Human Cutaneous Leishmaniasis in North Africa and Its Threats to Public Health: A Statistical Study Focused on Djelfa (Algeria)
Abstract
1. Introduction
- Survey the situation of CL in the Djelfa province over a period of 16 years and identify potential risk factors, such as climatic parameters that facilitate the spread of the disease;
- Identify specific strains of Leishmania since different regions may harbour different Leishmania species. This study will help to identify the specific strains present in Djelfa and to understand the genetic diversity of the parasite, which is crucial for the development of control strategies;
- Determine the potential influence of local factors, such as geography, climate, ecology and human behaviour, on the spread of the disease by focusing on Djelfa. In order to explore how these specific local factors may constitute risks for the transmission of cutaneous leishmaniasis, this study takes into account that these factors may differ from those in other regions of Algeria and even other North African countries;
- Clarify the impact of cutaneous leishmaniasis on the local population by understanding its extent in the region so that public health authorities will be able to allocate resources more appropriately for prevention, screening and treatment;
- Address the local concerns of Djelfa residents who may have specific concerns about the incidence of cutaneous leishmaniasis in their region in order to respond directly to these concerns, leading to a greater social acceptance of prevention and control measures.
2. Materials and Methods
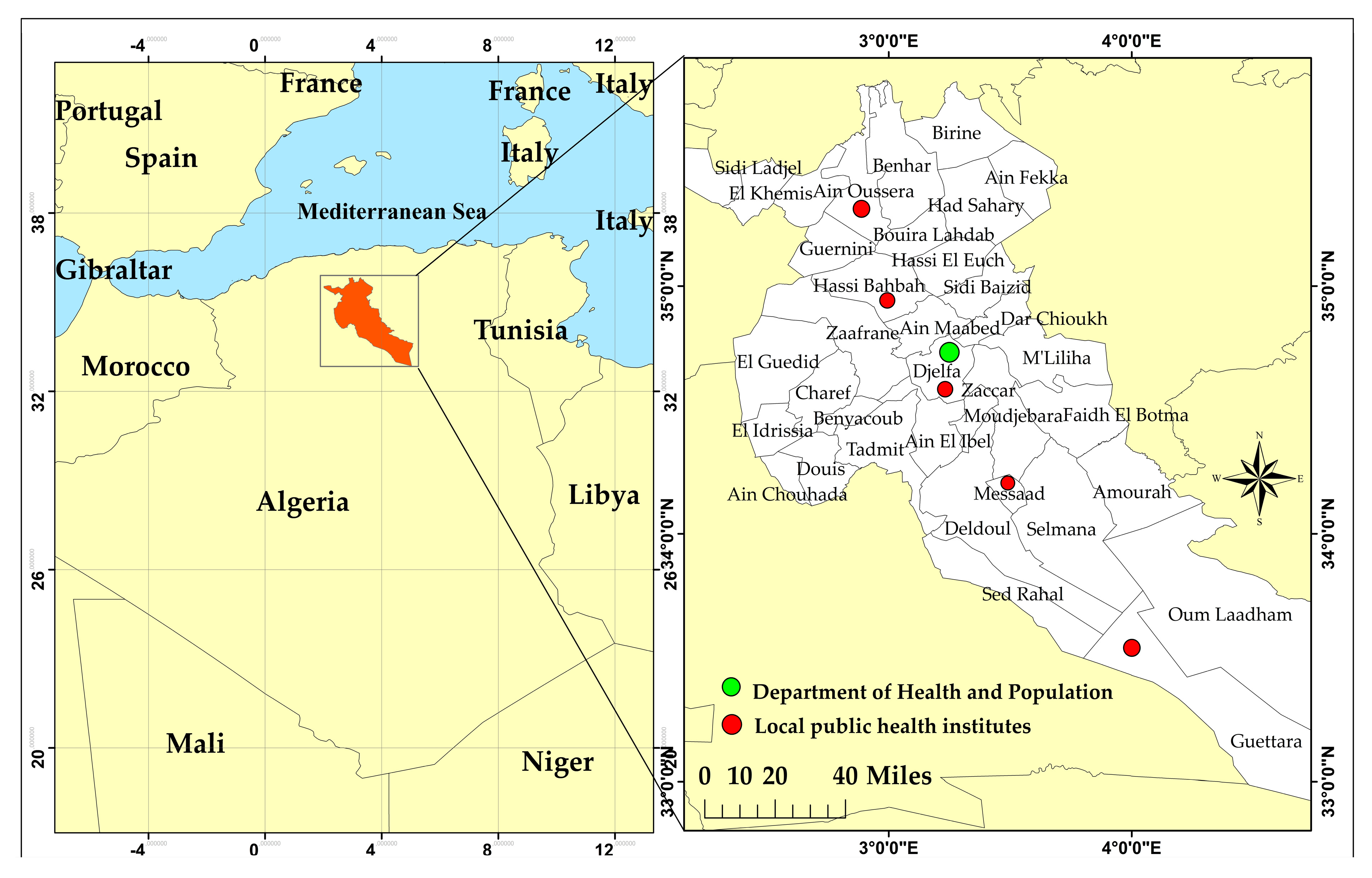
2.1. The Study Area
2.2. Data Collection
2.3. Diagnostic Investigations
2.4. Data Mapping
2.5. Statistical Analysis Tools
3. Results
3.1. Annual Distribution of Cutaneous Leishmaniasis Cases in Djelfa
3.2. Monthly Variations of Cutaneous Leishmaniasis Cases in Djelfa Province (2009–2021)
3.3. Distribution of Cutaneous Leishmaniasis Cases According to Age Groups and Gender (2009–2021)
3.4. Distribution of Cutaneous Leishmaniasis Cases by Commune in Djelfa Province (2006–2021)
3.5. Analysis and Interpretation of the Findings
- -
- With the populated density, there is a very strong presumption against the null hypothesis The two variables are strongly positively related . Among the 36 communes surveyed, Djelfa (533.5 people/km2), Ain Oussera (125.1 people/km2), Hassi Bahbah (111.7 people/km2) and Messaad (693.4 people/km2) are the most populous in the Djelfa province (Figure 4);
- -
- With the temperature minimum, there is a very strong presumption against the null hypothesis The is so close to −1. The two variables are strongly negatively related. Tmin was ranging between 1 °C in January and 20.7 °C in July;
- -
- With the temperature maximum and mean, there is a strong presumption against the null hypothesis . The two variables are moderately negatively related, and the r of the two relatives is, respectively, ( and ). The temperature trends showed that the Tmax varied from 9.7 in January to 35 in July, while the monthly mean temperature ranged from 5.3 °C in January to 35.8 °C;
- -
- With the precipitation, there is a weak presumption against the null hypothesis . The two variables are weakly positively related The range of precipitation was 32.7 mm in September, and the lowest value was 7.7 mm in July (Figure 5).
