Multidrug-Resistant Escherichia coli Isolate of Chinese Bovine Origin Carrying the blaCTX-M-55 Gene Located in IS26-Mediated Composite Translocatable Units
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Isolation and Identification
2.2. Antimicrobial Susceptibility Testing
2.3. Conjugation Assay and Determination of Conjugation Frequency
2.4. Whole-Genome Sequencing and Bioinformatics Analysis of CTX-M E. coli Producer
2.5. Plasmid Stability
2.6. Statistical Analysis
2.7. Nucleotide Sequence Accession Numbers
3. Results
3.1. Characteristics of CTX-M-Positive E. coli
3.2. Genetic Environment of the blaCTX-M-55-Harboring IncHI2 Plasmid
3.3. Transconjugative Frequencies of Plasmids Carrying blaCTX-M-55
3.4. Plasmid Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moffat, J.; Chalmers, G.; Reid-Smith, R.; Mulvey, M.R.; Agunos, A.; Calvert, J.; Cormier, A.; Ricker, N.; Scott Weese, J.; Boerlin, P. Resistance to Extended-Spectrum Cephalosporins in Escherichia coli and Other Enterobacterales from Canadian Turkeys. PLoS ONE 2020, 15, e0236442. [Google Scholar] [CrossRef]
- Jeanvoine, A.; Bouxom, H.; Leroy, J.; Gbaguidi-Haore, H.; Bertrand, X.; Slekovec, C. Resistance to Third-Generation Cephalosporins in Escherichia coli in the French Community: The Times They Are a-Changin’? Int. J. Antimicrob. Agents 2020, 55, 105909. [Google Scholar] [CrossRef]
- Wei, B.; Cha, S.Y.; Shang, K.; Zhang, J.F.; Jang, H.K.; Kang, M. Genetic Diversity of Extended-Spectrum Cephalosporin Resistance in Salmonella enterica and E. coli Isolates in a Single Broiler Chicken. Vet. Microbiol. 2021, 254, 109010. [Google Scholar] [CrossRef]
- Bevan, E.R.; Jones, A.M.; Hawkey, P.M. Global Epidemiology of CTX-M β-Lactamases: Temporal and Geographical Shifts in Genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar] [CrossRef]
- Dantas Palmeira, J.; Ferreira, H.M.N. Extended-Spectrum Beta-Lactamase (ESBL)-Producing Enterobacteriaceae in Cattle Production—A Threat around the World. Heliyon 2020, 6, e03206. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Chen, P.X.; Yang, L.; Cai, R.M.; Zhu, J.H.; Fang, L.X.; Webber, M.A.; Jiang, H.X. Transmission of Plasmid-Borne and Chromosomal BlaCTX-M-64 among Escherichia coli and Salmonella Isolates from Food-Producing Animals via ISEcp1-Mediated Transposition. J. Antimicrob. Chemother. 2020, 75, 1424–1427. [Google Scholar] [CrossRef]
