The Effect of Bifidobacterium animalis subsp. lactis Bl-04 on Influenza A Virus Infection in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mice and Infection Model
2.3. Viral Load Analysis
2.4. Microbial DNA Extraction and Quantitative Real-Time PCR (qPCR)
2.5. Transcriptomics
2.6. Microbiota Sequencing and Analysis
2.7. Statistics
3. Results
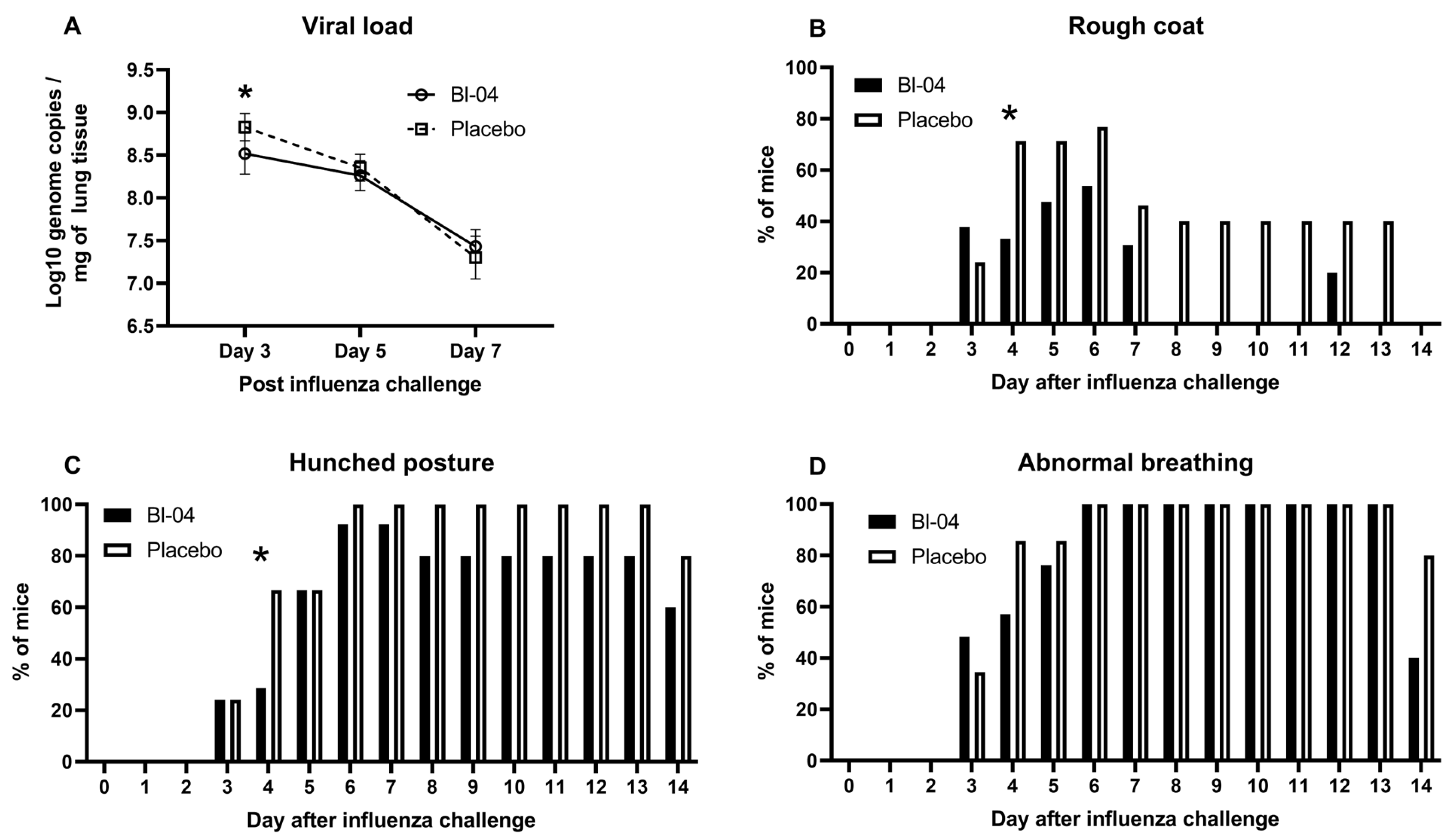
3.1. Bl-04 Decreases Viral Load and Modestly Improves Health Scores in H1N1-Infected Mice
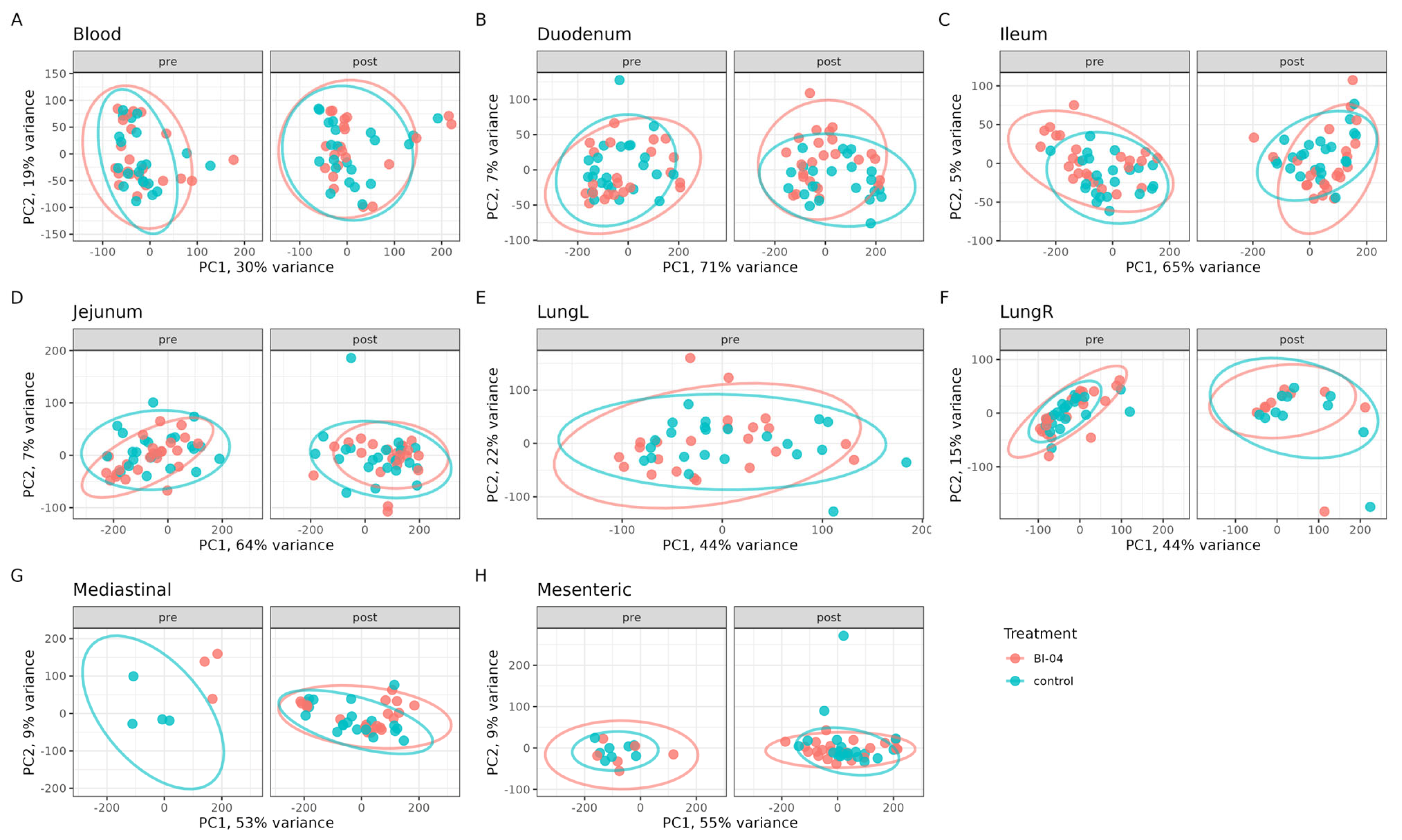
3.2. Bl-04 Does Not Have a Broad Influence on Tissue Transcriptomes
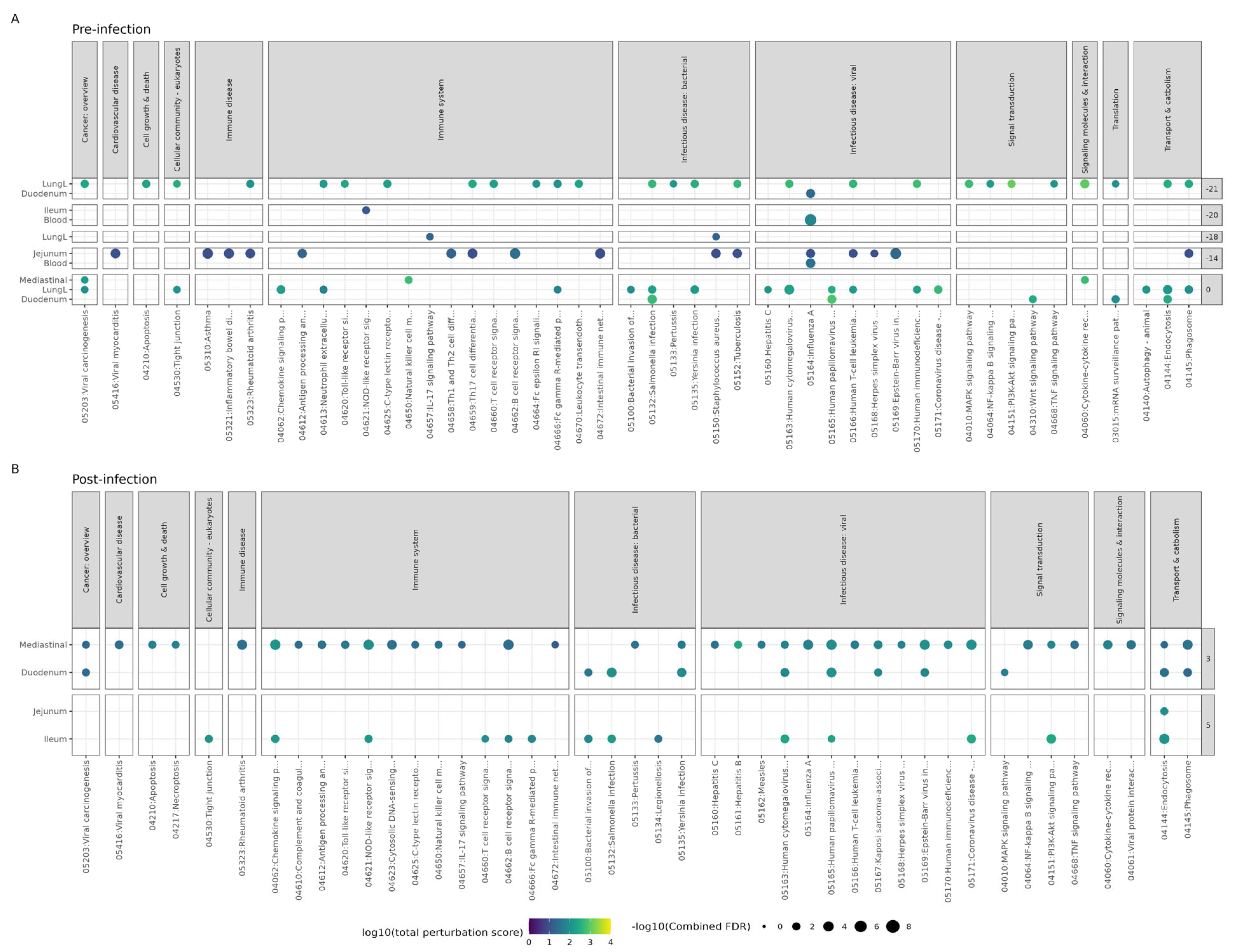
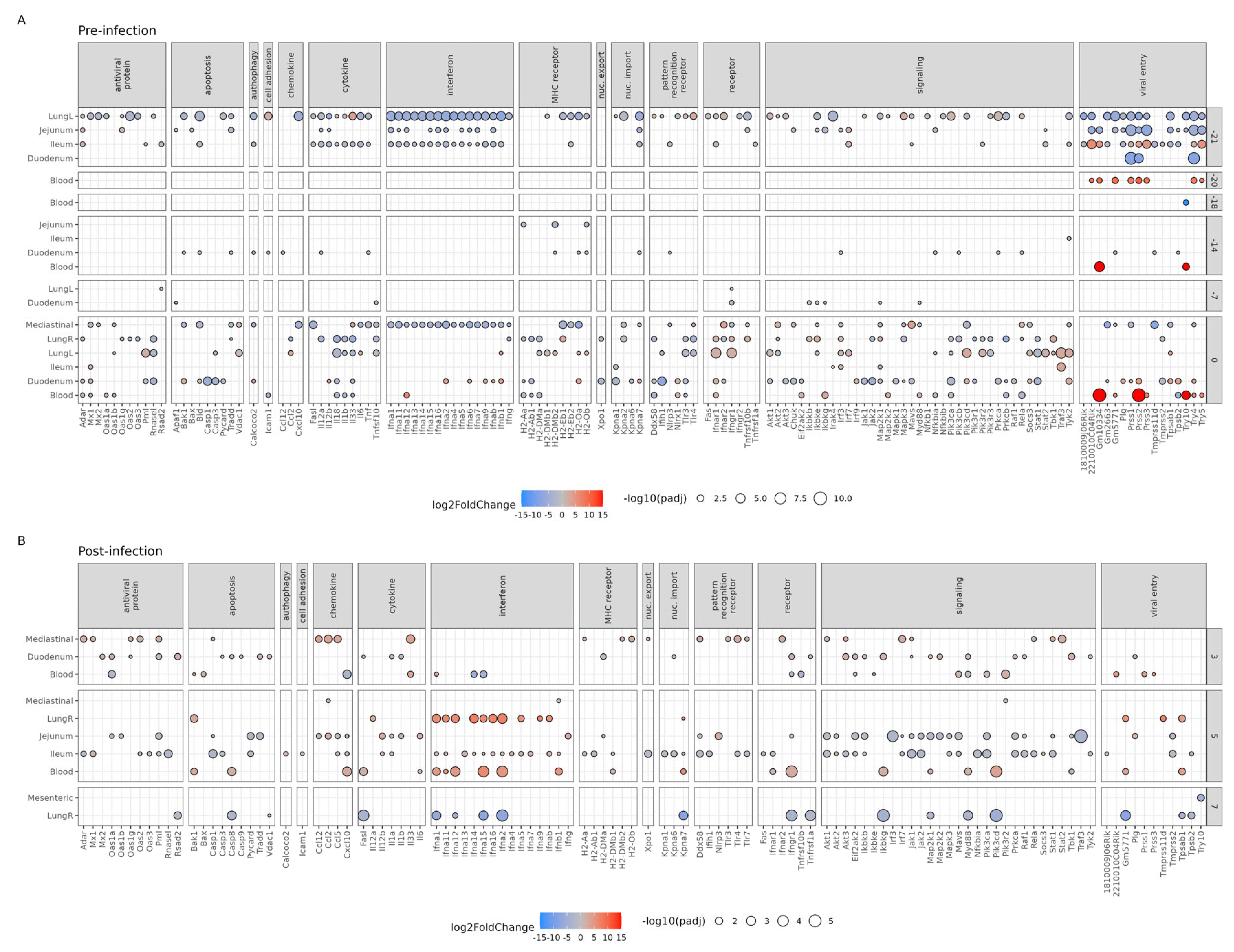
3.3. Effect of Bl-04 on Influenza A and Immune Pathways
3.4. Bl-04 Does Not Cause Major Shifts in the Murine Intestinal Microbiota Composition
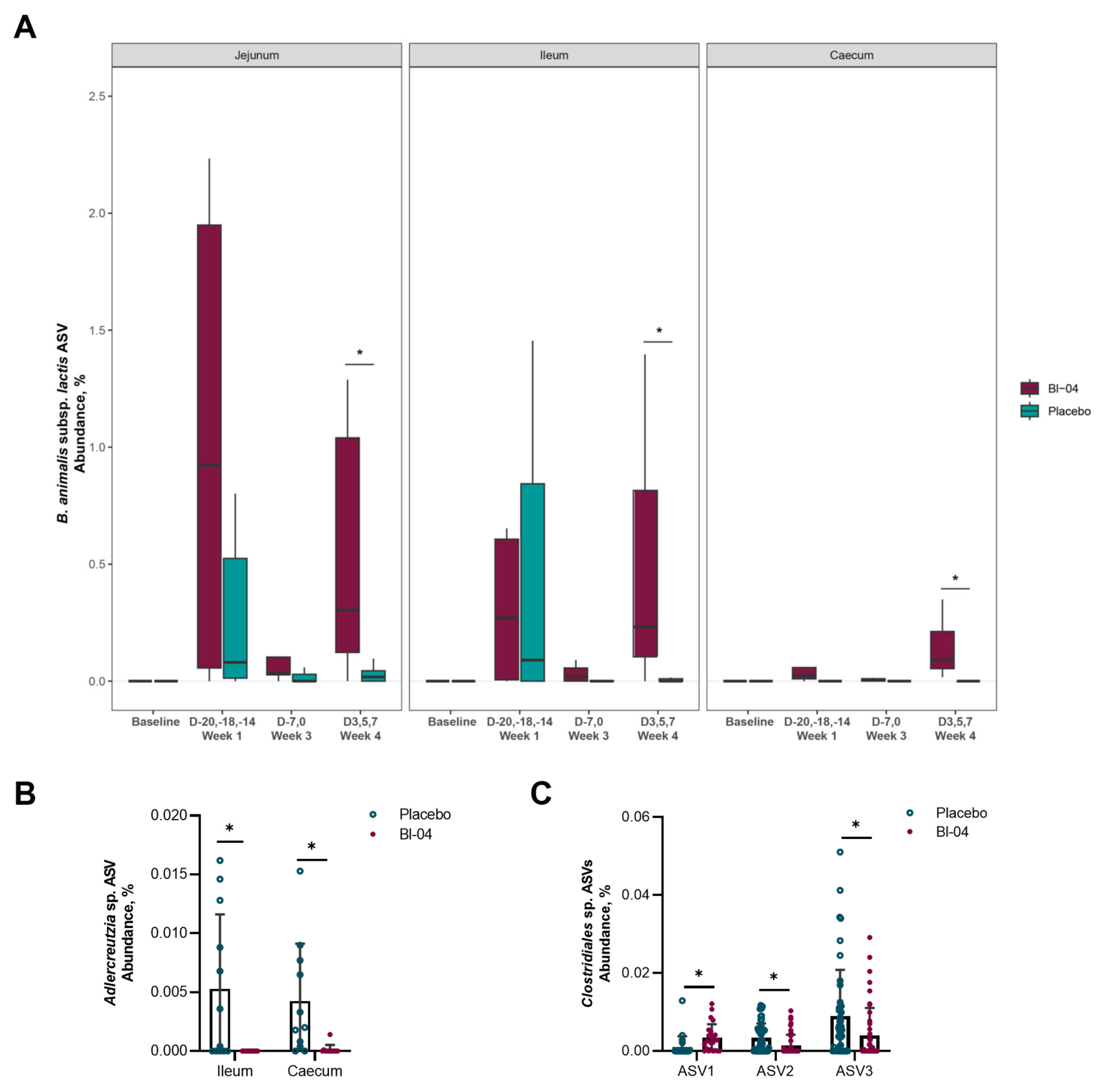
3.5. H1N1 Infection Associated with Higher Prevalence of Bl-04 in the Intestine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Gordon, A.; Shedden, K.; Kuan, G.; Ng, S.; Balmaseda, A.; Foxman, B. The respiratory microbiome and susceptibility to influenza virus infection. PLoS ONE 2019, 14, e0207898. [Google Scholar] [CrossRef]
- Tsang, T.K.; Lee, K.H.; Foxman, B.; Balmaseda, A.; Gresh, L.; Sanchez, N.; Ojeda, S.; Lopez, R.; Yang, Y.; Kuan, G.; et al. Association Between the Respiratory Microbiome and Susceptibility to Influenza Virus Infection. Clin. Infect. Dis. 2020, 71, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Van Kerkhove, M.D.; Vandemaele, K.A.H.; Shinde, V.; Jaramillo-Gutierrez, G.; Koukounari, A.; Donnelly, C.A.; Carlino, L.O.; Owen, R.; Paterson, B.; Pelletier, L.; et al. Risk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis. PLoS Med. 2011, 8, e1001053. