Differential Immune-Modulating Activities of Cell Walls and Secreted Metabolites from Probiotic Bacillus coagulans JBI-YZ6.3 under Normal versus Inflamed Culture Conditions
Abstract
:1. Introduction
| Features | Function | Mechanisms of Action | References |
|---|---|---|---|
| Physical | Acidophilic | Intracellular pH is kept stable in acid environments by basic amino acids, proton-efflux systems, and highly impermeable cell membranes. | [13,14,15,16] |
| Thermophilic | Thermal stability is attributed to the presence of saturated and straight-chain fatty acids, temperature-stable amino acids, and high guanine–cytosine content in DNA. | [14,15,16] | |
| Cell wall | Immune modulation | Bacterial cell wall components bind to Toll-like receptors (TLRs) and NOD-like receptors (NLRs) and activate the host immune system. | [9,43,44,45] |
| Metabolites | |||
| Bacteriocin | Anti-microbial | Target bacterial cell membranes are disrupted via the induction of cell permeabilization and pore formation. | [15,19,30] |
| Galactosidases | Improvement in carbohydrate metabolism. | Bacterial enzymes improve digestibility and carbohydrate metabolism by hydrolyzing non-digestible galactosides in food in the gut. | [18,20] |
| Fatty acids | Anti-fungal | Fungal cell membranes are disrupted using detergent-like properties of fatty acids and inhibiting the synthesis of membrane components such as ergosterol. | [9,32,33,35] |
| Regulation of inflammation | Signaling molecules and chemical messengers are involved in gut-brain communication. | [46,47,48] | |
| Biosurfactants | Anti-microbial | Pathogenic biofilm formation is inhibited on/in the gut mucosa. | [36,37] |
| Metabolites of unknown | Epithelial barrier protection | Stimulation of epithelial mucin secretion prevents microbial adhesion. | [23,24] |
| Chemical composition | Integrity of intestinal barriers is improved via the modulation of expression of tight junction proteins by host epithelial cells. | [23,24,25,26] | |
