Identification of Linear Epitopes in the C-Terminal Region of ASFV p72 Protein
Abstract
:1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Cells and Animals
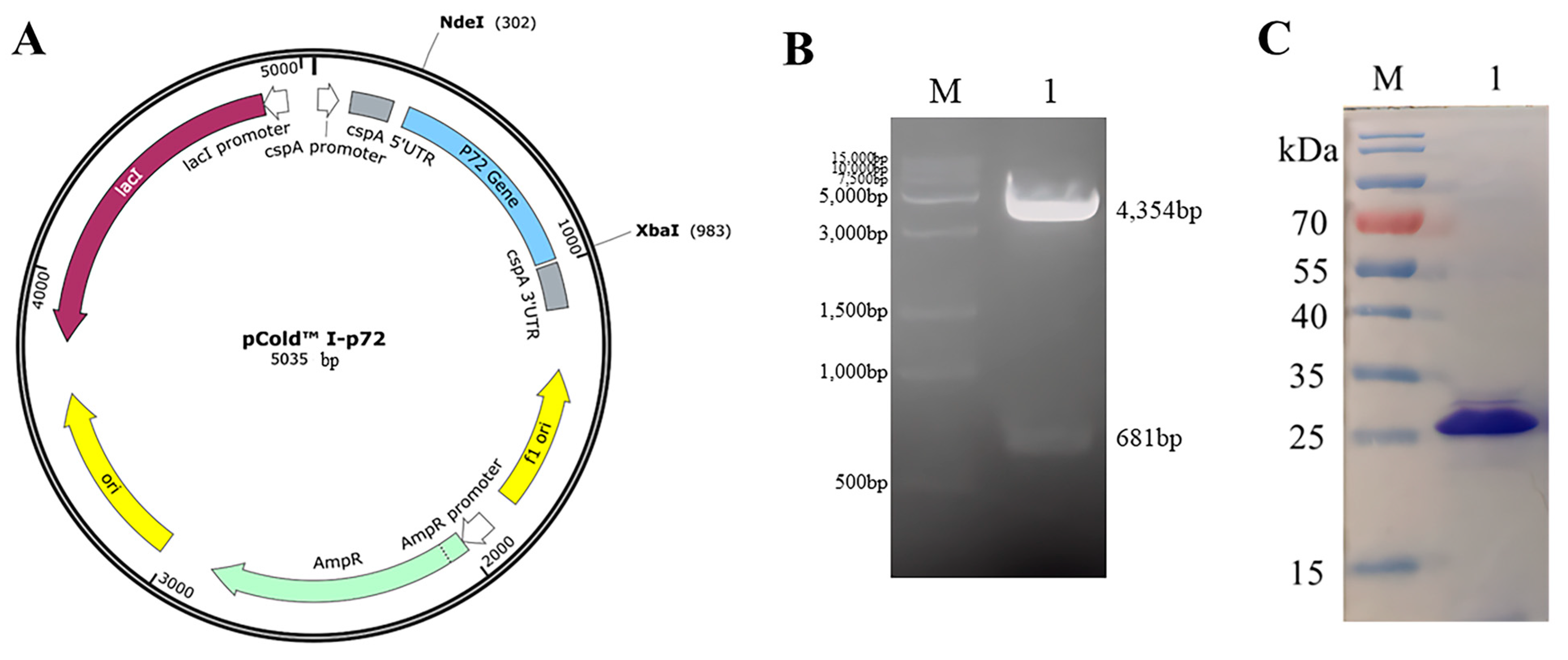
2.3. Expression and Purification of the Truncated C-Terminal p72 Protein
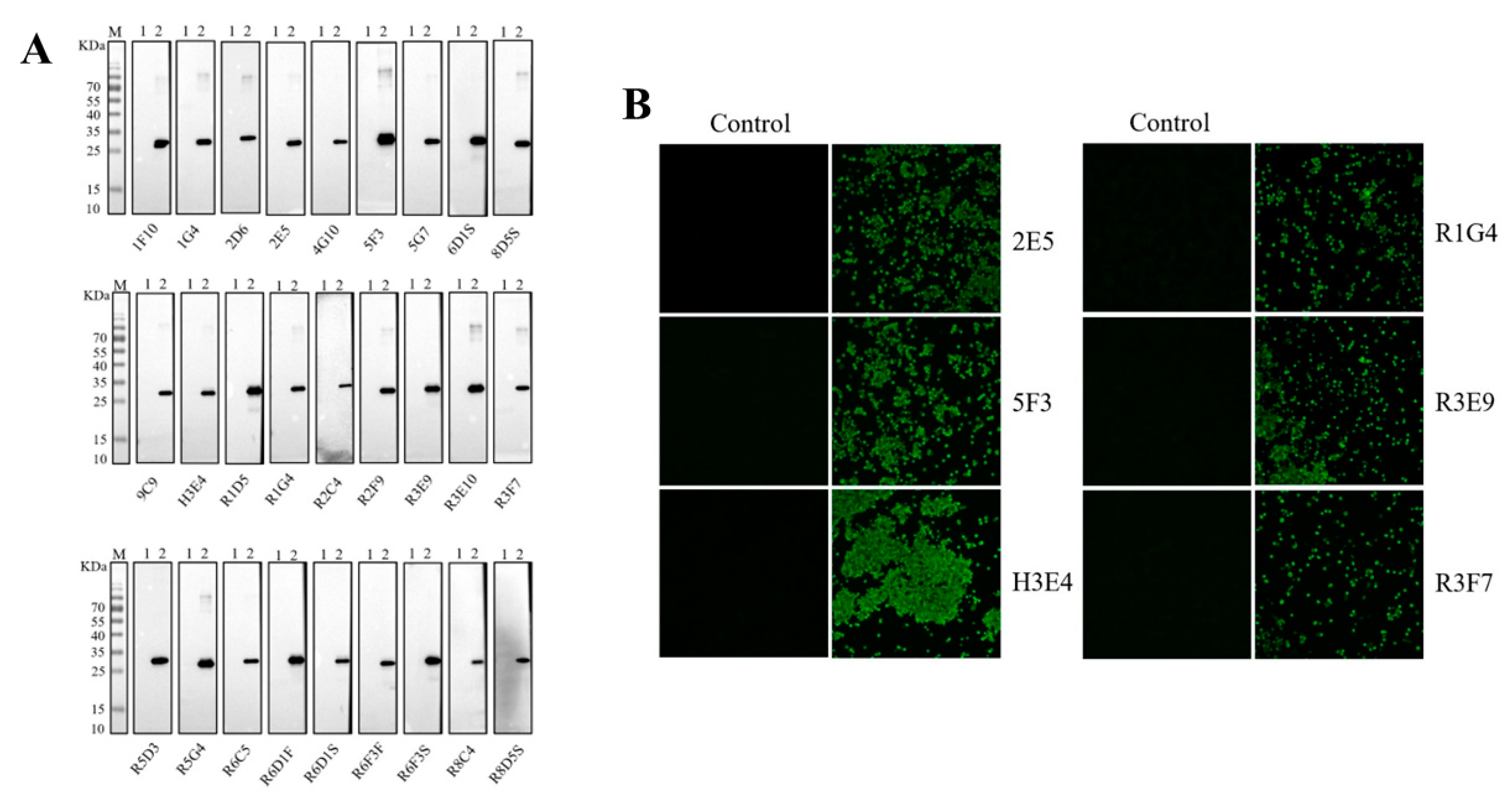
2.4. Generation of Monoclonal Antibodies against P72 Protein
2.5. Indirect ELISA
2.6. Western Blotting Assay
2.7. Immunofluorescence Assay (IFA)
2.8. Analysis of the Mab’s Antigenic Epitope
2.9. Homology Analysis
3. Results
3.1. Expression, Purification, and Identification of Recombinant p72 Protein
3.2. Production and Characterization of Monoclonal Antibodies against P72 Protein
3.3. Identify the Antigenic Epitope Recognized by the Mab
3.4. Structural Analysis of p72 Epitopes
3.5. Homology Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pikalo, J.; Zani, L.; Hühr, J.; Beer, M.; Blome, S. Pathogenesis of African swine fever in domestic pigs and European wild boar—Lessons learned from recent animal trials. Virus Res. 2019, 271, 197614. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current State of Global African Swine Fever Vaccine Development under the Prevalence and Transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Li, S.-H.; Yu, S.; Wang, Q.; Zhang, K.; Qu, L.; Sun, Y.; Bi, Y.; Tang, F.; et al. Transcriptome profiling in swine macrophages infected with African swine fever virus at single-cell resolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2201288119. [Google Scholar] [CrossRef]
- Keßler, C.; Forth, J.H.; Keil, G.M.; Mettenleiter, T.C.; Blome, S.; Karger, A. The intracellular proteome of African swine fever virus. Sci. Rep. 2018, 8, 14714. [Google Scholar] [CrossRef] [PubMed]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Andrés, G.; García-Escudero, R.; Viñuela, E.; Salas, M.L.; Rodríguez, J.M. African Swine Fever Virus Structural Protein pE120R Is Essential for Virus Transport from Assembly Sites to Plasma Membrane but Not for Infectivity. J. Virol. 2001, 75, 6758–6768. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef]