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Salk, H.M.; Simon, W.L.; Lambert, N.D.; Kennedy, R.B.; Grill, D.E.; Kabat, B.F.; Poland, G.A. Taxa of the Nasal Microbiome Are Associated with Influenza-Specific IgA Response to Live Attenuated Influenza Vaccine. PLoS ONE 2016, 11, e0162803. [Google Scholar] [CrossRef] [PubMed]
- Sencio, V.; Machado, M.G.; Trottein, F. The lung–gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 21, Cd006895. [Google Scholar] [CrossRef]
- Rashidi, K.; Razi, B.; Darand, M.; Dehghani, A.; Janmohammadi, P.; Alizadeh, S. Effect of probiotic fermented dairy products on incidence of respiratory tract infections: A systematic review and meta-analysis of randomized clinical trials. Nutr. J. 2021, 20, 61. [Google Scholar] [CrossRef]
- Wang, F.; Pan, B.; Xu, S.; Xu, Z.; Zhang, T.; Zhang, Q.; Bao, Y.; Wang, Y.; Zhang, J.; Xu, C.; et al. A meta-analysis reveals the effectiveness of probiotics and prebiotics against respiratory viral infection. Biosci. Rep. 2021, 41, BSR20203638. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Horn, P.L.; Pyne, D.B.; Gebski, V.J.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin. Nutr. 2014, 33, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Horn, P.L.; Lehtinen, M.J.; Koerbin, G.; Pyne, D.B.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Effects of probiotic supplementation over 5 months on routine haematology and clinical chemistry measures in healthy active adults. Eur. J. Clin. Nutr. 2014, 68, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Woodfolk, J.A.; Borish, L.; Steinke, J.W.; Patrie, J.T.; Muehling, L.M.; Lahtinen, S.; Lehtinen, M.J. