From Traditional Dairy Product “Katak” to Beneficial Lactiplantibacillus plantarum Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Home-Made Samples of “Katak” and LAB Microbiota Characterization
2.2. Molecular Identification of Newly Isolated Lactic Acid Bacteria
2.3. Antibacterial and Antibiofilm Activities
2.4. Test for the Evaluation of the Swimming Motility of Pseudomonas aeruginosa PAO1
sample/Diameter of control sample) × 100
2.5. In Vitro Evaluation of Anti-QS Activity of CFS from LAB by Agar Diffusion Assay
2.6. Gas-Chromatographic Analyses of Produced Volatile Fatty Acids (VFAs) in Growth Media
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Autochthonous Microbiota of “Katak”
3.2. Newly Isolated Lactobacilli from “Katak” as Active Antagonists of Pathogens
3.2.1. Anti-Microbial Activity of L. plantarum Strains from “Katak” against E. coli
3.2.2. Initial Characterization of Metabolites Produced during the Fermentation in MRS Broth
3.2.3. Antagonistic Activity of L. plantarum Strains against Staphylococcus aureus
3.2.4. Can Postbiotics Produced from L. plantarum Strains Inhibit Virulence Factors and Quorum-Sensing Controlled Pathogenesis?
- In vitro effects of LABs on the growth, biofilms, and motility of Pseudomonas aeruginosa PAO1.
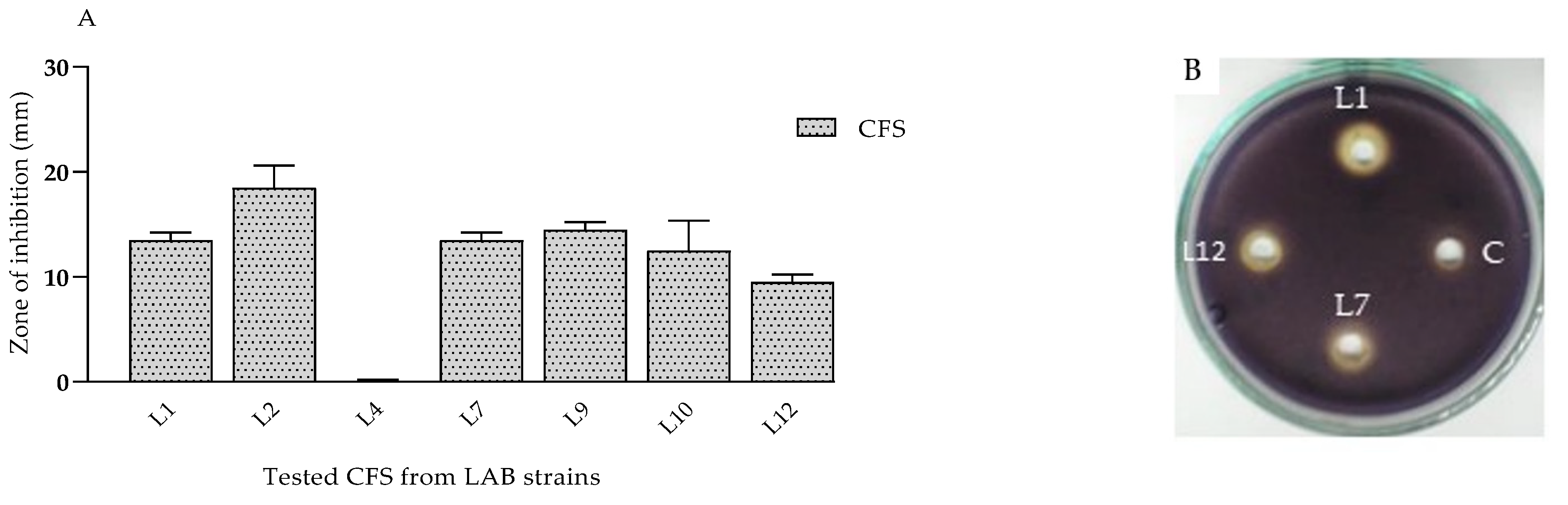
- Anti-quorum-sensing (QS) effects of CFSs from lactobacilli on violacein synthesis.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolova, D.; Hoxha, R.; Atanasov, N.; Trifonova, E.; Dobreva, L.; Nemska, V.; Evstatieva, Y.; Danova, S. Dairy Processing—From Basics to Advances; Salam, I., Ed.; IntechOpen: Rijeka, Croatia, 2022; pp. 14–155. [Google Scholar]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and cons for using non-starter lactic acid bacteria (nslab) as secondary/adjunct starters for cheese ripening. Trends Food Sci. Technol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Ramos, C.L.; Schwan, R.F. Technological and nutritional aspects of indigenous Latin America fermented foods. Curr. Opin. Food Sci. 2017, 13, 97–102. [Google Scholar] [CrossRef]
- Lortal, S.; Licitra, G.; Valence, F. Wooden tools: Reservoirs of microbial biodiversity in traditional cheesemaking. Microbiol. Spectr. 2014, 2, CM-0008-2012. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L. In vitro selection of probiotic lactobacilli: A critical appraisal. Curr. Issues Intest. Microbiol. 2000, 1, 59–67. [Google Scholar] [PubMed]
- Gavrilova, E.; Kostenko, V.; Zadorina, I.; Khusnutdinova, D.; Yarullina, D.; Ezhkova, A.; Bogachev, M.; Kayumov, A.; Nikitina, E. Repression of Staphylococcus aureus and Escherichia coli by Lactiplantibacillus plantarum strain ag10 in Drosophila melanogaster in vivo model. Microorganisms 2023, 11, 1297. [Google Scholar] [CrossRef] [PubMed]
- Danova, S.; Nemska, V.; Tropcheva, R. Bulgarian yogurt-like product “katak”. In Yogurt in Health and Disease Prevention, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 307–329. [Google Scholar]