| Regulation of inflammation | Production of anti-inflammatory cytokine production by gut epithelial cells is stimulated. | [23,39,43,44,45] | |
| Anti-cancer effects | Cancer cell growth is inhibited via increased expression of pro-apoptotic genes. | [9,27,28] | |
| Antioxidant protection | Increased production of host antioxidant enzymes provides protection. | [39,49,50,51] |
2. Materials and Methods
2.1. Reagents
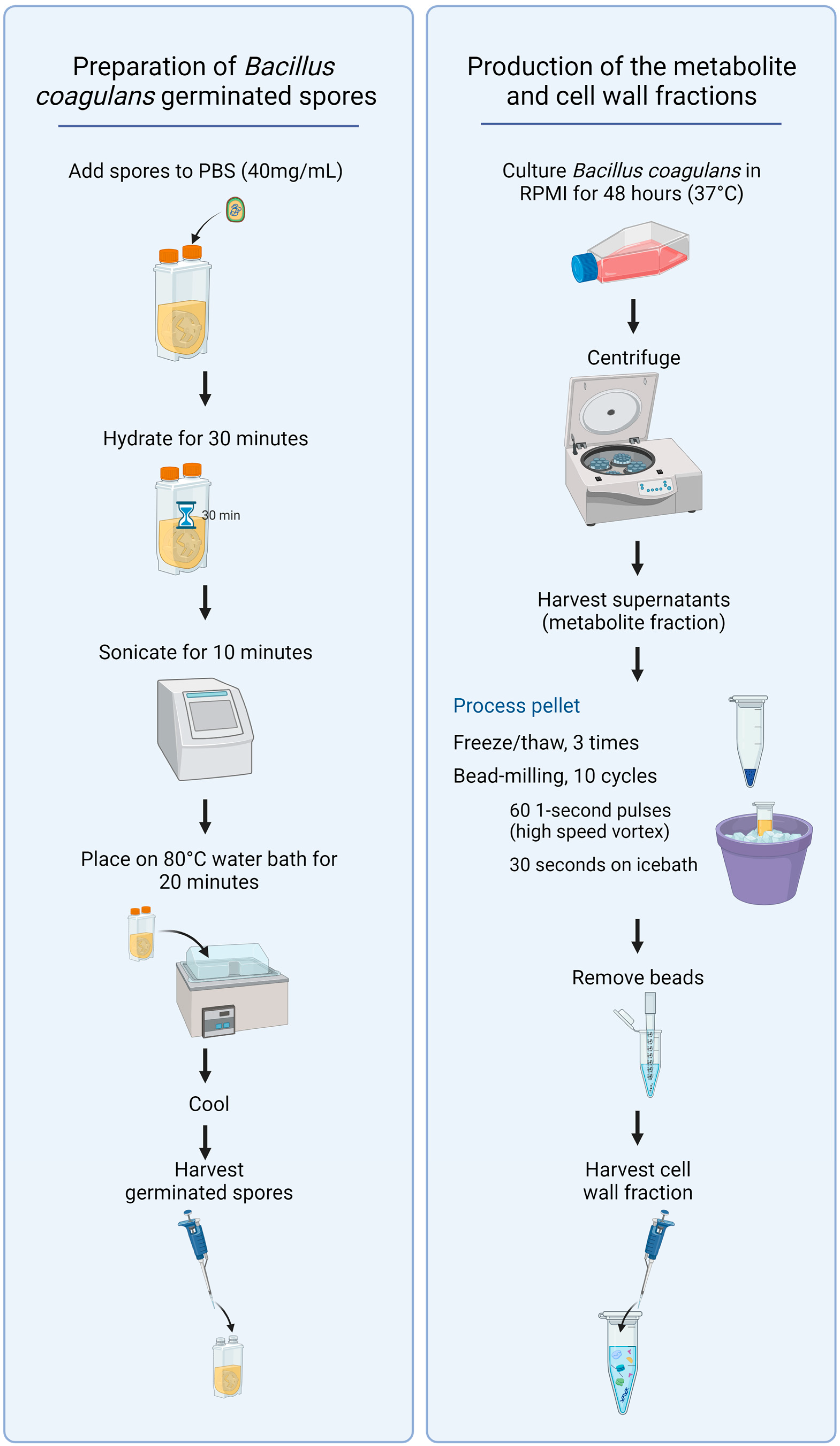
2.2. Bacillus Coagulans Germinated Spores, Metabolites, and Cell Wall Fractions
2.3. Immune Cell Activation
2.4. Production of Cytokines, Chemokines, and Growth Factors
2.5. Statistical Analysis
3. Results
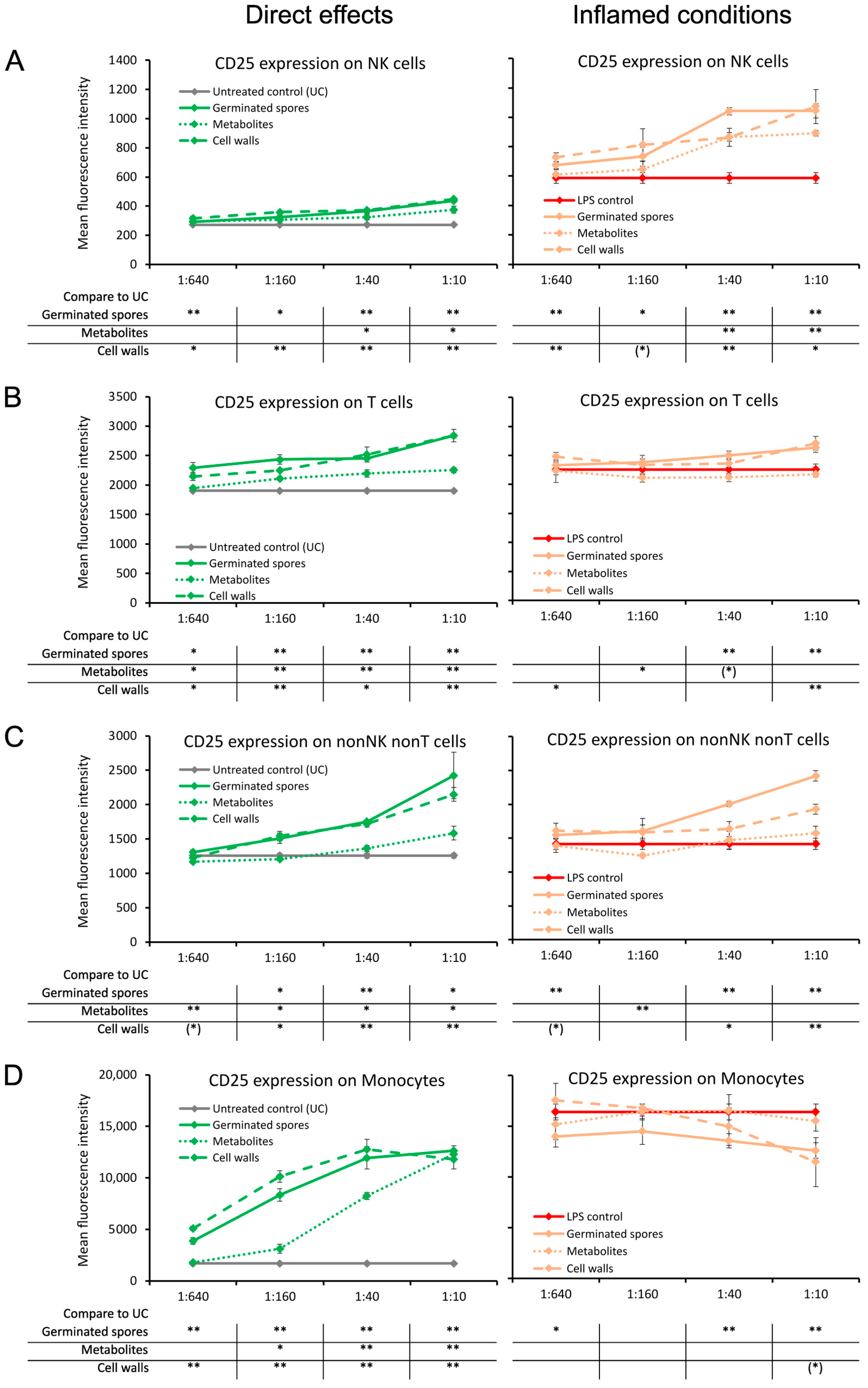
3.1. Induction of the CD25 and CD69 Activation Markers on Immune Cell Subsets
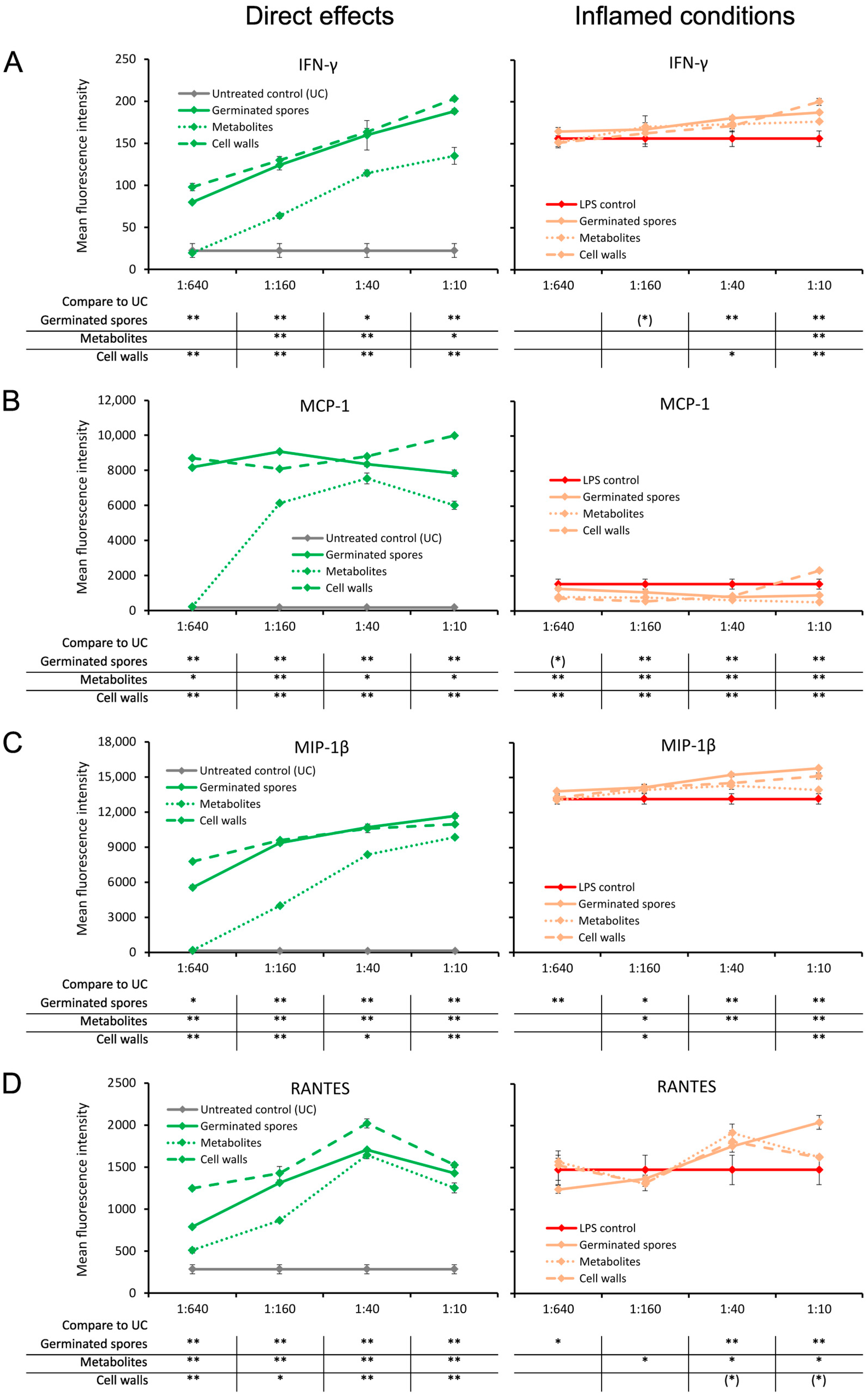
3.2. Increased Pro-Activating Cytokine Production
3.3. Increased Interferon and Chemokine Production
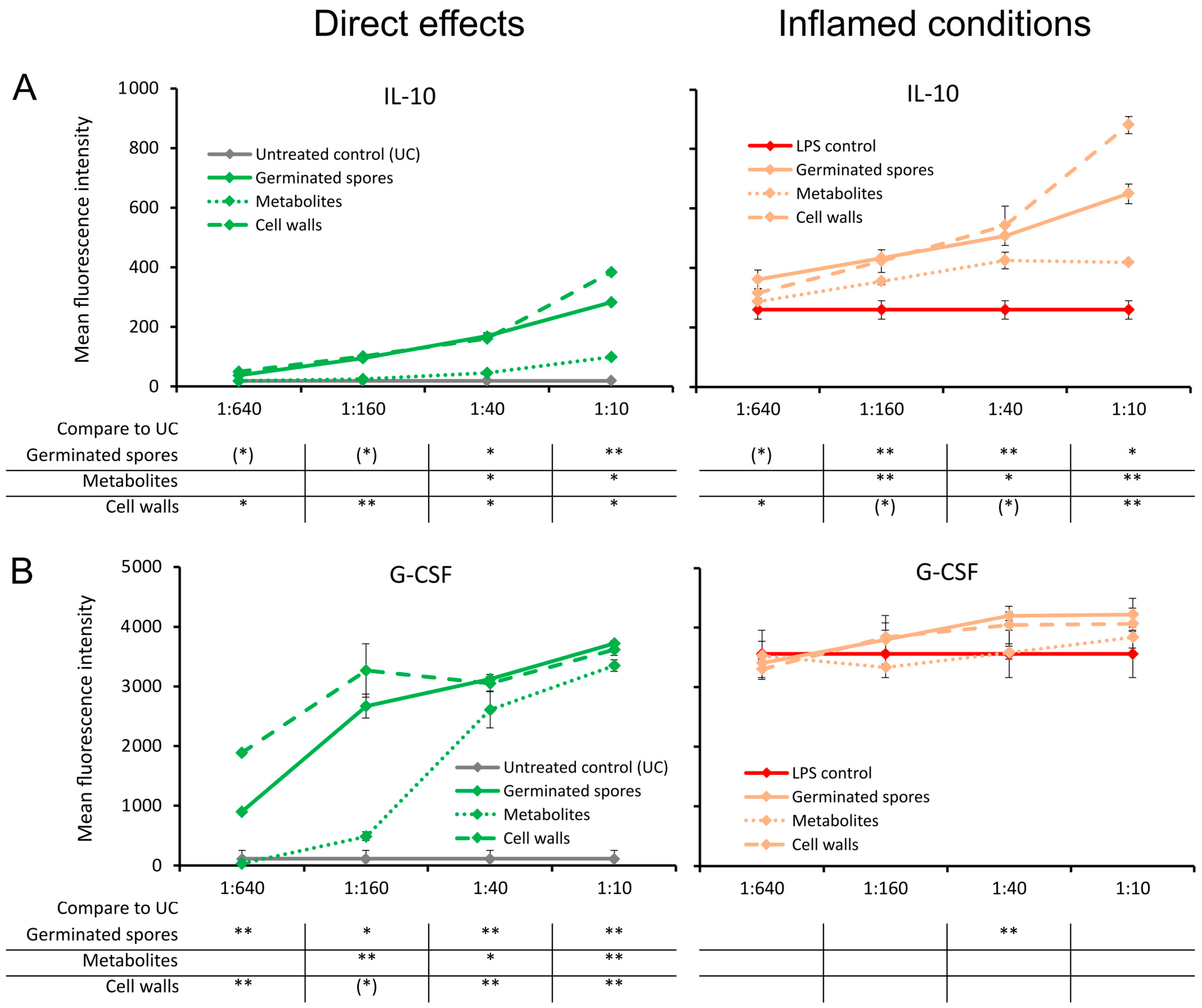
3.4. Cytokines Involved in the Return to Homeostasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDermott, A.J.; Huffnagle, G.B. The Microbiome and Regulation of Mucosa Immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef]
- Talebi, S.; Makhdoumi, A.; Bahreini, M.; Matin, M.M.; Moradi, H.S. Three novel Bacillus strains from a traditional lacto-fermented pickle as potential probiotics. J. Appl. Microbiol. 2018, 125, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Upadrasta, A.; Pitta, S.; Madempudi, R.S. Draft Genome Sequence of the Spore-Forming Probiotic Strain Bacillus coagulans Unique IS-2. Genome Announc. 2016, 4, e00225-16. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Konuray, G.; Erginkaya, Z. Potential Use of Bacillus coagulans in the Food Industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Saroj, D.B.; Gupta, A.K. Genome based safety assessment for Bacillus coagulans strain LBSC (DSM 17654) for probiotic application. Int. J. Food Microbiol. 2020, 318, 108523. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Forrester, I.T.; Wicken, A.J. The chemical composition of the cell walls of some thermophilic bacilli. J. Gen. Microbiol. 1966, 42, 147–154. [Google Scholar] [CrossRef]