- Revilla, Y.; Perez-Nunez, D.; Richt, J.A. African Swine Fever Virus Biology and Vaccine Approaches. Adv. Virus Res. 2018, 100, 41–74. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, B.; Qian, N.; Zhang, F.; Tan, X.; Lei, J.; Xiang, Y. Structure of the African swine fever virus major capsid protein p72. Cell Res. 2019, 29, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, Y.; Wang, Y.; Li, S.; Zhao, Z.; Bi, Y.; Sun, J.; Peng, R.; Song, H.; Zhu, D.; et al. Cryo-EM Structure of the African Swine Fever Virus. Cell Host Microbe 2019, 26, 836–843e3. [Google Scholar] [CrossRef] [PubMed]
- Epifano, C.; Krijnse-Locker, J.; Salas, M.L.; Rodríguez, J.M.; Salas, J. The African Swine Fever Virus Nonstructural Protein pB602L Is Required for Formation of the Icosahedral Capsid of the Virus Particle. J. Virol. 2006, 80, 12260–12270. [Google Scholar] [CrossRef]
- Kollnberger, S.D.; Gutierrez-Castañeda, B.; Foster-Cuevas, M.; Corteyn, A.; Parkhouse, R.M.E. Identification of the principal serological immunodeterminants of African swine fever virus by screening a virus cDNA library with antibody. J. Gen. Virol. 2002, 83, 1331–1342. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Glas, P.S.; Schumann, K.R. Diagnostic specificity of the African swine fever virus antibody detection enzyme-linked immunosorbent assay in feral and domestic pigs in the United States. Transbound. Emerg. Dis. 2017, 64, 1665–1668. [Google Scholar] [CrossRef]
- Geng, R.; Sun, Y.; Li, R.; Yang, J.; Ma, H.; Qiao, Z.; Lu, Q.; Qiao, S.; Zhang, G. Development of a p72 trimer–based colloidal gold strip for detection of antibodies against African swine fever virus. Appl. Microbiol. Biotechnol. 2022, 106, 2703–2714. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Richt, J.A. Subunit Vaccine Approaches for African Swine Fever Virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ge, S.; Zhang, Y.; Wu, X.; Wang, Z. A systematic review of genotypes and serogroups of African swine fever virus. Virus Genes 2022, 58, 77–87. [Google Scholar] [CrossRef]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef]
