Contribution of the Rapid LAMP-Based Diagnostic Test (RLDT) to the Evaluation of Enterotoxigenic Escherichia coli (ETEC) and Shigella in Childhood Diarrhea in the Peri-Urban Area of Ouagadougou, Burkina Faso
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Methods
- Collection of stool specimens, isolation, and identification of Shigella and ETEC
- ETEC and Shigella RLDTs analysis.
2.3. Statistical Analysis
3. Results
3.1. Stool Samples Used for Shigella and ETEC Testing
3.2. Stool Samples Positivity for Shigella and ETEC According to Diagnostic Tool
- Coinfection of Shigella and ETEC
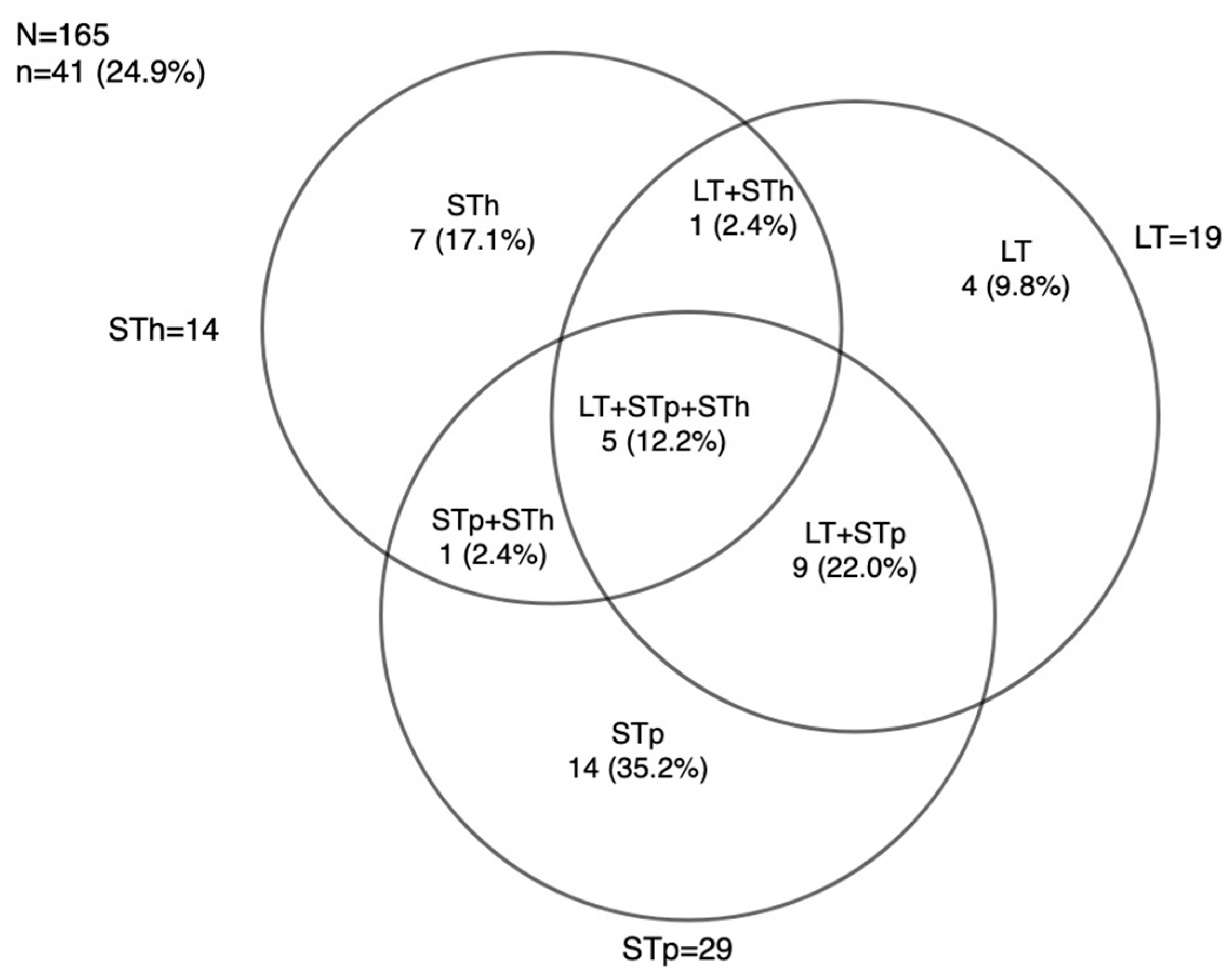
3.3. ETEC Toxins Genes Distribution using RLDT
3.4. Performance of the RLDT against Culture for Shigella and ETEC Detection
- Performance of RLDT against culture for Shigella detection
- Performance of the RLDT against culture followed by PCR for ETEC detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EDCTP | European and Developing countries Clinical trial Partnership |
| ETEC | Enterotoxigenic Escherichia coli |
| LT | Heat-labile enterotoxin |
| STp | Heat-stable enterotoxin Porcine |
| STh | Heat-stable enterotoxin Human |
| PCR | Polymerase chain reaction |
| RLDT | Rapid LAMP-based diagnostic test |
| Sd | Standard deviation |
| WHO | World Health Organisation |
References
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. Available online: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(18)30362-1/fulltext (accessed on 26 July 2022). [CrossRef] [PubMed]
- Zelelie, T.Z.; Gebreyes, D.S.; Tilahun, A.T.; Craddock, H.A.; Gishen, N.Z. Enteropathogens in Under-Five Children with Diarrhea in Health Facilities of Debre Berhan Town, North Shoa, Ethiopia. Ethiop. J. Health Sci. 2019, 29, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Sansonetti, P.J.; Marteyn, B.S. Shigella Diversity and Changing Landscape: Insights for the Twenty-First Century. Front. Cell. Infect. Microbiol. 2016, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- McQuade, E.T.R.; Shaheen, F.; Kabir, F.; Rizvi, A.; Platts-Mills, J.A.; Aziz, F.; Kalam, A.; Qureshi, S.; Elwood, S.; Liu, J.; et al. Epidemiology of Shigella infections and diarrhea in the first two years of life using culture-independent diagnostics in 8 low-resource settings. PLoS Neglected Trop. Dis. 2020, 14, e0008536. [Google Scholar]
- Hosangadi, D.; Smith, P.G.; Giersing, B.K. Considerations for using ETEC and Shigella disease burden estimates to guide vaccine development strategy. Vaccine 2019, 37, 7372–7380. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6892262/ (accessed on 26 June 2023). [CrossRef]
- Vidal, R.M.; Muhsen, K.; Tennant, S.M.; Svennerholm, A.-M.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; Saha, D.; Adegbola, R.; et al. Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS). PLoS Neglected Trop. Dis. 2019, 13, e0007037. Available online: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0007037 (accessed on 28 May 2023). [CrossRef]