- Zeng, S.; Luo, J.; Li, X.; Zhuo, C.; Wu, A.; Chen, X.; Huang, L.S. Molecular Epidemiology and Characteristics of CTX-M-55 Extended-Spectrum β-Lactamase-Producing Escherichia coli from Guangzhou, China. Front. Microbiol. 2021, 12, 730012. [Google Scholar] [CrossRef]
- Veldman, K.; Tulden, P.; Kant, A.; Testerink, J.; Mevius, D. Characteristics of cefotaxime-resistant Escherichia coli from wild birds in the Netherlands. Appl. Environ. Microbiol. 2013, 79, 7556–7561. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Liu, Y.; Wang, J.; Lv, L.; Chen, X.; He, D.; Yang, T.; Hou, J.; Tan, Y.; et al. F33: A-: B-, IncHI2/ST3, and IncI1/ST71 Plasmids Drive the Dissemination of FosA3 and BlaCTX-M-55/-14/-65 in Escherichia coli from Chickens in China. Front. Microbiol. 2014, 5, 688. [Google Scholar] [CrossRef]
- Jing, W.; ZhenLing, Z.; XinYi, H.; ZhenBao, M.; ZeWen, G.; LuChao, L.; YingBi, X.; Li, Z.; QianHua, S.; JianHua, L. Evolution and Comparative Genomics of F33:A-:B- Plasmids Carrying BlaCTX-M-55 or BlaCTX-M-65 in Escherichia coli and Klebsiella pneumoniae Isolated from Animals, Food Products, and Humans in China. mSphere 2018, 3, e00137-18. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, S.; Xu, J.; Li, Y.; Pu, L.; Han, X.; Feng, Y. Genetic Context Diversity of Plasmid-Borne BlaCTX-M-55 in Escherichia coli Isolated from Waterfowl. J. Glob. Antimicrob. Resist. 2022, 28, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Massé, J.; Lardé, H.; Fairbrother, J.M.; Roy, J.P.; Francoz, D.; Dufour, S.; Archambault, M. Prevalence of Antimicrobial Resistance and Characteristics of Escherichia coli Isolates from Fecal and Manure Pit Samples on Dairy Farms in the Province of Québec, Canada. Front. Vet. Sci. 2021, 8, 654125. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Oh, S.S.; Kim, J.; Park, S.; Shin, J. Clinically Relevant Extended-Spectrum β-Lactamase–Producing Escherichia coli Isolates from Food Animals in South Korea. Front. Microbiol. 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Lupo, A.; Saras, E.; Madec, J.Y.; Haenni, M. Emergence of BlaCTX-M-55 Associated with FosA, RmtB and Mcr Gene Variants in Escherichia coli from Various Animal Species in France. J. Antimicrob. Chemother. 2018, 73, 867–872. [Google Scholar] [CrossRef]