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M. den Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Leishmaniasis in High-Burden Countries: An Epidemiological Update Based on Data Reported in 2014. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2016, 91, 286–296. [Google Scholar]
- Piscopo, T.V.; Azzopardi, C.M. Leishmaniasis. Postgrad. Med. J. 2006, 83, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Leishmaniasis Emergence in Europe. Eurosurveillance 2010, 15, 19505. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, E. Current Treatment for Cutaneous Leishmaniasis: A Review. Am. J. Ther. 2009, 16, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine Sandflies and the Spreading of Leishmaniases and Other Diseases of Public Health Concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.P.; Croft, S.L. Management of Trypanosomiasis and Leishmaniasis. Br. Med. Bull. 2012, 104, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, P. Leishmaniasis: Current Situation and New Perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef]
- Hamel, H. Étude Comparée Des Boutons d’Alep et de Biskra; Rozier, V., Ed.; Bibliothèque François-Mitterrand: Paris, France, 1860. [Google Scholar]
- Aoun, K.; Bouratbine, A. Cutaneous Leishmaniasis in North Africa: A Review. Parasite 2014, 21, 14. [Google Scholar] [CrossRef]
- Saadene, Y.; Salhi, A.; Mliki, F.; Bouslama, Z. Climate Change and Cutaneous Leishmaniasis in the Province of Ghardaïa in Algeria: A Model-Based Approach to Predict Disease Outbreaks. Ann. Saudi Med. 2023, 43, 263–276. [Google Scholar] [CrossRef]
- Abdrebbi, S.B.; Henaoui, L.; Chabni, N.; Meguenni, K. Cutaneous Leishmaniasis: Endemic Regions and Epidemiological Characteristics of Cases Declared at University Hospital Center of Tlemcen, Algeria. J. Pharm. Pharmacol. 2019, 7, 249–254. [Google Scholar]
- Messahel, N.E.; Lafri, I.; Moualek, I.; Houali, K.; Hakem, A. Epidemiological Situation Analysis of Cutaneous Leishmaniasis in Batna (Northeast): An Important Focus in Algeria. Vet. Parasitol. Reg. Stud. Rep. 2021, 26, 100621. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R.W. Leishmaniasis Reservoirs and Their Significance in Control. Clin. Dermatol. 1996, 14, 523–532. [Google Scholar] [CrossRef]
- Ashford, R.W. The Leishmaniases as Emerging and Reemerging Zoonoses. Int. J. Parasitol. 2000, 30, 1269–1281. [Google Scholar] [CrossRef]
- Belazzoug, S. Découverte d’un Meriones Shawi (Rongeur, Gerbillidé) Naturellement Infesté Par Leishmania Dans Le Nouveau Foyer de Leishmaniose Cutanée de Ksar Chellala (Algérie). Bull. La Société Pathol. Exot. 1986, 79, 630–633. [Google Scholar]
- Ben-Ismail, R.; Helal, H.; Kouzena, N.; Ben Rachid, M.S. Natural Infestation of Meriones Libycus in a Focus of Cutaneous Zoonotic Leishmaniasis in Douara (Tunisia). In Proceedings of the Annales de la Société Belge de Médecine Tropicale, Bruxellles, Belgium, 1 January 1987; Instituut voor Tropische Geneeskunde: : Antwerpen, Belgium, 1987; Volume 67, pp. 201–202. [Google Scholar]
- Benikhlef, R.; Harrat, Z.; Toudjine, M.; Djerbouh, A.; Bendali-Braham, S.; Belkaid, M. Detection of Leishmania Infantum MON-24 in the Dog. Med. Trop. Rev. Du Corps Sante Colon. 2004, 64, 381–383. [Google Scholar]