- Bader, J.; Albin, A.; Stahl, U. Spore-forming bacteria and their utilisation as probiotics. Benef. Microbes 2012, 3, 67–75. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F. Evaluation of the Stability of Bacillus coazgulans MTCC 5856 during Processing and Storage of Functional Foods. Int. J. Food Sci. Technol. 2016, 51, 894–901. [Google Scholar] [CrossRef]
- Bora, P.S.; Puri, V.; Bansal, A.K. Physicochemical Properties and Excipient Compatibility studies of Probiotic Bacillus coagulans Spores. Sci. Pharm. 2009, 77, 625–638. [Google Scholar] [CrossRef]
- Patel, M.A.; Ou, M.S.; Harbrucker, R.; Aldrich, H.C.; Buszko, M.L.; Ingram, L.O.; Shanmugam, K.T. Isolation and characterization of acid-tolerant, thermophilic bacteria for effective fermentation of biomass-derived sugars to lactic acid. Appl. Environ. Microbiol. 2006, 72, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Hyronimus, B.; Le Marrec, C.; Sassi, A.H.; Deschamps, A. Acid and bile tolerance of spore-forming lactic acid bacteria. Int. J. Food Microbiol. 2000, 61, 193–197. [Google Scholar] [CrossRef]
- Endres, J.R.; Clewell, A.; Jade, K.A.; Farber, T.; Hauswirth, J.; Schauss, A.G. Safety assessment of a proprietary preparation of a novel Probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 2009, 47, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; Orrù, L.; Capozzi, V.; Martina, A.; Lamontanara, A.; Keller, D.; Cash, H.; Felis, G.E.; Cattivelli, L.; Torriani, S.; et al. Integrate genome-based assessment of safety for probiotic strains: Bacillus coagulans GBI-30, 6086 as a case study. Appl. Microbiol. Biotechnol. 2016, 100, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Bang, W.Y.; Ban, O.H.; Lee, B.S.; Oh, S.; Park, C.; Park, M.K.; Jung, S.K.; Yang, J.; Jung, Y.H. Genomic-, phenotypic-, and toxicity-based safety assessment and probiotic potency of Bacillus coagulans IDCC 1201 isolated from green malt. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab026. [Google Scholar] [CrossRef]