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection—A randomised controlled trial. Benef. Microbes 2017, 8, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Lehtoranta, L.; Hibberd, A.; Mannikko, S.; Zabel, B.; Yeung, N.; Huttunen, T.; Burns, F.R.; Lehtinen, M.J. Effect of Bifidobacterium animalis spp. lactis Bl-04 on Rhinovirus-Induced Colds: A Randomized, Placebo-Controlled, Single-Center, Phase II Trial in Healthy Volunteers. eClinicalMedicine 2022, 43, 101224. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Latvala, S.; Lehtinen, M.J. Role of Probiotics in Stimulating the Immune System in Viral Respiratory Tract Infections: A Narrative Review. Nutrients 2020, 12, 3163. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Rollon, R.; Choi, Y.-K. Animal Models for Influenza Research: Strengths and Weaknesses. Viruses 2021, 13, 1011. [Google Scholar] [CrossRef]
- Maines, T.R.; Jayaraman, A.; Belser, J.A.; Wadford, D.A.; Pappas, C.; Zeng, H.; Gustin, K.M.; Pearce, M.B.; Viswanathan, K.; Shriver, Z.H.; et al. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 2009, 325, 484–487. [Google Scholar] [CrossRef]
- Margine, I.; Krammer, F. Animal models for influenza viruses: Implications for universal vaccine development. Pathogens 2014, 3, 845–874. [Google Scholar] [CrossRef]
