHISB + 0.1 M glucose; 72 h | Form thick biofilm-like structures | [42] |
| R20291::spo0A178a | 24-WPP; BHISB + 0.1 M glucose; 24, 72, 120 h | Decreased biofilm formation. Form uneven and thick biofilm-like structures. Cellular form filamentous structures | [42] |
| R20291Δcwp84 | 24-WPP; BHISB + 0.1 M glucose; 24 h | Decreased in biofilm production | [42] |
| R20291- cdtA and cdtB, cwlD and cwlD * | 24-WPP; BHISB + 0.1 M glucose; 24, 72, 120 h | Unchanged | [42] |
| R20291ΔpilA1 | Glass jars with 8 mm glass beads and glass coverslips; BHISB; 24 h | Reduced thickness and biomass. Decreased live cell count and a decreased tendency to aggregate | [44] |
| 630Δerm::CD2214, ErmR TmS | 24-WMP; TYt, 48 h | Denser with several short-rod bacteria and smaller micro-aggregates | [46] |
| 630Δerm::pilA1, ErmR TmS, 630Δerm::CD2831 | 24-WMP; TYt, 24 h | Unchanged | [46] |
| 630Δerm ΔpilA1 (pRPF185 Ptet dccA), ErmR TmR | 96-well plate; TYt + anhydrotetracycline; 24 h | Form a dense and homogeneous, carpet-like biofilm, slightly decreased biomass | [46] |
| 630ΔermΔCD2831 (pRPF185 Ptet dccA), ErmR TmR | 96-well plate; TYt + anhydrotetracycline; 24 h | Form a dense and homogeneous, carpet-like biofilm without change in biofilm production | [46] |
| 630ΔermΔcwp84 and R20291Δcwp84 | 24-WPP; BHISB + 1.8% glucose; 72 h | Increased 72-fold. Denser and thicker. Protein abundance in biofilm was altered | [48] |
| 630Δerm::2831, 630Δerm::0183, 630Δerm::3392, 630Δerm::CbpA | 24-WMP; BHISB; 24 h | Decreased biofilm production | [51] |
| 630∆PEPP-1 | 24-WMP; BHISB, 24 h | Unchanged | [51] |
| 630∆PEPP-1(Ptet2831), 630∆PEPP-1(Ptet3246) | 24-WMP; BHISB, 24 h | Increased biofilm production | [51] |
| R20291::spo0A | 24-WMP or 24-WMP with coverslips; BHISB, 72 and 144 h | Decrease in depth and breadth of the biofilm. Decrease the number of spores | [51] |
| JIR8094:: lcpB | 24-WPP; BHISB + 1.8% glucose; 72 h | Robust biofilm | [52] |
| JIR8094:: lcpA | 24-WPP; BHISB + 1.8% glucose; 72 h | Unchanged | [52] |
| 630Δerm (pRPF185 Ptet dccA), TmR | 96 well polystyrene plate; TYt, 48 h | Increased biofilm production. Biovolume increase of 1.6-fold. Highly homogeneous and dense | [46,53] |
| R20291::lrp, 630Δerm::lrp | 24-WPP; BHISB + 0.1 M glucose; 72 h | Unchanged | [54] |
| R20291::lexA238a | 24-WPP; BHISB + 20 μg/mL lincomycin: 24 h | Increased biofilm production | [55] |
| 20291::luxS161a | 24-WPP; BHISB + 0.1 M glucose; 24 y 72 h | Decreased biofilm production | [56] |
| 630Δerm::dnaK723a | 96-well flat-bottom polystyrene plate; BHISB + 0.9% glucose; 24, 48 and 72 h | Increased biofilm production | [57] |
| 630Δerm::CD1687, JIR8094::codY, JIR8094::ccpA, 630Δerm::spo0A | 24-WPTCTP; BHISB + 100 mM glucose + 240 µM DOC; 48 h | Decreased biofilm production | [58] |
| 630Δerm::CD1688, 630Δerm::sigB, 630Δerm::sigE, 630Δerm::sigF | 24-WPTCTP; BHISB + 100 mM glucose + 240 µM DOC; 48 h | Unchanged | [58] |
| 630Δerm::cwp19 | 24-WPTCTP; BHISB + 100 mM glucose + 240 µM DOC; 48 h | Failed to form a biofilm | [58] |
| R20291ΔcmrR | 24-WPP; BHISB + 1% glucose + 50 mM sodium phosphate buffer; 24 h | [59] | |
| R20291ΔcmrT | 24-WPP; BHISB + 1% glucose + 50 mM sodium phosphate buffer; 24 h | Unchanged | [59] |
| 630ΔermΔprkC | 24-WMP; BHISB + 0.1 M glucose + polymyxin B (20 μg·mL−1) or DOC (0.01%); 48 h | Produce 6- and 10-fold more biofilm than the WT | [60] |
| 630∆erm∆pilW, 630∆ermΔpilA1, 630∆ermΔT4P2 cluster, 630∆ermΔsinR, 630∆ermΔCD630_08650, 630∆ermΔcwp29, 630∆ermΔcysk, 630∆ermΔagrBD, 630∆ermΔluxS, 630∆ermΔhprk, 630∆ermΔfumAB, 630∆ermΔnanEAT, 630∆ermΔprdB, 630∆ermΔfur, 630∆ermΔrex | 24-WPTCTP; BHISB + 100 mM glucose + 240 µM DOC; 48 h | Unchanged | [61] |
| 630∆ermΔbcsA, 630∆ermΔfliC | 24-WPTCTP; BHISB + 100 mM glucos e+ 240 µM DOC; 24 or 48 h | Unchanged | [61] |
| 630∆ermΔsigD | 24-WPTCTP; BHISB + 100 mM glucose + 240 µM DOC; 24 h | Unchanged | [61] |
| 630∆ermΔT4P1 cluster, 630∆ermΔcdsB 630∆ermΔspo0A, 630∆ermΔsigH, 630∆ermΔsigL, | 24-WPTCTP; BHISB + 100 mM glucose + 240 µM DOC; 48 h | Decreased biofilm production | [61] |