- Adibpour, N.; Hosseininezhad, M.; Pahlevanlo, A.; Hussain, M. A review on Bacillus coagulans as a Spore-Forming Probiotic. Appl. Food Biotechnol. 2019, 6, 91–100. [Google Scholar]
- Aulitto, M.; Strazzulli, A.; Sansone, F.; Cozzolino, F.; Monti, M.; Moracci, M.; Fiorentino, G.; Limauro, D.; Bartolucci, S.; Contursi, P. Prebiotic properties of Bacillus coagulans MA-13: Production of galactoside hydrolyzing enzymes and characterization of the transglycosylation properties of a GH42 β-galactosidase. Microb. Cell Factories 2021, 20, 71. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Fernandez, E.M.; Valenti, V.; Rockel, C.; Hermann, C.; Pot, B.; Boneca, I.G.; Grangette, C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 2011, 60, 1050–1059. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Zhang, Z.; Liu, T.; Fan, Y.; Liu, T.; Peng, N. Bacillus coagulans in Combination with Chitooligosaccharides Regulates Gut Microbiota and Ameliorates the DSS-Induced Colitis in Mice. Microbiol. Spectr. 2022, 10, e00641-22. [Google Scholar] [CrossRef]
- Jung, S.M.; Ha, A.W.; Choi, S.J.; Kim, S.Y.; Kim, W.K. Effect of Bacillus coagulans SNZ 1969 on the Improvement of Bowel Movement in Loperamide-Treated SD Rats. Nutrients 2022, 14, 3710. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, B.; Wang, N.; Yang, J.; Zhou, Q.; Sun, C.; Zhao, Y. Low fish meal diet supplemented with probiotics ameliorates intestinal barrier and immunological function of Macrobrachium rosenbergii via the targeted modulation of gut microbes and derived secondary metabolites. Front. Immunol. 2022, 13, 1074399. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Yang, Y.; Ding, L.; Yao, W. The synbiotic mixture of lactulose and Bacillus coagulans protects intestinal barrier dysfunction and apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechnol. 