- Ghosh, H.; Doijad, S.; Bunk, B.; Falgenhauer, L.; Yao, Y.; Spröer, C.; Gentil, K.; Schmiedel, J.; Imirzalioglu, C.; Overmann, J.; et al. Detection of Translocatable Units in a BlaCTX-M-15 Extended-Spectrum β-Lactamase-Producing ST131 Escherichia coli Isolate Using a Hybrid Sequencing Approach. Int. J. Antimicrob. Agents 2016, 47, 245–247. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Ding, X.M.; Lin, X.L.; Sun, R.Y.; Lu, Y.W.; Cai, R.M.; Webber, M.A.; Ding, H.Z.; Jiang, H.X. The Emergence of Chromosomally Located BlaTX-M-55 in Salmonella from Foodborne Animals in China. Front. Microbiol. 2019, 10, 1268. [Google Scholar] [CrossRef]
- Hassen, B.; Abbassi, M.S.; Ruiz-Ripa, L.; Mama, O.M.; Hassen, A.; Torres, C.; Hammami, S. High Prevalence of Mcr-1 Encoding Colistin Resistance and First Identification of BlaCTX-M-55 in ESBL/CMY-2-Producing Escherichia coli Isolated from Chicken Faeces and Retail Meat in Tunisia. Int. J. Food Microbiol. 2020, 318, 108478. [Google Scholar] [CrossRef]
- Shawa, M.; Furuta, Y.; Mulenga, G.; Mubanga, M.; Mulenga, E.; Zorigt, T.; Kaile, C.; Simbotwe, M.; Paudel, A.; Hang’ombe, B.; et al. Novel Chromosomal Insertions of ISEcp1-Bla CTX-M-15 and Diverse Antimicrobial Resistance Genes in Zambian Clinical Isolates of Enterobacter cloacae and Escherichia coli. Antimicrob. Resist. Infect. Control 2021, 10, 79. [Google Scholar] [CrossRef]
- Frost, L.S.; Leplae, R.; Summers, A.O.; Toussaint, A. Mobile Genetic Elements: The Agents of Open Source Evolution. Nat. Rev. Microbiol. 2005, 3, 722–732. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; Martínez, J.L.; Aracil-Gisbert, S.; Lanza, V.F. Gene Transmission in the One Health Microbiosphere and the Channels of Antimicrobial Resistance. Front. Microbiol. 2019, 10, 2892. [Google Scholar] [CrossRef]
- Cheng, K.; Fang, L.X.; Ge, Q.W.; Wang, D.; He, B.; Lu, J.Q.; Zhong, Z.X.; Wang, X.R.; Yu, Y.; Lian, X.L.; et al. Emergence of FosA3 and BlaCTX–M–14 in Multidrug-Resistant Citrobacter freundii Isolates from Flowers and the Retail Environment in China. Front. Microbiol. 2021, 12, 586504. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Olsen, R.H.; Song, A.; Xiao, J.; Wang, C.; Meng, H.; Shi, L. First Report of a Foodborne Salmonella Enterica Serovar Gloucester (4:I:L,w) ST34 Strain Harboring BlaCTX–M–55 and QnrS Genes Located in IS26-Mediated Composite Transposon. Front. Microbiol. 