- Khalil, I.; Walker, R.; Porter, C.K.; Muhib, F.; Chilengi, R.; Cravioto, A.; Guerrant, R.; Svennerholm, A.-M.; Qadri, F.; Baqar, S.; et al. Enterotoxigenic Escherichia coli (ETEC) vaccines: Priority activities to enable product development, licensure, and global access. Vaccine 2021, 39, 4266–4277. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8273896/ (accessed on 20 September 2022). [CrossRef]
- Wang, H.; Zhong, Z.; Luo, Y.; Cox, E.; Devriendt, B. Heat-Stable Enterotoxins of Enterotoxigenic Escherichia coli and Their Impact on Host Immunity. Toxins 2019, 11, 24. Available online: https://www.mdpi.com/2072-6651/11/1/24 (accessed on 21 October 2023). [CrossRef]
- Nasrin, S.; Haque, A.; Palit, P.; Das, R.; Mahfuz, M.; Faruque, A.S.G.; Ahmed, T. Incidence of Asymptomatic Shigella Infection and Association with the Composite Index of Anthropometric Failure among Children Aged 1–24 Months in Low-Resource Settings. Life 2022, 12, 607. Available online: https://www.mdpi.com/2075-1729/12/5/607 (accessed on 12 March 2023). [CrossRef]
- Hasso-Agopsowicz, M.; Lopman, B.A.; Lanata, C.F.; McQuade, E.T.R.; Kang, G.; Prudden, H.J.; Khalil, I.; Platts-Mills, J.A.; Kotloff, K.; Jit, M.; et al. World Health Organization Expert Working Group: Recommendations for assessing morbidity associated with enteric pathogens. Vaccine 2021, 39, 7521–7525. Available online: https://www.sciencedirect.com/science/article/pii/S0264410X2101478X (accessed on 22 June 2023). [CrossRef]
- Dembélé, R.; Konaté, A.; Traoré, O.; Kaboré, W.A.D.; Soulama, I.; Kagambèga, A.; Traoré, A.S.; Guessennd, N.K.; Aidara-Kane, A.; Gassama-Sow, A.; et al. Extended spectrum beta-lactamase and fluoroquinolone resistance genes among Escherichia coli and Salmonella isolates from children with diarrhea, Burkina Faso. BMC Pediatr. 2020, 20, 459. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, A.S.; Jean Pierre, H.; Bañuls, A.L.; Ouédraogo, R.; Godreuil, S. Emergence and spread of antibiotic resistance in West Africa: Contributing factors and threat assessment. Médecine Et Santé Trop. 2017, 27, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Troeger, C.E.; Blacker, B.F.; Reiner, R.C. Capturing the true burden of Shigella and ETEC: The way forward. Vaccine 2019, 37, 4784–4786. [Google Scholar] [CrossRef]
- Houpt, E.; Gratz, J.; Kosek, M.; Zaidi, A.K.M.; Qureshi, S.; Kang, G.; Babji, S.; Mason, C.; Bodhidatta, L.; Samie, A.; et al. Microbiologic Methods Utilized in the MAL-ED Cohort Study. Clin. Infect. Dis. 2014, 59 (Suppl 4), S225–S232. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4204609/ (accessed on 7 November 2021). [CrossRef] [PubMed]