- Tropcheva, R.; Nikolova, D.; Evstatieva, Y.; Danova, S. Antifungal activity and identification of lactobacilli, isolated from traditional dairy product “katak”. Anaerobe 2014, 28, 78–84. [Google Scholar] [CrossRef]
- Nemska, V.; Lazarova, N.; Georgieva, N.; Danova, S. Lactobacillus spp. from traditional bulgarian dairy products. J. Chem. Technol. Metall. 2016, 51, 693–704. [Google Scholar]
- Beijerinck, M.W. Uber oligonitrophile mikroben. Hyg. Abt. II 1901, 7, 561–582. [Google Scholar]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Dobreva, L. Functional Characteristic of Lactic Acid Bacteria from Different Habitats. Ph.D. Dissertation, The Stephan Angeloff Institute of Microbiology, Sofia, Bulgaria, 2023. [Google Scholar]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, R.N.; Iliev, I.N.; Chipeva, V.A.; Dimitonova, S.P.; Samelis, J.; Danova, S.T. Identification and in vitro characterisation of Lactobacillus plantarum strains from artisanal Bulgarian white brined cheeses. J. Basic. Microbiol. 2008, 48, 234–244. [Google Scholar] [CrossRef]
- Tagg, J.R.; McGiven, A.R. Assay system for bacteriocins. Appl. Microbiol. 1971, 21, 943. [Google Scholar] [CrossRef] [PubMed]
- De Soyza, A.; Hall, A.J.; Mahenthiralingam, E.; Drevinek, P.; Kaca, W.; Drulis-Kawa, Z.; Stoitsova, S.R.; Toth, V.; Coenye, T.; Zlosnik, J.E.; et al. Developing an international Pseudomonas aeruginosa reference panel. Microbiologyopen 2013, 2, 1010–1023. [Google Scholar] [CrossRef]
- Soto, S.M.; Smithson, A.; Horcajada, J.P.; Martinez, J.A.; Mensa, J.P.; Vila, J. Implication of biofilm formation in the persistence of urinary tract infection caused by uropathogenic Escherichia coli. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2006, 12, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Nacef, M.; Chevalier, M.; Chollet, S.; Drider, D.; Flahaut, C. Maldi-tof mass spectrometry for the identification of lactic acid bacteria isolated from a french cheese: The maroilles. Int. J. Food Microbiol. 2017, 247, 2–8. [Google Scholar] [CrossRef]
- Jussiaux, F.; Miot-Sertier, C.; Nguyen-Lopez, D.; Badet, C.; Samot, J. Reliability of maldi-tof mass spectrometry to identify oral isolates of Streptococcus salivarius and Lactobacillus spp. Arch. Oral. Biol. 2021, 121, 104983. [Google Scholar] [CrossRef] [PubMed]
- Petkova, M.; Gotcheva, V.; Dimova, M.; Bartkiene, E.; Rocha, J.M.; Angelov, A. Screening of Lactiplantibacillus plantarum strains from sourdoughs for biosuppression of Pseudomonas syringae pv. Syringae and Botrytis cinerea in table grapes. Microorganisms 2022, 10, 2094. [Google Scholar] [CrossRef]
- Danova, S. Biodiversity and Probiotic Potential of Lactic Acid Bacteria from Different Ecological Niches. Ph.D. Thesis, The Stephan Angeloff Institute of Microbiology, Sofia, Bulgaria, 2015. [Google Scholar]
- Tropcheva, R.; Hristova, J.; Georgieva, R.; Salageanu, A.; Sgouras, D.; Danova, S. In vitro assessment of prebiotic utilization by dairy lactobacilli. Bulg. J. Agric. Sci. 2013, 19, 105–107. [Google Scholar]