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Dolati, M.; Tafvizi, F.; Salehipour, M.; Komeili Movahed, T.; Jafari, P. Biogenic copper oxide nanoparticles from Bacillus coagulans induced reactive oxygen species generation and apoptotic and anti-metastatic activities in breast cancer cells. Sci. Rep. 2023, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Dolati, M.; Tafvizi, F.; Salehipour, M.; Movahed, T.K.; Jafari, P. Inhibitory effects of probiotic Bacillus coagulans against MCF7 breast cancer cells. Iran. J. Microbiol. 2021, 13, 839–847. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Moll, G.N.; Konings, W.N.; Driessen, A.J. Bacteriocins: Mechanism of membrane insertion and pore formation. Antonie Van Leeuwenhoek 1999, 76, 185–198. [Google Scholar] [CrossRef]
- Le Marrec, C.; Hyronimus, B.; Bressollier, P.; Verneuil, B.; Urdaci, M.C. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans I(4). Appl. Environ. Microbiol. 2000, 66, 5213–5220. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Khadke, S.K.; Lee, J. Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol. Microb. Biotechnol. 2021, 14, 1353–1366. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Current Perspectives on Antifungal Lactic Acid Bacteria as Natural Bio-Preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Sjögren, J.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 2003, 69, 7554–7557. [Google Scholar] [CrossRef]
- Riazi, S.; Wirawan, R.E.; Badmaev, V.; Chikindas, M.L. Characterization of lactosporin, a novel antimicrobial protein produced by Bacillus coagulans ATCC 7050. J. Appl. Microbiol. 2009, 106, 1370–1377. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Dongari-Bagtzoglou, A. Anticandidal Activities by Lactobacillus Species: An Update on Mechanisms of Action. Front. Oral Health 2021, 2, 689382. [Google Scholar] [CrossRef] [PubMed]
- Huszcza, E.; Burczyk, B. Surfactin isoforms from Bacillus coagulans. Z. Für Naturforschung C 2006, 61, 727–733. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Cheng, Z.; Wang, T.; Chen, J.; Long, M. Bacillus coagulans TL3 Inhibits LPS-Induced Caecum Damage in Rat by Regulating the TLR4/MyD88/NF-κB and Nrf2 Signal Pathways and Modulating Intestinal Microflora. Oxidative Med. Cell. Longev. 2022, 2022, 5463290. [Google Scholar] [CrossRef] [PubMed]