2021, 12, 646101. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A. Plasmids and the Spread of Resistance. Int. J. Med. Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, S.; White, D.G.; Schroeder, C.M.; Lu, R.; Yang, H.; McDermott, P.F.; Ayers, S.; Meng, J. Characterization of Multiple-Antimicrobial-Resistant Salmonella Serovars Isolated from Retail Meats. Appl. Environ. Microbiol. 2004, 70, 1–7. [Google Scholar] [CrossRef]
- Liu, C.; Qin, S.; Xu, H.; Xu, L.; Zhao, D.; Liu, X.; Lang, S.; Feng, X.; Liu, H.M. New Delhi Metallo-β-Lactamase 1(NDM-1), the Dominant Carbapenemase Detected in Carbapenem-Resistant Enterobacter Cloacae from Henan Province, China. PLoS ONE 2015, 10, e0135044. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Lee, E.H.; Yoon, Y.; Chua, B.; Son, A. Portable Lysis Apparatus for Rapid Single-Step DNA Extraction of Bacillus subtilis. J. Appl. Microbiol. 2016, 120, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A Genome Comparison Visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple Prokaryote Genome Comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef]
- Zhao, Q.-Y.; Zhu, J.-H.; Cai, R.-M.; Zheng, X.-R.; Zhang, L.-J.; Chang, M.-X.; Lu, Y.-W.; Fang, L.-X.; Sun, J.; Jiang, H.-X. IS26 Is Responsible for the Evolution and Transmission of BlaNDM-Harboring Plasmids in Escherichia coli of Poultry Origin in China. mSystems 2021, 6, e0064621. [Google Scholar] [CrossRef]
- Lv, L.; Wan, M.; Wang, C.; Gao, X.; Yang, Q.; Partridge, S.R.; Wang, Y.; Zong, Z.; Doi, Y.; Shen, J.; et al. Emergence of a Plasmid-Encoded Resistance-Nodulation-Division Efflux Pump Conferring Resistance to Multiple Drugs, Including Tigecycline, in Klebsiella pneumoniae. mBio 2020, 11, e02930-19. [Google Scholar] [CrossRef]
- He, D.D.; Zhao, S.Y.; Wu, H.; Hu, G.Z.; Zhao, J.F.; Zong, Z.Y.; Pan, Y.S. Antimicrobial Resistance-Encoding Plasmid Clusters with Heterogeneous MDR Regions Driven by IS26 in a Single Escherichia coli Isolate. J. Antimicrob. Chemother. 2019, 74, 1511–1516. [Google Scholar] [CrossRef]