- Connor, S.; Velagic, M.; Zhang, X.; Johura, F.-T.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Alam, M.; Sack, D.A.; Wierzba, T.F.; et al. Evaluation of a simple, rapid and field-adapted diagnostic assay for enterotoxigenic E. coli and Shigella. PLoS Neglected Trop. Dis. 2022, 16, e0010192. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8853640/ (accessed on 6 July 2023). [CrossRef] [PubMed]
- Chakraborty, S.; Connor, S.; Velagic, M. Development of a simple, rapid, and sensitive diagnostic assay for enterotoxigenic E. coli and Shigella spp. applicable to endemic countries. PLoS Neglected Trop. Dis. 2022, 16, e0010180. Available online: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0010180 (accessed on 23 February 2023). [CrossRef]
- Silwamba, S.; Chilyabanyama, O.N.; Liswaniso, F.; Chisenga, C.C.; Chilengi, R.; Dougan, G.; Kwenda, G.; Chakraborty, S.; Simuyandi, M. Field evaluation of a novel, rapid diagnostic assay, and molecular epidemiology of enterotoxigenic E. coli among Zambian children presenting with diarrhea. PLoS Neglected Trop. Dis. 2022, 16, e0010207. Available online: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0010207 (accessed on 23 February 2023). [CrossRef]
- Aranda, K.R.S.; Fagundes-Neto, U.; Scaletsky, I.C.A. Evaluation of Multiplex PCRs for Diagnosis of Infection with Diarrheagenic Escherichia coli and Shigella spp. J. Clin. Microbiol. 2004, 42, 5849–5853. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC535216/ (accessed on 24 June 2023). [CrossRef]
- Nitiema, L.W.; Nordgren, J.; Ouermi, D.; Dianou, D.; Traore, A.S.; Svensson, L.; Simpore, J. Burden of rotavirus and other enteropathogens among children with diarrhea in Burkina Faso. Int. J. Infect. Dis. 2011, 15, e646–e652. [Google Scholar] [CrossRef]
- Bonkoungou, I.; Lienemann, T.; Martikainen, O.; Dembelé, R.; Sanou, I.; Traoré, A.; Siitonen, A.; Barro, N.; Haukka, K. Diarrhoeagenic Escherichia coli detected by 16-plex PCR in children with and without diarrhoea in Burkina Faso. Clin. Microbiol. Infect. 2012, 18, 901–906. [Google Scholar] [CrossRef]
- Konaté, A.; Dembélé, R.; Kagambèga, A.; Soulama, I.; Kaboré, W.A.; Sampo, E.; Cissé, H.; Sanou, A.; Serme, S.; Zongo, S.; et al. Molecular Characterization of Diarrheagenic Escherichia coli in Children Less Than 5 Years of Age with Diarrhea in Ouagadougou, Burkina Faso. Eur. J. Microbiol. Immunol. 2017, 7, 220–228. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5632749/ (accessed on 17 September 2022). [CrossRef] [PubMed]
- Cissé, H.; Kagambèga, A.; Bouda, S.C.; Sawadogo, A.; Barro, N. Phenotypic and Genotypic Antibiotic Resistant Diarrheagenic Escherichia coli Isolated from Patients with Diarrhea in Ouagadougou, Burkina Faso. Adv. Microbiol. 2023, 13, 347–359. Available online: http://www.scirp.org/Journal/Paperabs.aspx?paperid=126150 (accessed on 9 July 2023). [CrossRef]