- Pommerenke, C.; Wilk, E.; Srivastava, B.; Schulze, A.; Novoselova, N.; Geffers, R.; Schughart, K. Global Transcriptome Analysis in Influenza-Infected Mouse Lungs Reveals the Kinetics of Innate and Adaptive Host Immune Responses. PLoS ONE 2012, 7, e41169. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.J.; Hibberd, A.A.; Männikkö, S.; Yeung, N.; Kauko, T.; Forssten, S.; Lehtoranta, L.; Lahtinen, S.J.; Stahl, B.; Lyra, A.; et al. Nasal microbiota clusters associate with inflammatory response, viral load, and symptom severity in experimental rhinovirus challenge. Sci. Rep. 2018, 8, 11411. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Voichita, C.; Draghici, S. ROntoTools: The R Onto-Tools Suite: R Package, version 2.14; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package: R Package, version 2.5-2; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Million, M.; Tomas, J.; Wagner, C.; Lelouard, H.; Raoult, D.; Gorvel, J.-P. New insights in gut microbiota and mucosal immunity of the small intestine. Hum. Microbiome J. 2018, 7–8, 23–32. [Google Scholar] [CrossRef]
- Lkhagva, E.; Chung, H.-J.; Hong, J.; Tang, W.H.W.; Lee, S.-I.; Hong, S.-T.; Lee, S. The regional diversity of gut microbiome along the GI tract of male C57BL/6 mice. BMC Microbiol. 2021, 21, 44. [Google Scholar] [CrossRef]
- Groeger, D.; Schiavi, E.; Grant, R.; Kurnik-Łucka, M.; Michalovich, D.; Williamson, R.; Beinke, S.; Kiely, B.; Akdis, C.A.; Hessel, E.M.; et al. Intranasal Bifidobacterium longum protects against viral-induced lung inflammation and injury in a murine model of lethal influenza infection. eBioMedicine 2020, 60, 102981. [Google Scholar] [CrossRef]