- Harrat, Z.; Boubidi, S.C.; Pratlong, F.; Benikhlef, R.; Selt, B.; Dedet, J.P.; Ravel, C.; Belkaid, M. Description of a Dermatropic Leishmania Close to L. Killicki (Rioux, Lanotte & Pratlong 1986) in Algeria. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 716–720. [Google Scholar] [PubMed]
- Bachi, F.; Icheboudene, K.; Benzitouni, A.; Taharboucht, Z.; Zemmouri, M. Epidemiology of Cutaneous Leishmaniasis in Algeria through Molecular Characterization. Bull. Soc. Pathol. Exot. 2019, 112, 147–152. [Google Scholar] [CrossRef]
- Boubidi, S.; Benallal, K.; Boudrissa, A.; Bouiba, L.; Bouchareb, B.; Garni, R.; Bouratbine, A.; Ravel, C.; Dvorak, V.; Votypka, J.; et al. Phlebotomus Sergenti (Parrot, 1917) Identified as Leishmania Killicki Host in Ghardaïa, South Algeria. Microbes Infect. 2011, 13, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Izri, M.A.; Belazzoug, S. Phlebotomus (Larroussius) Perfiliewi Naturally Infected with Dermotropic Leishmania Infantum at Tenes, Algeria. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 399. [Google Scholar] [CrossRef]
- Izri, M.A.; Belazzoug, S.; Boudjebla, Y.; Dereure, J.; Pratlong, S.; Delalbre-Belmonte, A.; Rioux, J.-A. Leishmania Infantum Mon-1 Isole de Phlebotomus Perniciosus, En Kabylie (Algerie). Ann. Parasitol. Hum. Comparée 1990, 65, 150. [Google Scholar] [CrossRef]
- Berdjane-Brouk, Z.; Charrel, R.N.; Hamrioui, B.; Izri, A. First Detection of Leishmania Infantum DNA in Phlebotomus Longicuspis Nitzulescu, 1930 from Visceral Leishmaniasis Endemic Focus in Algeria. Parasitol. Res. 2012, 111, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Boudrissa, A.; Cherif, K.; Kherrachi, I.; Benbetka, S.; Bouiba, L.; Boubidi, S.C.; Benikhlef, R.; Arrar, L.; Hamrioui, B.; Harrat, Z. Extension de Leishmania Major Au Nord de l’Algérie. Bull. La Société Pathol. Exot. 2012, 105, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Guessous-Idrissi, N.; Hamdani, A.; Rhalem, A.; Riyad, M.; Sahibi, H.; Dehbi, F.; Bichichi, M.; Essari, A.; Berrag, B. Epidemiology of Human Visceral Leishmaniasis in Taounate, a Northern Province of Morocco. Parasite 1997, 4, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. New Developments in Diagnosis of Leishmaniasis. Indian J. Med. Res. 2006, 123, 311. [Google Scholar]
- Atta-ur-Rahman, A.B.R.; Choudhary, M.I. Frontiers in Medicinal Chemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2013; Volume 6, ISBN 1608054640. [Google Scholar]
- Nylén, S.; Eidsmo, L. Tissue Damage and Immunity in Cutaneous Leishmaniasis. Parasite Immunol. 2012, 34, 551–561. [Google Scholar] [CrossRef]
- Gaouaoui, R.; Zeroual, S.; Boudjelida, H. Association between Climatic Changes and Leishmaniasis Incidence in Biskra District, Algeria. J. Entomol. Zool. Stud 2017, 5, 43–49. [Google Scholar]
- Benikhlef, R.; Aoun, K.; Boudrissa, A.; Ben Abid, M.; Cherif, K.; Aissi, W.; Benrekta, S.; Boubidi, S.C.; Späth, G.F.; Bouratbine, A.; et al. Cutaneous Leishmaniasis in Algeria; Highlight on the Focus of M’Sila. Microorganisms 2021, 9, 962. [Google Scholar] [CrossRef]
- Yabrir, B.; Laoun, A.; Chenouf, N.S.; Mati, A. Characteristics of Sheep Farms in Middle Algerian Area Steppe in Relationship with the Aridity of the Environment: Case of the Region of Djelfa. Livest. Res. Rural Dev. 2015, 27, 23. [Google Scholar]