- Duan, X.; Liu, Y.; Chen, Z.; Xie, Z.; Tian, C.; Li, Y.; Lv, L.; Wang, R.; Liu, J.; Chen, H. Identification of monoclonal antibody targeting epitope on p72 trimeric spike of African swine fever virus. Virus Genes 2023, 59, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Geng, R.; Shao, H.; Ye, J.; Qian, K.; Chen, H.; Qin, A. Identification of novel linear epitopes in P72 protein of African swine fever virus recognized by monoclonal antibodies. Front. Microbiol. 2022, 13, 1055820. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Yang, S.; Shao, J.; Zhou, G.; Ma, Y.; Wen, S.; Hou, Z.; Peng, D.; Guo, H.; Liu, W.; et al. Identification of p72 epitopes of African swine fever virus and preliminary application. Front. Microbiol. 2023, 14, 1126794. [Google Scholar] [CrossRef]
- Heimerman, M.E.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R. Linear epitopes in African swine fever virus p72 recognized by monoclonal antibodies prepared against baculovirus-expressed antigen. J. Veter- Diagn. Investig. 2018, 30, 406–412. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, Y.; Li, J.; Wang, A.; Meng, X.; Chen, L.; Wei, J.; Tong, W.; Kong, N.; Yu, L.; et al. Screening and identification of the dominant antigens of the African swine fever virus. Front. Vet. Sci. 2023, 10, 1175701. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Hu, Y.; Meng, X.; Chen, L.; Tong, W.; Shan, T.; Yu, H.; Kong, N.; Liu, X.; et al. Construction and Identification of Recombinant Baculovirus Expressing P72 Protein of African Swine Fever Virus. Chin. J. Anim. Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef]
- Gavier-Widén, D.; Ståhl, K.; Dixon, L. No hasty solutions for African swine fever. Science 2020, 367, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Mighell, E.; Ward, M.P. African Swine Fever spread across Asia, 2018–2019. Transbound. Emerg. Dis. 2021, 68, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Li, J.; Fan, X.; Liu, F.; Li, L.; Wang, Q.; Ren, W.; Bao, J.; Liu, C.; Wang, H.; et al. Molecular Characterization of African Swine Fever Virus, China, 2018. Emerg. Infect. Dis. 2018, 24, 2131–2133. [Google Scholar] [CrossRef] [PubMed]