- Corsetti, A.; Gobbetti, M. Lactobacillus spp.|Lactobacillus plantarum. In Encyclopedia of Dairy Sciences; Roginski, H., Ed.; Elsevier: Oxford, UK, 2002; pp. 1501–1507. [Google Scholar]
- Sulmiyati; Said, N.; Fahrodi, D.U.; Malaka, R.; Maruddin, F. Assessment of the antibacterial activity of goat milk kefir on Escherichia coli ATTC 8739 and Salmonella enteric subsp. Enterica serovar Typhimurium ATTC 14028 using a well diffusion method. IOP Conf. Ser. Earth Environ. Sci. 2019, 247, 012051. [Google Scholar] [CrossRef]
- Lindgren, S.E.; Dobrogosz, W.J. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol. Rev. 1990, 7, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Vasiee, A.R.; Yazdi, F.; Mortazavi, A.; Edalatian Dovom, M.R. Isolation, identification and characterization of probiotic lactobacilli spp. From tarkhineh. Int. Food Res. J. 2014, 21, 2487–2492. [Google Scholar]
- Minervini, F.; Missaoui, J.; Celano, G.; Calasso, M.; Achour, L.; Saidane, D.; Gobbetti, M.; De Angelis, M. Use of autochthonous lactobacilli to increase the safety of zgougou. Microorganisms 2019, 8, 29. [Google Scholar] [CrossRef]
- Dinev, T.; Beev, G.; Tzanova, M.; Denev, S.; Dermendzhieva, D.; Stoyanova, A. Antimicrobial activity of Lactobacillus plantarum against pathogenic and food spoilage microorganisms: A review. Bulg. J. Vet. Med. 2018, 21, 253–268. [Google Scholar] [CrossRef]
- Lanciotti, R.; Patrignani, F.; Bagnolini, F.; Guerzoni, M.E.; Gardini, F. Evaluation of diacetyl antimicrobial activity against Escherichia coli, Listeria monocytogenes and Staphylococcus aureus. Food Microbiol. 2003, 20, 537–543. [Google Scholar] [CrossRef]
- Haller, D.; Colbus, H.; Gänzle, M.G.; Scherenbacher, P.; Bode, C.; Hammes, W.P. Metabolic and functional properties of lactic acid bacteria in the gastro-intestinal ecosystem: A comparative in vitro study between bacteria of intestinal and fermented food origin. Syst. Appl. Microbiol. 2001, 24, 218–226. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D.; Łepecka, A.; Długosz, E.; Kołożyn-Krajewska, D. Lactobacillus plantarum strains isolated from polish regional cheeses exhibit anti-staphylococcal activity and selected probiotic properties. Probiotics Antimicrob. Proteins 2020, 12, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Setyawardani, T.; Rahayu, W.; Maheswari, R.R.; Palupi, N. Antimicrobial activity and adhesion ability of indogenous lactic acid bacteria isolated from goat milk. Int. Food Res. J. 2014, 21, 959–964. [Google Scholar]
- Setyawardani, T.; Sumarmono, J. Isolation and antimicrobial activities of lactic acid bacteria originated from Indonesian local goat’s colostrum. Anim. Prod. 2018, 20, 173–181. [Google Scholar] [CrossRef]
- Makete, G.; Aiyegoro, O.A.; Thantsha, M.S. Isolation, identification and screening of potential probiotic bacteria in milk from south african saanen goats. Probiotics Antimicrob. Proteins 2017, 9, 246–254. [Google Scholar] [CrossRef]