- Kawahara, T.; Takahashi, T.; Oishi, K.; Tanaka, H.; Masuda, M.; Takahashi, S.; Takano, M.; Kawakami, T.; Fukushima, K.; Kanazawa, H.; et al. Consecutive oral administration of Bifidobacterium longum MM-2 improves the defense system against influenza virus infection by enhancing natural killer cell activity in a murine model. Microbiol. Immunol. 2015, 59, 12210. [Google Scholar] [CrossRef]
- Nakayama, Y.; Moriya, T.; Sakai, F.; Ikeda, N.; Shiozaki, T.; Hosoya, T.; Nakagawa, H.; Miyazaki, T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci. Rep. 2014, 4, 4638. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Huang, Y.; Liu, G.; Li, X.; Xiao, Y.; Liu, C.; Zhang, Y.; Li, J.; Xu, J.; Lu, S.; et al. A Novel Immunobiotics Bacteroides dorei Ameliorates Influenza Virus Infection in Mice. Front. Immunol. 2022, 12, 828887. [Google Scholar] [CrossRef] [PubMed]
- Kiso, M.; Takano, R.; Sakabe, S.; Katsura, H.; Shinya, K.; Uraki, R.; Watanabe, S.; Saito, H.; Toba, M.; Kohda, N.; et al. Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against influenza A virus. Sci. Rep. 2013, 3, 1563. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Kiyoshima, J.; Shida, K.; Yasui, H. Effect of intranasal administration of Lactobacillus casei Shirota on influenza virus infection of upper respiratory tract in mice. Clin. Diagn. Lab. Immunol. 2001, 8, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Izumo, T.; Maekawa, T.; Ida, M.; Noguchi, A.; Kitagawa, Y.; Shibata, H.; Yasui, H.; Kiso, Y. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int. Immunopharmacol. 2010, 10, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, Y.T.; Ngo, V.L.; Cho, Y.H.; Ko, E.J.; Hong, S.M.; Kim, K.H.; Jang, J.H.; Oh, J.S.; Park, M.K.; et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 17360. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Tada, A.; Vizoso-Pinto, M.G.; Salva, S.; Kanmani, P.; Agüero, G.; Alvarez, S.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015, 64, 589–602. [Google Scholar] [CrossRef]