- Platts-Mills, J.A.; Liu, J.; Rogawski, E.T.; Kabir, F.; Lertsethtakarn, P.; Siguas, M.; Khan, S.S.; Praharaj, I.; Murei, A.; Nshama, R.; et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: A reanalysis of the MAL-ED cohort study. Lancet Glob Health 2018, 6, e1309-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-M.; Ma, J.-C.; Hao, Z.-Y.; Zhang, Z.-Y.; Mason, C.; Sethabutr, O.; von Seidlein, L.; Wang, X.-Y.; Xu, Z.-Y. Surveillance of shigellosis by real-time PCR suggests underestimation of shigellosis prevalence by culture-based methods in a population of rural China. J. Infect. 2010, 61, 471–475. [Google Scholar] [CrossRef] [PubMed]

| ETEC Toxin/Virulence Factor | Target Gene | Primer Name | Primer Sequences (5′ to 3′) | Amplicon Size (bp) |
|---|---|---|---|---|
| LT | eltB | LTF | ACG GCG TTA CTA TCC TCT C | 274 |
| LTR | TGG TCT CGG TCA GAT ATG TG | |||
| STp | estA1 | STpF1 | TCT TTC CCC TCT TTT AGT CAG | 166 |
| STpR2 | ACA GGC AGG ATT ACA ACA AAG | |||
| STh | estA2 | SThnyF | TTCACCTTTCCCTCAGGATG | 120 |
| SThnyR | CTATTATTCATGCTTTCAGGACCA |
| Well | A | B | C | D | E | F | G | H |
| Gene | ipaH | control | ipaH | control | ipaH | control | ipaH | control |
| Well | A | B | C | D | E | F | G | H |
| Gene | LT | STh | STp | control | LT | STh | STp | control |
| Shigella n = 263 (44.87) | ETEC n = 165 (23.19) | |
| Characteristics | ||
| Age (Months) | ||
| 0–11 | 57 (21.7) | 38 (23.0) |
| 12–23 | 81 (30.8) | 49 (29.7) |
| 24+ | 125 (47.5) | 78 (47.3) |
| Gender | ||
| Male | 134 (51.0) | 79 (47.9) |
| Female | 129 (49.1) | 86 (52.1) |
| Type of stool | ||
| Diarrheal | 155 (58.9) | 104 (63.0) |
| Non-diarrheal | 108 (41.1) | 61 (37.0) |
| Severity scoring (Vesikari score for diarrheal cases) | ||
| Mild | 144 (93.0) | 98 (24.6) |
| Moderate | 5 (3.2) | 2 (24.6) |
| Severe | 6 (3.9) | 4 (24.6) |
| Stunted (HAZ < −2.0) | ||
| Yes | 67 (25.5) | 48 (29.1) |
| No | 196 (74.5) | 117 (70.9) |
| RLDT | Culture | ||
|---|---|---|---|
| Characteristics | Proportion of Positive Shigella using RLDT | Proportion of Positive Shigella by Culture | p-Value |
| Overall | 118 (44.9 *) | 61 (23.2 *) | <0.001 |
| Age (Months) | |||
| 0–11 | 29 (50.9) | 13 (22.8) | <0.001 |
| 12–23 | 41 (50.6) | 17 (21.0) | |
| 24+ | 48 (38.4) | 31 (24.8) | |
| Gender | |||
| Male | 61 (45.5) | 27 (20.2) | <0.001 |
| Female | 57 (44.2) | 34 (26.4) | |
| Type of stool | |||
| Diarrheal | 75 (48.4) | 29 (18.7) | <0.001 |
| Non-diarrheal | 43 (39.8) | 32 (29.6) | |
| Severity scoring (Vesikari score for diarrheal cases) | |||
| Mild | 69 (47.9) | 28 (19.4) | <0.001 |
| Moderate | 2 (40.0) | 0 | |
| Severe | 4 (66.7) | 1 (16.7) | |
| Stunted (HAZ < −2.0) | |||
| Yes | 29 (43.3) | 13 (19.4) | <0.001 |
| No | 89 (45.4) | 48 (24.5) | |
| Shigella Culture (as the Gold Standard) vs. RLDT | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total Samples Screened | Samples Positive via RLDT (%) | Samples Positive via Culture (%) | False Positive | False Negative | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) |