- Talbi, F.Z.; Aarab, L.; Faraj, C.; Janati Idrissi, A.; El Ouali Lalami, A. Monitoring of Vector-Borne Diseases: Investigation of Feeding Preferences of the Sand Fly, Phlebotomus Perniciosus (Diptera: Psychodidae) in a Focus of Cutaneous Leishmaniasis in Aichoun, North Center of Morocco. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 48–52. [Google Scholar]
- Benidir, M.; Ghozlane, F.; Belkheir, B.; Bousbia, A.; Yakhlef, H.; Kali, S. Conduite Alimentaire Du Bovin Laitier Chez Les Agro-Pasteurs Sédentaires En Zone Steppique Algérienne. Cas de La Wilaya de Djelfa Feeding Management of Dairy Cattle in Steppe Area of Djelfa, Algeria. In Proceedings of the Rencontres autour des Recherches sur les Ruminants, Paris, France, 7–8 December 2011; Institut de l’élevage: Paris, France, 2011; p. 129. [Google Scholar]
- Boukraa, S.; Boubidi, S.; Zimmer, J.-Y.; Francis, F.; Haubruge, E.; Alibenali-Lounaci, Z.; Doumandji, S. Surveillance Des Populations de Phlébotomes (Diptera: Psychodidae), Vecteurs Des Agents Responsables Des Leishmanioses Dans La Région Du M’Zab-Ghardaïa (Algérie). Entomol. Faun. Faun. Entomol. 2011, 63, 97–101. [Google Scholar]
- Faber, W.R.; Oskam, L.; van Gool, T.; Kroong, N.C.M.; Knegt-Junk, K.J.; Hofwegenf, H.; van der Wal, A.C.; Kager, P.A. Value of Diagnostic Techniques for Cutaneous Leishmaniasis. J. Am. Acad. Dermatol. 2003, 49, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Zait, H.; Hamrioui, B. Leishmanioses Cutanées En Algérie Bilan de 386 Cas Diagnostiqués Au CHU Mustapha d’Alger de 1998 à 2007. Rev. Francoph. Des Lab. 2009, 2009, 33–39. [Google Scholar] [CrossRef]
- Watt, T.A.; McCleery, R.H.; Hart, T. Introduction to Statistics for Biology; CRC Press: Boca Raton, FL, USA, 2007; ISBN 1420011529. [Google Scholar]
- Rohatgi, V.K.; Saleh, A.K.M.E. An Introduction to Probability and Statistics. John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1118799658. [Google Scholar]
- Kanoun, A.; Kanoun, M.; Yakhlef, H.; Cherfaoui, M.A. Pastoralisme En Algérie: Systèmes d’élevage et Stratégies d’adaptation Des Éleveurs Ovins. Renc. Rech. Ruminants 2007, 14, 181–184. [Google Scholar]
- Souttou, K.; Sekour, M.; Gouissem, K.; Hadjoudj, M.; Guezoul, O.; Doumandji, S.; Denys, C. Paramètres Écologiques Des Rongeurs Recensés Dans Un Milieu Semi Aride à Djelfa (Algérie). Alger. J. Arid Environ. “AJAE” 2012, 2, 28–41. [Google Scholar]
- Cherif, K.; Boudrissa, A.; Cherif, M.H.; Harrat, Z. A Social Program for the Control of Zoonotic Cutaneous Leishmaniasis in M’Sila, Algeria. Sante Publique 2012, 24, 511–522. [Google Scholar] [CrossRef]
- Balaska, S.; Calzolari, M.; Grisendi, A.; Scremin, M.; Dottori, M.; Mavridis, K.; Bellini, R.; Vontas, J. Monitoring of Insecticide Resistance Mutations and Pathogen Circulation in Sand Flies from Emilia-Romagna, a Leishmaniasis Endemic Region of Northern Italy. Viruses 2023, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.H.N.; Werneck, G.L.; Rodrigues, L.; Santos, M.V.; Araújo, I.B.; Moura, L.S.; Moreira, S.; Gomes, R.B.B.; Lima, S.S. Household Structure and Urban Services: Neglected Targets in the Control of Visceral Leishmaniasis. Ann. Trop. Med. Parasitol. 2005, 99, 229–236. [Google Scholar] [CrossRef]