- Asama, T.; Uematsu, T.; Kobayashi, N.; Tatefuji, T.; Hashimoto, K. Oral administration of heat-killed Lactobacillus kunkeei YB38 improves murine influenza pneumonia by enhancing IgA production. Biosci. Microbiota Food Health 2017, 36, 1–9. [Google Scholar] [CrossRef]
- Belkacem, N.; Serafini, N.; Wheeler, R.; Derrien, M.; Boucinha, L.; Couesnon, A.; Cerf-Bensussan, N.; Gomperts Boneca, I.; Di Santo, J.P.; Taha, M.K.; et al. Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS ONE 2017, 12, e0184976. [Google Scholar] [CrossRef]
- Song, J.A.; Kim, H.J.; Hong, S.K.; Lee, D.H.; Lee, S.W.; Song, C.S.; Kim, K.T.; Choi, I.S.; Lee, J.B.; Park, S.Y. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J. Microbiol. Immunol. Infect. 2016, 49, 16–23. [Google Scholar] [CrossRef]
- Zhang, H.; Miao, J.; Su, M.; Liu, B.Y.; Liu, Z. Effect of fermented milk on upper respiratory tract infection in adults who lived in the haze area of Northern China: A randomized clinical trial. Pharm. Biol. 2021, 59, 645–650. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Loo, Y.M.; Fornek, J.; Crochet, N.; Bajwa, G.; Perwitasari, O.; Martinez-Sobrido, L.; Akira, S.; Gill, M.A.; García-Sastre, A.; Katze, M.G.; et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 2008, 82, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Kosaka, A.; Yan, H.; Guo, Z.; Uchiyama, R.; Fukui, R.; Kaneko, D.; Kumagai, Y.; You, D.