| Fragments | Primer Sequences | Target Fragments Location |
|---|---|---|
| A1-F | cgggatccCCCGAGATCCACAACCTGTTC | aa421–567 |
| A1-R | ccgctcgagttaGCCGTAGTGGAATGGGATGTAG | |
| A2-F | cggaattcGGCCACGTGGTGAACGCCA | aa491–646 |
| A2-R | ccgctcgagttaGGTGCTGTACCTCAGCACGG | |
| B1-F | cgggatccGGCCACGTGGTGAACGC | aa491–544 |
| B1-R | ccgctcgagttaGCGGTCACGGAGAT | |
| B2-F | cggaattcGCCCTGCCAGACGCTTG | aa514–567 |
| B2-R | ccgctcgagttaGTAGTGGAATGGGATGTAG | |
| B3-F | cgggatccGGCAACGCCATCAAGACC | aa568–622 |
| B3-R | ccgctcgagttaCAGCGGTGGTGATGC | |
| B4-F | cggaattcTCCGGACACATCAACGTGAG | aa595–646 |
| B4-R | ccgctcgagttaGGTGCTGTACCTCAGCACGG | |
| B2–1-F | gatccGCCCTGCCAGACGCTTGCTCCAGCATCTCCGACATCAGCCCAGTGACCTAC CCCATCACCCTGCCAATCATCAAGAACATCTCCGTGACCGCCCACGGCtaac | aa514–546 |
| B2–1-R | tcgagttaGCCGTGGGCGGTCACGGAGATGTTCTTGATGATTGGCAGGGTGATGGGG TAGGTCACTGGGCTGATGTCGGAGATGCTGGAGCAAGCGTCTGGCAGGGCg | |
| B2–2-F | gatccCCAATCATCAAGAACATCTCCGTGACCGCCCACGGCATCAACCTGATCGAC AAGTTCCCCAGCAAGTTCTGCTCCAGCTACATCCCATTCCACTACGGCtaac | aa535–567 |
| B2–2-R | tcgagttaGCCGTAGTGGAATGGGATGTAGCTGGAGCAGAACTTGCTGGGGAACTTG TCGATCAGGTTGATGCCGTGGGCGGTCACGGAGATGTTCTTGATGATTGGg | |
| C1-F | ggatccCACGGCATCAACCTGATCGACAAGTTCCCCAGCAAGc | aa545–556 |
| C1-R | ctcgagCTTGCTGGGGAACTTGTCGATCAGGTTGATGCCGTGg | |
| C2-F | gatccAACCTGATCGACAAGTTCCCCAGCAAGTTCTGCc | aa548–558 |
| C2-R | tcgagGCAGAACTTGCTGGGGAACTTGTCGATCAGGTTg | |
| C3-F | gatccGACAAGTTCCCCAGCAAGTTCTGCTCCAGCc | aa551–560 |
| C3-R | tcgagGCTGGAGCAGAACTTGCTGGGGAACTTGTCg | |
| C4-F | gatccAGCAAGTTCTGCTCCAGCTACATCCCATTCc | aa555–564 |
| C4-R | tcgagGAATGGGATGTAGCTGGAGCAGAACTTGCTg | |
| C5-F | aattcTTCTGCTCCAGCTACATCCCATTCCACTACGGCc | aa557–567 |
| C5-R | tcgagGCCGTAGTGGAATGGGATGTAGCTGGAGCAGAAg | |
| C6-F | gatccCAAGAACATCTCCGTGACCGCCCACGGCATCAACCTGATCGACAAGTTtaac | aa537–552 |
| C6-R | tcgagttaAACTTGTCGATCAGGTTGATGCCGTGGGCGGTCACGGAGATGTTCTTGg | |