| 630∆ermΔptsI | 24-WPTCTP; BHISB + 100 mM glucose + 240 µM DOC; 48 h | Induction of biofilm abolished by DOC | [61] |
| Effect on Biofilm | Effect on Spores/Planktonic Cells | Changes in Gene Expression |
|---|---|---|
| Fidaxomicin | ||
| Penetrates biofilms within 2 min [120]. 0.03x–0.25x MICs exhibit a dose-dependent inhibitory effect on biofilm formation [121]. 0.09x and 0.25x MICs cause thickness and biomass reduction [121]. 0.50x MIC decreases vegetative cell growth and biofilm formation [121]. 25x MIC decreases the spore count and kills vegetative cells within mature biofilms [120]. | Spores 0.25x and 0.125x MICs during the stationary phase prevent the production of spores. 2x MIC decreases the outgrowth of vegetative cells [122]. Planktonic cells 0.25x MIC reduces viability [123]. | fliC expression increases, but no change occurs in the expression of pilA1, cwp84, luxS, dccA, and spo0A [121]. 0.25x MIC decreases spo0A transcription [123]. No accumulation of spoIIR or spoIIID mRNA occurs [122]. In the non-biofilm state, it suppresses the expression of both tcdA and tcdB, with maximal repression at 1/4x MIC [123]. |
| Vancomycin | ||
| 0.25x MIC is associated with reduced biomass [121]. 12.5 mg/mL reduces the viable vegetative cell count in intact biofilms with an enhanced effect by adding DNase [51]. It does not affect spore viability, irrespective of biofilm disruption [51]. | Spores 0.5x MIC may affect the spore count [124]. 0.25x and 0.125x MICs do not affect sporulation during the stationary phase [122]. 0.25x and 0.125x MICs reduce spore production in 48 h cultures [123]. 2.5x MIC inhibits the outgrowth of vegetative cells and does not affect spore germination [125]. Planktonic cells It only inhibits the growth of vegetative cells [122]. | 0.25x MIC does not change the mARN expression of pilA1, cwp84, luxS, dccA, and spo0A [121]. In a biofilm state, 0.5x MIC increases the transcription of tcdA and tcdB toxins [124]. |
| Metronidazole | ||
| 0.25x and 0.5x MICs increase in vitro biofilm formation. It stimulates the production of a thick biofilm composed of layered aggregates and influences extracellular matrix production a [121,126]. | Spores 0.5x MIC does not affect sporulation [124]. 0.25x MIC does not inhibit sporulation [122]. 0.25x–0.125x and 0.0625x MICs stimulate sporulation in strain 5325 [123]. 0.25x–0.125x and 0.0625x MICs suppress spore formation in strain 9689 [123]. | |
| Surotomycin | Planktonic cells | |
| It penetrates C. difficile biofilms in less than one hour and starts accumulating. It exhibits a disruptive activity on biofilm structure at 24 h [120]. 100x, 50x, and 25x MICs kill vegetative C. difficile strain ATCC BAA-1382 within biofilms in vitro [120]. | 8x and 80x MICs kill vegetative exponential-phase cells. 80x MIC kills stationary-phase cells [127]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Mendoza, D.; Martínez-Meléndez, A.; Maldonado-Garza, H.J.; Córdova-Fletes, C.; Garza-González, E. Review of the Impact of Biofilm Formation on Recurrent Clostridioides difficile Infection. Microorganisms 2023, 11, 2525. https://doi.org/10.3390/microorganisms11102525
Rubio-Mendoza D, Martínez-Meléndez A, Maldonado-Garza HJ, Córdova-Fletes C, Garza-González E. Review of the Impact of Biofilm Formation on Recurrent Clostridioides difficile Infection. Microorganisms. 2023; 11(10):2525. https://doi.org/10.3390/microorganisms11102525
Chicago/Turabian StyleRubio-Mendoza, Daira, Adrián Martínez-Meléndez, Héctor Jesús Maldonado-Garza, Carlos Córdova-Fletes, and Elvira Garza-González. 2023. "Review of the Impact of Biofilm Formation on Recurrent Clostridioides difficile Infection" Microorganisms 11, no. 10: 2525. https://doi.org/10.3390/microorganisms11102525
APA StyleRubio-Mendoza, D., Martínez-Meléndez, A., Maldonado-Garza, H. J., Córdova-Fletes, C., & Garza-González, E. (2023). Review of the Impact of Biofilm Formation on Recurrent Clostridioides difficile Infection. Microorganisms, 11(10), 2525. https://doi.org/10.3390/microorganisms11102525