- Macnaughtan, J.; Figorilli, F.; García-López, E.; Lu, H.; Jones, H.; Sawhney, R.; Suzuki, K.; Fairclough, S.; Marsden, J.; Moratella, A.; et al. A Double-Blind, Randomized Placebo-Controlled Trial of Probiotic Lactobacillus casei Shirota in Stable Cirrhotic Patients. Nutrients 2020, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Abada, E.A.E. Isolation and characterization of a antimicrobial compound from Bacillus coagulans. Anim. Cells Syst. 2008, 12, 41–46. [Google Scholar] [CrossRef]
- Abdhul, K.; Ganesh, M.; Shanmughapriya, S.; Vanithamani, S.; Kanagavel, M.; Anbarasu, K.; Natarajaseenivasan, K. Bacteriocinogenic potential of a probiotic strain Bacillus coagulans [BDU3] from Ngari. Int. J. Biol. Macromol. 2015, 79, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Benson, K.F.; Carter, S.G.; Endres, J.R. GanedenBC30™ cell wall and metabolites: Anti-inflammatory and immune modulating effects in vitro. BMC Immunol. 2010, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.F.; Redman, K.A.; Carter, S.G.; Keller, D.; Farmer, S.; Endres, J.R.; Jensen, G.S. Probiotic metabolites from Bacillus coagulans GanedenBC30™ support maturation of antigen-presenting cells in vitro. World J. Gastroenterol. 2012, 18, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Cash, H.A.; Farmer, S.; Keller, D. Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. J. Inflamm. Res. 2017, 10, 107–117. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, T.; Wu, Y.; Huang, X.; Teng, J.; Zhao, N.; Zheng, X.; Yan, F. Bacillus coagulans (Weizmannia coagulans) XY2 attenuates Cu-induced oxidative stress via DAF-16/FoxO and SKN-1/Nrf2 pathways and gut microbiota regulation. J. Hazard. Mater. 2023, 457, 131741. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G.; Ali, S.A. Postbiotics Implication in the Microbiota-Host Intestinal Epithelial Cells Mutualism. Probiotics Antimicrob. Proteins 2023, 1–16. [Google Scholar] [CrossRef]