| 263 | 118 | 57 | 61 | 4 | 93.4 (90.5, 96.4) | 69.8 (64.3, 75.4) | 48.3 (42.3, 54.3) | 97.2 (95.3, 99.2) |
| Cohen’s Kappa RLDT Stool Culture for Shigella Detection | |||||
|---|---|---|---|---|---|
| Agreement | Expected Agreement | Kappa | Std. error | z | p Value |
| 75.29% | 52.75% | 0.4769 | 0.0548 | 8.70 | <0.001 |
| Cohen’s Kappa RLDT Stool Culture for ETEC Detection | |||||
|---|---|---|---|---|---|
| Agreement | Expected Agreement | Kappa | Std. err. | z | p Value |
| 78.18% | 73.02% | 0.1914 | 0.0503 | 3.81 | <0.001 |
| ETEC Culture (as the Gold Standard *) vs. RLDT | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total Samples Screened | Samples Positive via ETEC RLDT | Samples Positive via Culture | False Positive | False Negative | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) |
| 165 | 41 | 7 | 35 | 1 | 83.7 (80.37, 91.1) | 77.9 (71.5, 84.2) | 14.6 (9.2, 20.0) | 99.2 (97.8, 100.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Héma, A.; Sermé, S.S.; Sawadogo, J.; Diarra, A.; Barry, A.; Ouédraogo, A.Z.; Nébié, I.; Tiono, A.B.; Houard, S.; Chakraborty, S.; et al. Contribution of the Rapid LAMP-Based Diagnostic Test (RLDT) to the Evaluation of Enterotoxigenic Escherichia coli (ETEC) and Shigella in Childhood Diarrhea in the Peri-Urban Area of Ouagadougou, Burkina Faso. Microorganisms 2023, 11, 2809. https://doi.org/10.3390/microorganisms11112809
Héma A, Sermé SS, Sawadogo J, Diarra A, Barry A, Ouédraogo AZ, Nébié I, Tiono AB, Houard S, Chakraborty S, et al. Contribution of the Rapid LAMP-Based Diagnostic Test (RLDT) to the Evaluation of Enterotoxigenic Escherichia coli (ETEC) and Shigella in Childhood Diarrhea in the Peri-Urban Area of Ouagadougou, Burkina Faso. Microorganisms. 2023; 11(11):2809. https://doi.org/10.3390/microorganisms11112809
Chicago/Turabian StyleHéma, Alimatou, Samuel S. Sermé, Jean Sawadogo, Amidou Diarra, Aissata Barry, Amidou Z. Ouédraogo, Issa Nébié, Alfred B. Tiono, Sophie Houard, Subhra Chakraborty, and et al. 2023. "Contribution of the Rapid LAMP-Based Diagnostic Test (RLDT) to the Evaluation of Enterotoxigenic Escherichia coli (ETEC) and Shigella in Childhood Diarrhea in the Peri-Urban Area of Ouagadougou, Burkina Faso" Microorganisms 11, no. 11: 2809. https://doi.org/10.3390/microorganisms11112809
APA StyleHéma, A., Sermé, S. S., Sawadogo, J., Diarra, A., Barry, A., Ouédraogo, A. Z., Nébié, I., Tiono, A. B., Houard, S., Chakraborty, S., Ouédraogo, A., & Sirima, S. B. (2023). Contribution of the Rapid LAMP-Based Diagnostic Test (RLDT) to the Evaluation of Enterotoxigenic Escherichia coli (ETEC) and Shigella in Childhood Diarrhea in the Peri-Urban Area of Ouagadougou, Burkina Faso. Microorganisms, 11(11), 2809. https://doi.org/10.3390/microorganisms11112809