- Calderon-Anyosa, R.; Galvez-Petzoldt, C.; Garcia, P.J.; Carcamo, C.P. Housing Characteristics and Leishmaniasis: A Systematic Review. Am. J. Trop. Med. Hyg. 2018, 99, 1547–1554. [Google Scholar] [CrossRef]
- de Oliveira Guerra, J.A.; Ribeiro, J.A.S.; Coelho, L.I.D.A.R.D.C.; Barbosa, M.D.G.V.; Paes, M.G. Epidemiologia Da Leishmaniose Tegumentar Na Comunidade São João, Manaus, Amazonas, Brasil. Cad. Saude Publica 2006, 22, 2319–2327. [Google Scholar]
- Hamiroune, M.; Selt, F.; Senni, Z.; Saidani, K.; Djemal, M. Situation Épidémiologique de La Leishmaniose Cutanée Humaine Dans La Région Steppique de Djelfa En Algérie: Incidence et Facteurs de Variation. Int. J. Innov. Appl. Stud. 2019, 26, 253–261. [Google Scholar]
- Collis, S.; El-Safi, S.; Atia, A.A.; Bhattacharyya, T.; Hammad, A.; Den Boer, M.; Le, H.; Whitworth, J.A.; Miles, M.A. Epidemiological and Molecular Investigation of Resurgent Cutaneous Leishmaniasis in Sudan. Int. J. Infect. Dis. 2019, 88, 14–20. [Google Scholar] [CrossRef] [PubMed]
- EL Alaoui, Z.; Amayour, A.; El Aasri, A.; EL Kharim, K.; El Belghyti, D. Leishmaniose Cutanées À Ain Dfali, Aspects Épidémio-Cliniques Comparatifs De 132 Cas. Eur. Sci. Journal, ESJ 2017, 13, 60. [Google Scholar]
- Feliciangeli, M.D. Ecology of Sandflies (Diptera: Psychodidae) in a Restricted Focus of Cutaneous Leishmaniasis in Northern Venezuela: III. Seasonal Fluctuation. Mem. Inst. Oswaldo Cruz 1987, 82, 167–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ebi, K.L.; Hasegawa, T.; Hayes, K.; Monaghan, A.; Paz, S.; Berry, P. Health Risks of Warming of 1.5 °C, 2 °C, and Higher, above Pre-Industrial Temperatures. Environ. Res. Lett. 2018, 13, 063007. [Google Scholar] [CrossRef]
- Barati, H.; Lotfi, M.H.; Mozafari, G.; Dehghan, H.; Barati, M.; Tajfirouzeh, A. Epidemiological Aspects of Cutaneous Leishmaniasis in Yazd Province within 2004-2013. J. Community Heal. Res. 2016, 5, 131–139. [Google Scholar]
- Pérez-Flórez, M.; Ocampo, C.B.; Valderrama-Ardila, C.; Alexander, N. Spatial Modeling of Cutaneous Leishmaniasis in the Andean Region of Colombia. Mem. Inst. Oswaldo Cruz 2016, 111, 433–442. [Google Scholar] [CrossRef]
- Toumi, A.; Chlif, S.; Bettaieb, J.; Alaya, N.B.; Boukthir, A.; Ahmadi, Z.E.; Salah, A. Ben Temporal Dynamics and Impact of Climate Factors on the Incidence of Zoonotic Cutaneous Leishmaniasis in Central Tunisia. PLoS Negl. Trop. Dis. 2012, 6, e1633. [Google Scholar] [CrossRef]
- El-Raey, M. Impacts and Implications of Climate Change for the Coastal Zones of Egypt. Coast. Zo. Clim. Chang. 2010, 7, 31–50. [Google Scholar]
- World Health Organization. Urbanization: An Increasing Risk Factor for Leishmaniasis. Wkly. Epidemiol. Rec. Relev. épidémiologique Hebd. 2002, 77, 365–370. [Google Scholar]
- Kahime, K.; Boussaa, S.; Nhammi, H.; Boumezzough, A. Urbanization of Human Visceral Leishmaniasis in Morocco. Parasite Epidemiol. Control 2017, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, P. Global Control and Leishmania HIV Co-Infection. Clin. Dermatol. 1999, 17, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.J. Urban Poverty and Unavailable Family Members. J. Strateg. Syst. Ther. 1990, 9, 35–48. [Google Scholar] [CrossRef]