- Chen, J.; Cai, J.; Lin, J.; Cheng, Z.; Long, M. Inhibitory Effects of Bacillus coagulans TL3 on the Ileal Oxidative Stress and Inflammation Induced by Lipopolysaccharide in Rats. Curr. Microbiol. 2023, 80, 84. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Mu, G.; Xu, Y.; Wang, X.; Tuo, Y.; Qian, F. Protecting Effect of Bacillus coagulans T242 on HT-29 Cells Against AAPH-Induced Oxidative Damage. Probiotics Antimicrob. Proteins 2022, 14, 741–750. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Borrego, F.; Peña, J.; Solana, R. Regulation of CD69 expression on human natural killer cells: Differential involvement of protein kinase C and protein tyrosine kinases. Eur. J. Immunol. 1993, 23, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Poggi, A.; Pende, D.; Tripodi, G.; Orengo, A.M.; Pella, N.; Augugliaro, R.; Bottino, C.; Ciccone, E.; Moretta, L. CD69-mediated pathway of lymphocyte activation: Anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J. Exp. Med. 1991, 174, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Dons’koi, B.V.; Chernyshov, V.P.; Osypchuk, D.V. Measurement of NK activity in whole blood by the CD69 up-regulation after co-incubation with K562, comparison with NK cytotoxicity assays and CD107a degranulation assay. J. Immunol. Methods 2011, 372, 187–195. [Google Scholar] [CrossRef]
- Clausen, J.; Vergeiner, B.; Enk, M.; Petzer, A.L.; Gastl, G.; Gunsilius, E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology 2003, 207, 85–93. [Google Scholar] [CrossRef]
- Driesen, J.; Popov, A.; Schultze, J.L. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology 2008, 213, 849–858. [Google Scholar] [CrossRef]
- Wöbke, T.K.; von Knethen, A.; Steinhilber, D.; Sorg, B.L. CD69 is a TGF-β/1α,25-dihydroxyvitamin D3 target gene in monocytes. PLoS ONE 2013, 8, e64635. [Google Scholar] [CrossRef]