-J.; Carreras, J.; et al. Double-Stranded RNA of Intestinal Commensal but Not Pathogenic Bacteria Triggers Production of Protective Interferon-β. Immunity 2013, 38, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Preising, J.; Philippe, D.; Gleinser, M.; Wei, H.; Blum, S.; Eikmanns, B.J.; Niess, J.-H.; Riedel, C.U. Selection of Bifidobacteria Based on Adhesion and Anti-Inflammatory Capacity In Vitro for Amelioration of Murine Colitis. Appl. Environ. Microbiol. 2010, 76, 3048–3051. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riedel, C.U.; Foata, F.; Philippe, D.; Adolfsson, O.; Eikmanns, B.J.; Blum, S. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-kappaB activation. World J. Gastroenterol. 2006, 12, 3729–3735. [Google Scholar] [CrossRef]
- Killip, M.J.; Fodor, E.; Randall, R.E. Influenza virus activation of the interferon system. Virus Res. 2015, 209, 11–22. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Yang, Y.; Gong, W.; Sun, X.; Yang, L.; Zhang, Q.; Jin, M. Akkermansia muciniphila Improves Host Defense Against Influenza Virus Infection. Front. Microbiol. 2020, 11, 586476. [Google Scholar] [CrossRef]
- Yildiz, S.; Mazel-Sanchez, B.; Kandasamy, M.; Manicassamy, B.; Schmolke, M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 2018, 6, 9. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Feng, J.-W.; Hu, X.-T.; Wang, T.; Gong, W.-X.; Huang, K.; Guo, Y.-X.; Zou, Z.; Lin, X.; et al. Influenza infection elicits an expansion of gut population of endogenous Bifidobacterium animalis which protects mice against infection. Genome Biol. 2020, 21, 99. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Lehtinen, M.J.; Kumar, R.; Zabel, B.; Mäkelä, S.M.; Nedveck, D.; Tang, P.; Latvala, S.; Guery, S.; Budinoff, C.R. The effect of the probiotic consortia on SARS-CoV-2 infection in ferrets and on human immune cell response in vitro. iScience 2022, 25, 104445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabel, B.; Mäkelä, S.M.; Nedveck, D.; Hibberd, A.A.; Yeung, N.; Latvala, S.; Lehtoranta, L.; Junnila, J.; Walters, K.B.; Morovic, W.; et al. The Effect of Bifidobacterium animalis subsp. lactis Bl-04 on Influenza A Virus Infection in Mice. Microorganisms 2023, 11, 2582. https://doi.org/10.3390/microorganisms11102582
Zabel B, Mäkelä SM, Nedveck D, Hibberd AA, Yeung N, Latvala S, Lehtoranta L, Junnila J, Walters KB, Morovic W, et al. The Effect of Bifidobacterium animalis subsp. lactis Bl-04 on Influenza A Virus Infection in Mice. Microorganisms. 2023; 11(10):2582. https://doi.org/10.3390/microorganisms11102582
Chicago/Turabian StyleZabel, Bryan, Sanna M. Mäkelä, Derek Nedveck, Ashley A. Hibberd, Nicolas Yeung, Sinikka Latvala, Liisa Lehtoranta, Jouni Junnila, Kevin B. Walters, Wesley Morovic, and et al. 2023. "The Effect of Bifidobacterium animalis subsp. lactis Bl-04 on Influenza A Virus Infection in Mice" Microorganisms 11, no. 10: 2582. https://doi.org/10.3390/microorganisms11102582
APA StyleZabel, B., Mäkelä, S. M., Nedveck, D., Hibberd, A. A., Yeung, N., Latvala, S., Lehtoranta, L., Junnila, J., Walters, K. B., Morovic, W., & Lehtinen, M. J. (2023). The Effect of Bifidobacterium animalis subsp. lactis Bl-04 on Influenza A Virus Infection in Mice. Microorganisms, 11(10), 2582. https://doi.org/10.3390/microorganisms11102582