- Wei, X.; Wang, W.; Lu, N.; Wu, L.; Dong, Z.; Li, B.; Zhou, X.; Cheng, F.; Zhou, K.; Cheng, H.; et al. Prevalence of Multidrug-Resistant CTX-M Extended Spectrum Beta-Lactamase-Producing Escherichia coli from Different Bovine Faeces in China. Front. Vet. Sci. 2022, 9, 738904. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S.N.; Hilty, M.; Perreten, V.; Endimiani, A. Extended-Spectrum Cephalosporin-Resistant Gram-Negative Organisms in Livestock: An Emerging Problem for Human. Health? Drug Resist. Updates 2013, 16, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Mollenkopf, D.F.; Weeman, M.F.; Daniels, J.B.; Abley, M.J.; Mathews, J.L.; Gebreyes, W.A.; Wittum, T.E. Variable within- and between-Herd Diversity of CTX-M Cephalosporinase-Bearing Escherichia coli Isolates from Dairy Cattle. Appl. Environ. Microbiol. 2012, 78, 4552–4560. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Masani, K.; Sato, C.; Hiki, M.; Usui, M.; Baba, K.; Ozawa, M.; Harada, K.; Aoki, H.; Sawada, T. Phylogenetic Groups and Cephalosporin Resistance Genes of Escherichia coli from Diseased Food-Producing Animals in Japan. Acta Vet. Scand. 2011, 53, 52. [Google Scholar] [CrossRef]
- Hiroi, M.; Harada, T.; Kawamori, F.; Takahashi, N.; Kanda, T.; Sugiyama, K.; Masuda, T.; Yoshikawa, Y.; Ohashi, N. A Survey of β-Lactamase-Producing Escherichia coli in Farm Animals and Raw Retail Meat in Shizuoka Prefecture, Japan. Jpn. J. Infect. Dis. 2011, 64, 153–155. [Google Scholar] [CrossRef]
- Ho, P.L.; Chow, K.H.; Lai, E.L.; Lo, W.U.; Yeung, M.K.; Chan, J.; Chan, P.Y.; Yuen, K.Y. Extensive Dissemination of CTX-M-Producing Escherichia coli with Multidrug Resistance to “critically Important” Antibiotics among Food Animals in Hong Kong, 2008–2010. J. Antimicrob. Chemother. 2011, 66, 765–768. [Google Scholar] [CrossRef]
- Zheng, H.; Zeng, Z.; Chen, S.; Liu, Y.; Yao, Q.; Deng, Y.; Chen, X.; Lv, L.; Zhuo, C.; Chen, Z.; et al. Prevalence and Characterisation of CTX-M β-Lactamases amongst Escherichia coli Isolates from Healthy Food Animals in China. Int. J. Antimicrob. Agents 2012, 39, 305–310. [Google Scholar] [CrossRef]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant Gram-Negative Bacteria: The Role of High-Risk Clones in the Dissemination of Antibiotic Resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global Extraintestinal Pathogenic Escherichia coli (Expec) Lineages. Clin. Microbiol. Rev. 2019, 32, e00135-18. [Google Scholar] [CrossRef]