| C7-F | gatccCATCTCCGTGACCGCCCACGGCATCAACCTGATCGAtaac | aa539–550 |
| C7-R | tcgagttaTCGATCAGGTTGATGCCGTGGGCGGTCACGGAGATGg | |
| C8-F | gatccGGCAACGCCATCAAGACCCCAGACGACCCAGGAGCCATGc | aa568–580 |
| C8-R | tcgagCATGGCTCCTGGGTCGTCTGGGGTCTTGATGGCGTTGCCg | |
| C9-F | gatccGACGACCCAGGAGCCATGATGATCACCTTCGCCCTGc | aa575–586 |
| C9-R | tcgagCAGGGCGAAGGTGATCATCATGGCTCCTGGGTCGTCg | |
| C10-F | aattcATGATCACCTTCGCCCTGAAGCCAAGGGAGGAGTACCAGCCAc | aa581–594 |
| C10-R | tcgagTGGCTGGTACTCCTCCCTTGGCTTCAGGGCGAAGGTGATCATg | |
| D1-F | gatccAACATCTCCGTGACCGCCCACGGCATCAACCTGATCGACAAGTTCCCCAGC AAGTTCTGCTCCAGCTACATCtaac | aa539–562 |
| D1-R | tcgagttaGATGTAGCTGGAGCAGAACTTGCTGGGGAACTTGTCGATCAGGTTGATG CCGTGGGCGGTCACGGAGATGTTg | |
| D2-F | gatccAACATCTCCGTGACCGCCCACGGCATCAACCTGATCGACAAGTTCCCCAGC AAGtaac | aa539–556 |
| D2-R | tcgagttaCTTGCTGGGGAACTTGTCGATCAGGTTGATGCCGTGGGCGGTCACGGAG ATGTTg | |
| D3-F | gatccCACGGCATCAACCTGATCGACAAGTTCCCCAGCAAGTTCTGCTCCAGCTAC ATCtaac | aa545–562 |
| D3-R | tcgagttaGATGTAGCTGGAGCAGAACTTGCTGGGGAACTTGTCGATCAGGTTGATG CCGTGg | |
| D4-F | gatccGTGACCGCCCACGGCATCAACCTGATCGACAAGTTCtaac | aa542–553 |
| D4-R | tcgagttaGAACTTGTCGATCAGGTTGATGCCGTGGGCGGTCACg | |
| D4L-F | gatccACCGCCCACGGCATCAACCTGATCGACAAGTTCtaac | aa543–553 |
| D4L-R | tcgagttaGAACTTGTCGATCAGGTTGATGCCGTGGGCGGTg | |
| D4R-F | gatccGTGACCGCCCACGGCATCAACCTGATCGACAAGtaac | aa542–552 |
| D4R-R | tcgagttaCTTGTCGATCAGGTTGATGCCGTGGGCGGTCACg | |
| D5-F | gatccGGCAACGCCATCAAGACCCCAGACGACCCAGGAGCCATGc | aa568–574 |
| D5-R | tcgagCATGGCTCCTGGGTCGTCTGGGGTCTTGATGGCGTTGCCg | |
| D6-F | gatccTTCGCCCTGAAGCCAAGGGAGGAGTACtaac | aa584–592 |
| D6-R | tcgagtta GTACTCCTCCCTTGGCTTCAGGGCGAAg | |
| D6L-F | gatccCTGAAGCCAAGGGAGGAGTACtaac | aa586–592 |
| D6L-R | tcgagttaGTACTCCTCCCTTGGCTTCAGg | |
| D6M-F | gatccGCCCTGAAGCCAAGGGAGGAGtaac | aa585–591 |
| D6M-R | tcgagttaCTCCTCCCTTGGCTTCAGGGCg | |
| D6R-F | gatccTTCGCCCTGAAGCCAAGGGAGtaac | aa584–590 |
| D6R-R | tcgagttaCTCCCTTGGCTTCAGGGCGAAg |
| Fragements | Amino Acid Sequence |
|---|---|
| A1 | 421PEIHNLFVKRVRFSLIRVHKTQVTHTNNNHHDEKLMSALKWPIEYMFIGLKPTWNISDQNPHQ HRDWHKFGHVVNAIMQPTHHAEISFQDRDTALPDACSSISDISPVTYPITLPIIKNISVTAHGINLID KFPSKFCSSYIPFHYG567 |