- Weigel, M.M.; Armijos, R.X.; Zurita, C.; Racines, J.; Reddy, A.; Mosquera, J. Nutritional Status and Cutaneous Leishmaniasis in Rural Ecuadorian Children. J. Trop. Pediatr. 1995, 41, 22–28. [Google Scholar] [CrossRef]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef]
- Du, R.; Hotez, P.J.; Al-Salem, W.S.; Acosta-Serrano, A. Old World Cutaneous Leishmaniasis and Refugee Crises in the Middle East and North Africa. PLoS Negl. Trop. Dis. 2016, 10, e0004545. [Google Scholar] [CrossRef] [PubMed]
- El Aasri, A.; El Madhi, Y.; Najy, M.; El Rhaouat, O.; Belghyti, D. Epidemiology of Cutaneous Leishmaniasis in Sidi Kacem Province, Northwestern Morocco (2006–2014). Asian Pacific J. Trop. Dis. 2016, 6, 783–786. [Google Scholar] [CrossRef]
- El Miri, H.; Rhajaoui, M.; Himmi, O.; Ouahabi, S.; Benhoussa, A.; Faraj, C. Etude Entomologique de Cinq Foyers de Leishmaniose Cutanée Dans La Province de Sidi Kacem Au Nord Du Maroc. Ann. la Société Entomol. Fr. 2013, 49, 154–159. [Google Scholar] [CrossRef]
- Grove, S.S. Cutaneous Leishmaniasis in South West Africa. S. Afr. Med. J. 1970, 44, 206–207. [Google Scholar]
- Tabbabi, A. Review of Leishmaniasis in the Middle East and North Africa. Afr. Health Sci. 2019, 19, 1329. [Google Scholar] [CrossRef] [PubMed]
- Bachi, F. Aspects Épidémiologiques et Cliniques Des Leishmanioses En Algérie. La Lett. l’infectiologue 2006, 1, 9–15. [Google Scholar]
- Harrat, Z.; Hamrioui, B.; Belkaïd, M.; Tabet-Derraz, O. Point Actuel Sur l’épidémiologie. Bull. Soc. Path. Ex 1995, 88, 180–184. [Google Scholar]
- Mihoubi, I.; De Monbrison, F.; Romeuf, N.; Moulahem, T.; Picot, S. Outsourced Real-Time PCR Diagnosis of Cutaneous Leishmaniasis in the Outbreak Region of Constatine, Algeria. Med. Trop. Rev. du Corps sante Colon. 2006, 66, 39–44. [Google Scholar]
- Salah, A.B.; Kamarianakis, Y.; Chlif, S.; Alaya, N.B.; Prastacos, P. Zoonotic Cutaneous Leishmaniasis in Central Tunisia: Spatio Temporal Dynamics. Int. J. Epidemiol. 2007, 36, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Pratlong, F.; Hammami, M.; Kallel, K.; Dedet, J.P.; Chaker, E. Human Cutaneous Leishmaniasis Due to Leishmania Infantum in the Sidi Bourouis Focus (Northern Tunisia): Epidemiological Study and Isoenzymatic Characterization of the Parasites. Acta Trop. 2003, 85, 83–86. [Google Scholar] [CrossRef]
- Snoussi, S. Situation de l’élevage Ovin En Tunisie et Rôle de La Recherche Réflexions Sur Le Développement d’une Approche Système. Cah. Agric. 2003, 12, 419–428. [Google Scholar]
- Kahime, K.; Boussaa, S.; Idrissi, A.L.-E.; Nhammi, H.; Boumezzough, A. Epidemiological Study on Acute Cutaneous Leishmaniasis in Morocco. J. Acute Dis. 2016, 5, 41–45. [Google Scholar] [CrossRef]
- Rhajaoui, M.; Nasereddin, A.; Fellah, H.; Azmi, K.; Amarir, F.; Al-Jawabreh, A.; Ereqat, S.; Planer, J.; Abdeen, Z. New Clinicoepidemiologic Profile of Cutaneous Leishmaniasis, Morocco. Emerg. Infect. Dis. 2007, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- El Omari, H.; Chahlaoui, A.; Talbi, F.; Ouarrak, K.; El Ouali Lalami, A. Impact of Urbanization and Socioeconomic Factors on the Distribution of Cutaneous Leishmaniasis in the Center of Morocco. Interdiscip. Perspect. Infect. Dis. 2020, 2020. [Google Scholar] [CrossRef]