- Hayashi, W.; Ohsaki, Y.; Taniguchi, Y.; Koide, S.; Kawamura, K.; Suzuki, M.; Kimura, K.; Wachino, J.-i.; Nagano, Y.; Arakawa, Y.; et al. High Prevalence of BlaCTX-M-14 among Genetically Diverse Escherichia coli Recovered from Retail Raw Chicken Meat Portions in Japan. Int. J. Food Microbiol. 2018, 284, 98–104. [Google Scholar] [CrossRef]
- Zurfluh, K.; Albini, S.; Mattmann, P.; Kindle, P.; Nüesch-Inderbinen, M.; Stephan, R.; Vogler, B.R. Antimicrobial Resistant and Extended-Spectrum β-Lactamase Producing Escherichia coli in Common Wild Bird Species in Switzerland. Microbiologyopen 2019, 8, e845. [Google Scholar] [CrossRef]
- Varani, A.; He, S.; Siguier, P.; Ross, K.; Chandler, M. The IS6 Family, a Clinically Important Group of Insertion Sequences Including IS26. Mob. DNA 2021, 12, 11. [Google Scholar] [CrossRef]
- Harmer, C.J.; Hall, R.M. IS26 Cannot Move Alone. J. Antimicrob. Chemother. 2021, 76, 1428–1432. [Google Scholar] [CrossRef]
- He, S.; Hickman, A.B.; Varani, A.M.; Siguier, P.; Chandler, M.; Dekker, J.P.; Dyda, F. Insertion Sequence IS26 Reorganizes Plasmids in Clinically Isolated Multidrug-Resistant Bacteria by Replicative Transposition. mBio 2015, 6, e00762. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Harmer, C.J.; Hall, R.M. IS26-Mediated Precise Excision of the IS26-Apha1a Translocatable Unit. mBio 2015, 6, e01866-15. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; Hall, R.M. IS 26-Mediated Formation of Transposons Carrying Antibiotic Resistance Genes. mSphere 2016, 1, e00038-16. [Google Scholar] [CrossRef] [PubMed]
- Leão, C.; Clemente, L.; Moura, L.; Seyfarth, A.M.; Hansen, I.M.; Hendriksen, R.S.; Amaro, A. Emergence and Clonal Spread of CTX-M-65-Producing Escherichia coli from Retail Meat in Portugal. Front. Microbiol. 2021, 12, 653595. [Google Scholar] [CrossRef]
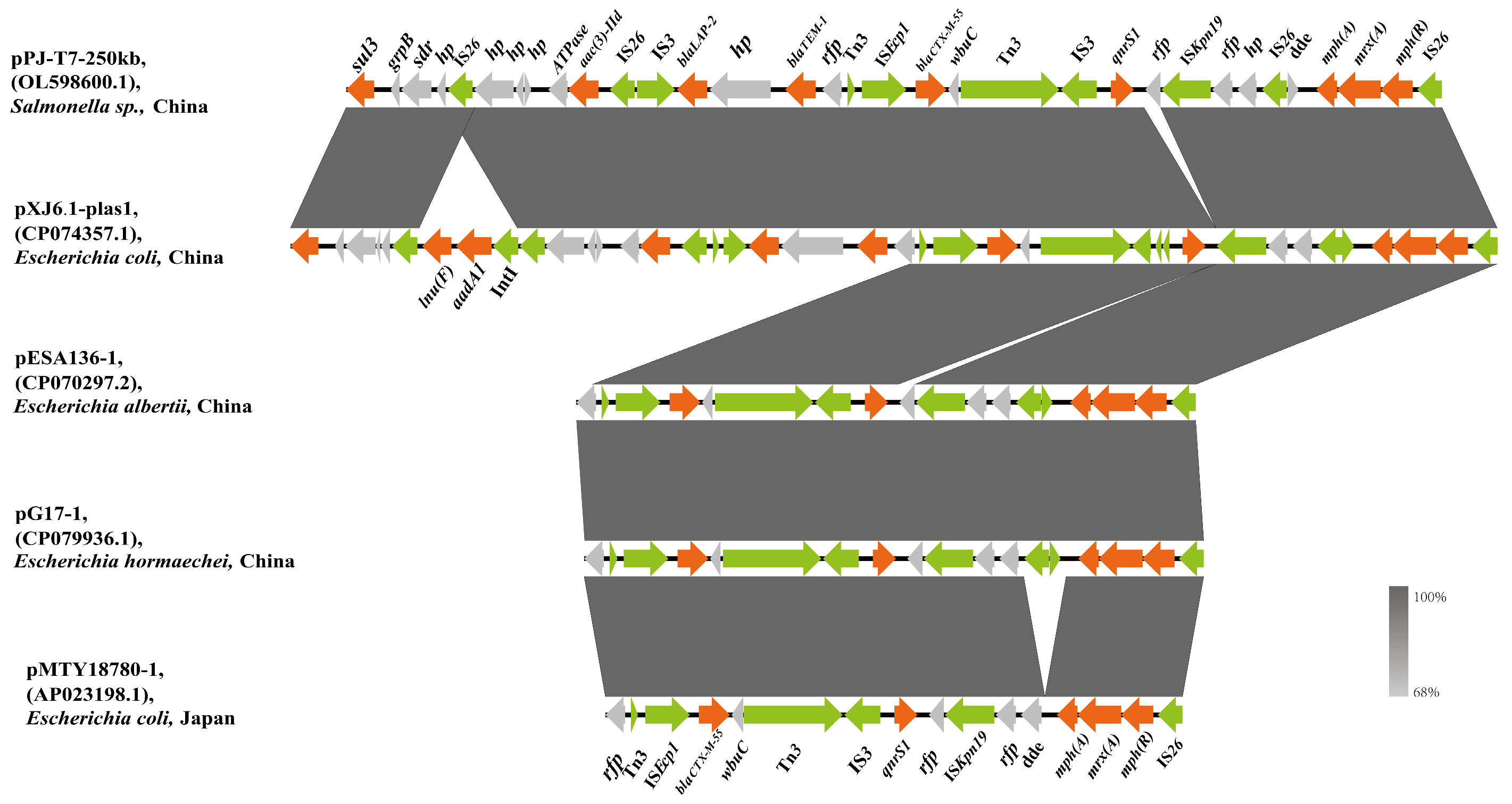


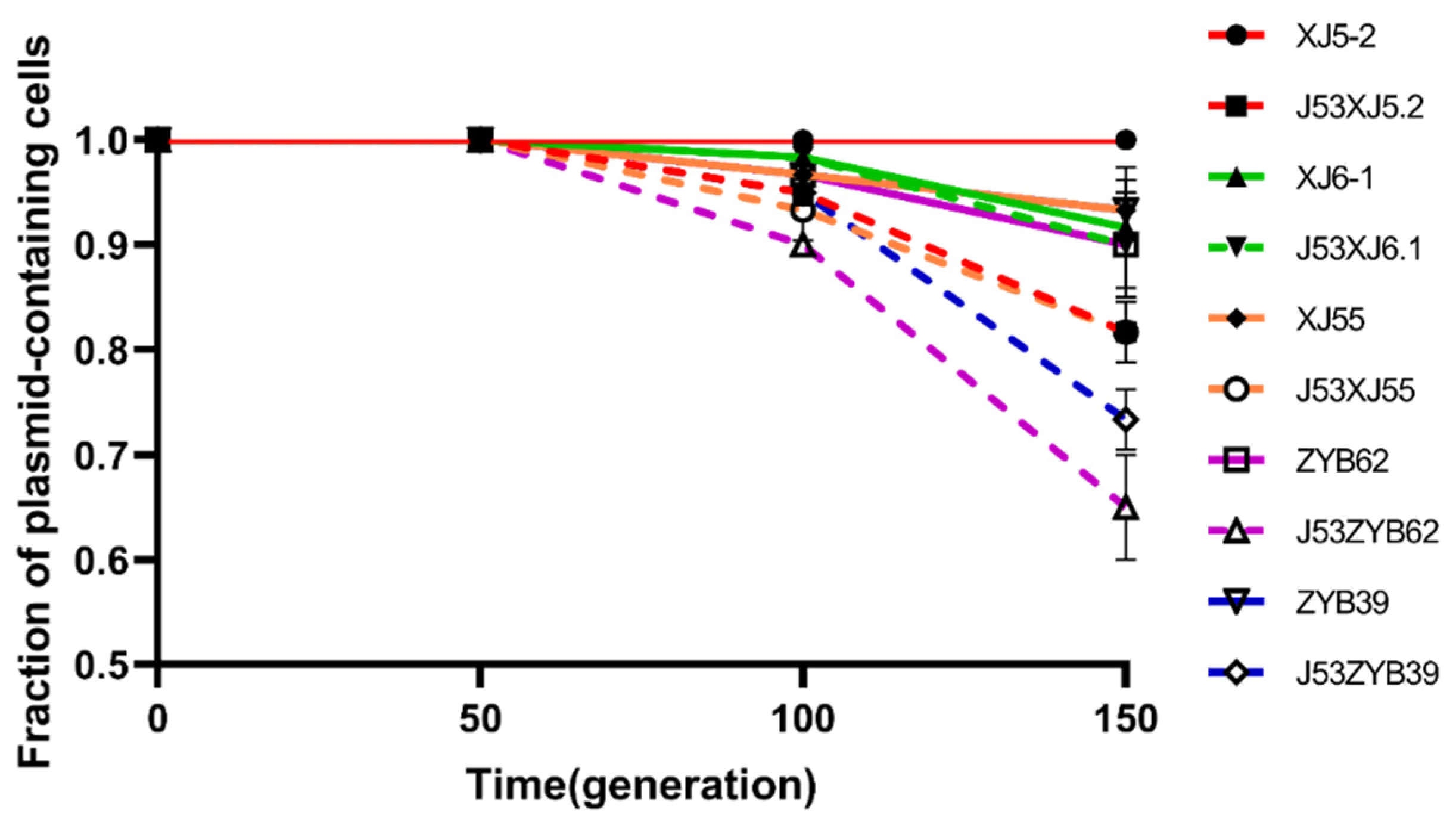
| Strain | Province | Cattle | MLST | Serotype | Resistance Profiles |
|---|---|---|---|---|---|
| XJ5.2 | Xinjiang | Dairy | ST5044 | O45:H45 | KF/CTX/CAZ/CRO/C/K/AMP/GN/ATM/ |
| XJ6.1 | Xinjiang | Dairy | NT | O23:H24 | KF/CTX/CAZ/CRO/TE/C/K/AMP/GN/DO/ATM/SXT |
| XJ55 | Xinjiang | Dairy | ST155 | O88:H25 | KF/CTX/CAZ/CRO/TE/C/AMP/GN/ATM/SXT |
| ZYB39 | Gansu | Beef | ST6345 | O83:H7 | KF/CTX/CAZ/CRO/TE/C/AMP/GN/DO/ATM/SXT |