| A2 | 491GHVVNAIMQPTHHAEISFQDRDTALPDACSSISDISPVTYPITLPIIKNISVTAHGINLIDKFPSKFC SSYIPFHYGGNAIKTPDDPGAMMITFALKPREEYQPSGHINVSRAREFYISWDTDYVGSITTADLVV SASAINFLLLQNGSAVLRYST646 |
| B1 | 491GHVVNAIMQPTHHAEISFQDRDTALPDACSSISDISPVTYPITLPIIKNISVTA544 |
| B2 | 514ALPDACSSISDISPVTYPITLPIIKNISVTAHGINLIDKFPSKFCSSYIPFHYG567 |
| B3 | 568GNAIKTPDDPGAMMITFALKPREEYQPSGHINVSRAREFYISWDTDYVGSITTAD622 |
| B4 | 595SGHINVSRAREFYISWDTDYVGSITTADLVVSASAINFLLLQNGSAVLRYST646 |
| B2–1 | 514ALPDACSSISDISPVTYPITLPIIKNISVTAHG546 |
| B2–2 | 535PIIKNISVTAHGINLIDKFPSKFCSSYIPFHYG567 |
| C1 | 545HGINLIDKFPSK556 |
| C2 | 548NLIDKFPSKFC558 |
| C3 | 551DKFPSKFCSS560 |
| C4 | 555SKFCSSYIPF564 |
| C5 | 557FCSSYIPFHYG567 |
| C6 | 537IKNISVTAHGINLIDK552 |
| C7 | 539NISVTAHGINLI550 |
| C8 | 568GNAIKTPDDPGAM580 |
| C9 | 575DDPGAMMITFAL586 |
| C10 | 581MITFALKPREEYQP594 |
| D1 | 539NISVTAHGINLIDKFPSKFCSSYI562 |
| D2 | 539NISVTAHGINLIDKFPSK556 |
| D3 | 545HGINLIDKFPSKFCSSYI562 |
| D4 | 542VTAHGINLIDKF553 |
| D4L | 543TAHGINLIDKF553 |
| D4R | 542VTAHGINLIDK552 |
| D5 | 568GNAIKTP574 |
| D6 | 584FALKPREEY592 |
| D6L | 586LKPREEY592 |
| D6M | 585ALKPREE591 |
| D6R | 584FALKPRE590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wang, A.; Yan, W.; Li, J.; Meng, X.; Chen, L.; Li, S.; Tong, W.; Kong, N.; Yu, L.; et al. Identification of Linear Epitopes in the C-Terminal Region of ASFV p72 Protein. Microorganisms 2023, 11, 2846. https://doi.org/10.3390/microorganisms11122846
Hu Y, Wang A, Yan W, Li J, Meng X, Chen L, Li S, Tong W, Kong N, Yu L, et al. Identification of Linear Epitopes in the C-Terminal Region of ASFV p72 Protein. Microorganisms. 2023; 11(12):2846. https://doi.org/10.3390/microorganisms11122846
Chicago/Turabian StyleHu, Yifan, Anchen Wang, Wanwan Yan, Junbo Li, Xin Meng, Lingchao Chen, Songnan Li, Wu Tong, Ning Kong, Lingxue Yu, and et al. 2023. "Identification of Linear Epitopes in the C-Terminal Region of ASFV p72 Protein" Microorganisms 11, no. 12: 2846. https://doi.org/10.3390/microorganisms11122846