- Dannull, J.; Schneider, T.; Lee, W.T.; de Rosa, N.; Tyler, D.S.; Pruitt, S.K. Leukotriene C4 induces migration of human monocyte-derived dendritic cells without loss of immunostimulatory function. Blood 2012, 119, 3113–3122. [Google Scholar] [CrossRef]
- Suárez, N.; Ferrara, F.; Rial, A.; Dee, V.; Chabalgoity, J.A. Bacterial Lysates as Immunotherapies for Respiratory Infections: Methods of Preparation. Front. Bioeng. Biotechnol. 2020, 8, 545. [Google Scholar] [CrossRef]
- Montazeri-Najafabady, N.; Ghasemi, Y.; Dabbaghmanesh, M.H.; Ashoori, Y.; Talezadeh, P.; Koohpeyma, F.; Abootalebi, S.N.; Gholami, A. Exploring the bone sparing effects of postbiotics in the post-menopausal rat model. BMC Complement. Med. Ther. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Hall, F.G.; Urbizo-Reyes, U.C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A.; Liceaga, A.M. In Silico Prediction and In Vitro Assessment of Multifunctional Properties of Postbiotics Obtained from Two Probiotic Bacteria. Probiotics Antimicrob. Proteins 2020, 12, 608–622. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iloba, I.; McGarry, S.V.; Yu, L.; Cruickshank, D.; Jensen, G.S. Differential Immune-Modulating Activities of Cell Walls and Secreted Metabolites from Probiotic Bacillus coagulans JBI-YZ6.3 under Normal versus Inflamed Culture Conditions. Microorganisms 2023, 11, 2564. https://doi.org/10.3390/microorganisms11102564
Iloba I, McGarry SV, Yu L, Cruickshank D, Jensen GS. Differential Immune-Modulating Activities of Cell Walls and Secreted Metabolites from Probiotic Bacillus coagulans JBI-YZ6.3 under Normal versus Inflamed Culture Conditions. Microorganisms. 2023; 11(10):2564. https://doi.org/10.3390/microorganisms11102564
Chicago/Turabian StyleIloba, Ifeanyi, Sage V. McGarry, Liu Yu, Dina Cruickshank, and Gitte S. Jensen. 2023. "Differential Immune-Modulating Activities of Cell Walls and Secreted Metabolites from Probiotic Bacillus coagulans JBI-YZ6.3 under Normal versus Inflamed Culture Conditions" Microorganisms 11, no. 10: 2564. https://doi.org/10.3390/microorganisms11102564