| ZYB62 | Gansu | Beef | ST58 | O8:H21 | KF/CTX/CAZ/CRO/TE/C/AMP/GN/ATM/SXT |
| Plasmids | Size (kb) | Replicon Type | Resistance Genes | Number of TUs |
|---|---|---|---|---|
| pXJ5.2_1 | 209 | IncHI2 | blaCTX-M-55/dfrA14/aadA5/sul2/floR | NE |
| pXJ6.1_1 | 267 | IncHI2 | blaCTX-M-55/dfrA14/qnrS1/blaΔTEM/lap/aac(3’)-III/aadA1/lnu(F)/sul3/tetA/tetR/floR/aph(3″)-Ib | 5 |
| pXJ55_1 | 230 | IncHI2 | blaCTX-M-55/aac(3’)-III/sul1/qnrB6/aac(6′)-Ib/floR/tetA/tetR/strB/aph(3″)-Ib/blaTEM-1 | 2 |
| pZYB39_2 | 230 | IncHI2 | blaCTX-M-55/aac(3’)-IId/sul1/qacE/qnrB6/dfrA27/arr-3/aac(6′)-Ib/floR/tetA/strB/aph(3″)-Ib/blaTEM-1 | 2 |
| pZYB62_1 | 226 | IncHI2 | blaCTX-M-55/aac(6′)-Ib/arr-3/dfrA27/qacE/sul1/floR/tetA/strB/aph(3″)/blaTEM-1 | 2 |
| E. coli Strain (Transconjugants) | Transconjugative Frequencies a |
|---|---|
| pJ53CTX-M b | |
| J53XJ5.2 | 4.7 × 10−5 |
| J53XJ6.1 | 3.6 × 10−8 |
| J53XJ55 | 1.2 × 10−6 |
| J53ZYB39 | 1.8 × 10−8 |
| J53ZYB62 | 4.3 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wei, X.; Arbab, S.; Wu, L.; Lu, N.; Zhu, Q.; Bai, Y.; Zhang, J. Multidrug-Resistant Escherichia coli Isolate of Chinese Bovine Origin Carrying the blaCTX-M-55 Gene Located in IS26-Mediated Composite Translocatable Units. Microorganisms 2023, 11, 2795. https://doi.org/10.3390/microorganisms11112795
Wang W, Wei X, Arbab S, Wu L, Lu N, Zhu Q, Bai Y, Zhang J. Multidrug-Resistant Escherichia coli Isolate of Chinese Bovine Origin Carrying the blaCTX-M-55 Gene Located in IS26-Mediated Composite Translocatable Units. Microorganisms. 2023; 11(11):2795. https://doi.org/10.3390/microorganisms11112795
Chicago/Turabian StyleWang, Weiwei, Xiaojuan Wei, Safia Arbab, Lingyu Wu, Ningning Lu, Qiqi Zhu, Yubin Bai, and Jiyu Zhang. 2023. "Multidrug-Resistant Escherichia coli Isolate of Chinese Bovine Origin Carrying the blaCTX-M-55 Gene Located in IS26-Mediated Composite Translocatable Units" Microorganisms 11, no. 11: 2795. https://doi.org/10.3390/microorganisms11112795
APA StyleWang, W., Wei, X., Arbab, S., Wu, L., Lu, N., Zhu, Q., Bai, Y., & Zhang, J. (2023). Multidrug-Resistant Escherichia coli Isolate of Chinese Bovine Origin Carrying the blaCTX-M-55 Gene Located in IS26-Mediated Composite Translocatable Units. Microorganisms, 11(11), 2795. https://doi.org/10.3390/microorganisms11112795