- World Health Organization. Report of the Consultative Meeting on Cutaneous Leishmaniasis, Geneva, WHO Headquarters, 30 April to 2 May 2007; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Abdellatif, M.Z.M.; El-Mabrouk, K.; Ewis, A.A. An Epidemiological Study of Cutaneous Leishmaniasis in Al-Jabal Al-Gharbi, Libya. Korean J. Parasitol. 2013, 51, 75. [Google Scholar] [CrossRef]
- El-Badry, A.A.; El-Dwibe, H.; Basyoni, M.M.A.; Al-Antably, A.S.A.; Al-Bashier, W.A. Molecular Prevalence and Estimated Risk of Cutaneous Leishmaniasis in Libya. J. Microbiol. Immunol. Infect. 2017, 50, 805–810. [Google Scholar] [CrossRef]
- Gaci, D.; Huguenin, J.; Kanoun, M.; Boutonnet, J.-P.; Abdelkrim, H. Nouvelles Mobilités Pastorales: Cas Des Éleveurs d’ovins de La Wilaya de Djelfa, Algérie. Rev. d’élevage Médecine Vétérinaire Des Pays Trop. 2021, 74, 3–11. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Mukherjee, S.; Ghosh, S.; Roy, S.; Saha, B.; Das, N.K.; Chatterjee, M. A Male Preponderance in Patients with Indian Post Kala-azar Dermal Leishmaniasis Is Associated with Increased Circulating Levels of Testosterone. Int. J. Dermatol. 2016, 55, e250–e255. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Status of Endemicity of Cutaneous Leishmaniasis. Available online: https://apps.who.int/neglected_diseases/ntddata/leishmaniasis/leishmaniasis.html (accessed on 10 December 2022).
- Bounoua, L.; Kahime, K.; Houti, L.; Blakey, T.; Ebi, K.; Zhang, P.; Imhoff, M.; Thome, K.; Dudek, C.; Sahabi, S.; et al. Linking Climate to Incidence of Zoonotic Cutaneous Leishmaniasis (L. Major) in Pre-Saharan North Africa. Int. J. Environ. Res. Public Health 2013, 10, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Atia, N.; Benzaoui, A.; Jacques, S.; Hamiane, M.; El Kourd, K.; Bouakaz, A.; Ouahabi, A. Particle Swarm Optimization and Two-Way Fixed-Effects Analysis of Variance for Efficient Brain Tumor Segmentation. Cancers 2022, 14, 4399. [Google Scholar] [CrossRef] [PubMed]
- Benlamoudi, A.; Bekhouche, S.E.; Korichi, M.; Bensid, K.; Ouahabi, A.; Hadid, A.; Taleb-Ahmed, A. Face Presentation Attack Detection Using Deep Background Subtraction. Sensors 2022, 22, 3760. [Google Scholar] [CrossRef] [PubMed]
- Adjabi, I.; Ouahabi, A.; Benzaoui, A.; Taleb-Ahmed, A. Past, Present, and Future of Face Recognition: A Review. Electronics 2020, 9, 1188. [Google Scholar] [CrossRef]
- Adjabi, I.; Ouahabi, A.; Benzaoui, A.; Jacques, S. Multi-Block Color-Binarized Statistical Images for Single-Sample Face Recognition. Sensors 2021, 21, 728. [Google Scholar] [CrossRef] [PubMed]
- El Morabit, S.; Rivenq, A.; Zighem, M.-E.; Hadid, A.; Ouahabi, A.; Taleb-Ahmed, A. Automatic Pain Estimation from Facial Expressions: A Comparative Analysis Using off-the-Shelf CNN Architectures. Electronics 2021, 10, 1926. [Google Scholar] [CrossRef]
- Khaldi, Y.; Benzaoui, A.; Ouahabi, A.; Jacques, S.; Taleb-Ahmed, A. Ear Recognition Based on Deep Unsupervised Active Learning. IEEE Sens. J. 2021, 21, 20704–20713. [Google Scholar] [CrossRef]
- Benzaoui, A.; Khaldi, Y.; Bouaouina, R.; Amrouni, N.; Alshazly, H.; Ouahabi, A. A Comprehensive Survey on Ear Recognition: Databases, Approaches, Comparative Analysis, and Open Challenges. Neurocomputing 2023. [Google Scholar] [CrossRef]
- Sid Ahmed, S.; Messali, Z.; Ouahabi, A.; Trepout, S.; Messaoudi, C.; Marco, S. Nonparametric Denoising Methods Based on Contourlet Transform with Sharp Frequency Localization: Application to Low Exposure Time Electron Microscopy Images. Entropy 2015, 17, 3461–3478. [Google Scholar] [CrossRef]
- Ouahabi, A. Signal and Image Multiresolution Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118568664. [Google Scholar]
- Ouahabi, A. A Review of Wavelet Denoising in Medical Imaging. In Proceedings of the 2013 8th International Workshop on Systems, Signal Processing and their Applications (WoSSPA), Piscataway, NJ, USA, 12–15 May 2013; pp. 19–26. [Google Scholar]
- El Mahdaoui, A.; Ouahabi, A.; Moulay, M.S. Image Denoising Using a Compressive Sensing Approach Based on Regularization Constraints. Sensors 2022, 22, 2199. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, A.; Ouahabi, A.; Jacques, S.; Hamiane, M. Concrete Cracks Detection and Monitoring Using Deep Learning-Based Multiresolution Analysis. Electronics 2021, 10, 1772. [Google Scholar] [CrossRef]
- Arbaoui, A.; Ouahabi, A.; Jacques, S.; Hamiane, M. Wavelet-Based Multiresolution Analysis Coupled with Deep Learning to Efficiently Monitor Cracks in Concrete. Frat. ed Integrità Strutt. 2021, 15, 33–47. [Google Scholar] [CrossRef]





| Evolution of the Annual Cases of CL in Djelfa Province (2006–2021) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Total |
| CL cases | 1407 | 154 | 40 | 33 | 49 | 255 | 210 | 44 | 34 | 45 | 50 | 222 | 445 | 251 | 354 | 271 | 3864 |
| Age Groups | Gender | Number of Cases | Percentage % | Total |
|---|---|---|---|---|
| 00–01 year | M | 31 | 1.37% | 2.34% |
| F | 22 | 0.97% | ||
| 02–04 years | M | 125 | 5.52% | 9.59% |
| F | 92 | 4.07% | ||
| 05–09 years | M | 153 | 6.76% | 12.15% |
| F | 122 | 5.39% | ||
| 10–19 years | M | 227 | 10.03% | 19.22% |
| F | 208 | 9.19% | ||
| 20–44 years | M | 430 | 19.00% | 34.87% |
| F | 359 | 15.86% | ||
| 45–64 years | M | 175 | 7.73% | 16.39% |
| F | 196 | 8.66% | ||
| Over 65 years | M | 72 | 3.18% | 5.44% |
| F | 51 | 2.25% | ||
| Total | M | 1213 | 53.60% | 100 % |
| F | 1050 | 46.40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messaoudene, F.; Boukraa, S.; Boubidi, S.C.; Guerzou, A.; Ouahabi, A. Human Cutaneous Leishmaniasis in North Africa and Its Threats to Public Health: A Statistical Study Focused on Djelfa (Algeria). Microorganisms 2023, 11, 2608. https://doi.org/10.3390/microorganisms11102608
Messaoudene F, Boukraa S, Boubidi SC, Guerzou A, Ouahabi A. Human Cutaneous Leishmaniasis in North Africa and Its Threats to Public Health: A Statistical Study Focused on Djelfa (Algeria). Microorganisms. 2023; 11(10):2608. https://doi.org/10.3390/microorganisms11102608
Chicago/Turabian StyleMessaoudene, Fatma, Slimane Boukraa, Said Chaouki Boubidi, Ahlem Guerzou, and Abdeldjalil Ouahabi. 2023. "Human Cutaneous Leishmaniasis in North Africa and Its Threats to Public Health: A Statistical Study Focused on Djelfa (Algeria)" Microorganisms 11, no. 10: 2608. https://doi.org/10.3390/microorganisms11102608
APA StyleMessaoudene, F., Boukraa, S., Boubidi, S. C., Guerzou, A., & Ouahabi, A. (2023). Human Cutaneous Leishmaniasis in North Africa and Its Threats to Public Health: A Statistical Study Focused on Djelfa (Algeria). Microorganisms, 11(10), 2608. https://doi.org/10.3390/microorganisms11102608