- Milioni, C.; Martínez, B.; Degl’Innocenti, S.; Turchi, B.; Fratini, F.; Cerri, D.; Fischetti, R. A novel bacteriocin produced by Lactobacillus plantarum lpu4 as a valuable candidate for biopreservation in artisanal raw milk cheese. Dairy. Sci. Technol. 2015, 95, 479–494. [Google Scholar] [CrossRef]
- Shokri, D.; Khorasgani, M.R.; Mohkam, M.; Fatemi, S.M.; Ghasemi, Y.; Taheri-Kafrani, A. The inhibition effect of lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2018, 10, 34–42. [Google Scholar] [CrossRef]
- Fangous, M.S.; Gosset, P.; Galakhoff, N.; Gouriou, S.; Guilloux, C.A.; Payan, C.; Vallet, S.; Hery-Arnaud, G.; Le Berre, R. Priming with intranasal lactobacilli prevents Pseudomonas aeruginosa acute pneumonia in mice. BMC Microbiol. 2021, 21, 195. [Google Scholar] [CrossRef]
- O’May, C.; Tufenkji, N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 2011, 77, 3061–3067. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Shukla, V.; Bhathena, Z. Broad spectrum anti-quorum sensing activity of tannin-rich crude extracts of Indian medicinal plants. Scientifica 2016, 2016, 5823013. [Google Scholar] [CrossRef]
- Dimitrova, P.D.; Damyanova, T.; Paunova-Krasteva, T. Chromobacterium violaceum: Model for evaluating anti-quorum sensing activity of plant substances. Sci. Pharm. 2023, 91, 33. [Google Scholar] [CrossRef]
- Díaz, M.A.; González, S.N.; Alberto, M.R.; Arena, M.E. Human probiotic bacteria attenuate Pseudomonas aeruginosa biofilm and virulence by quorum-sensing inhibition. Biofouling 2020, 36, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Siddiqui, A.J.; Ashraf, S.A.; Surti, M.; Awadelkareem, A.M.; Snoussi, M.; Hamadou, W.S.; Bardakci, F.; Jamal, A.; Jahan, S.; et al. Lactiplantibacillus plantarum-derived biosurfactant attenuates quorum sensing-mediated virulence and biofilm formation in Pseudomonas aeruginosa and Chromobacterium violaceum. Microorganisms 2022, 10, 1026. [Google Scholar] [CrossRef]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Reddy, D.; Mohanty, S.K.; Kumavath, R.; Murali, A.; Suchiang, K.; Busi, S. Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PAO1: Insights from in vitro, in vivo and in silico studies. Microbiol. Res. 2019, 226, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Alfano, A.; Donnarumma, G.; Cimini, D.; Fusco, A.; Marzaioli, I.; De Rosa, M.; Schiraldi, C. Lactobacillus plantarum: Microfiltration experiments for the production of probiotic biomass to be used in food and nutraceutical preparations. Biotechnol. Prog. 2015, 31, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Alfano, A.; Perillo, F.; Fusco, A.; Savio, V.; Corsaro, M.M.; Donnarumma, G.; Schiraldi, C.; Cimini, D. Lactobacillus brevis CD2: Fermentation Strategies and Extracellular Metabolites Characterization. Probiotics Antimicrob. Proteins 2020, 12, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
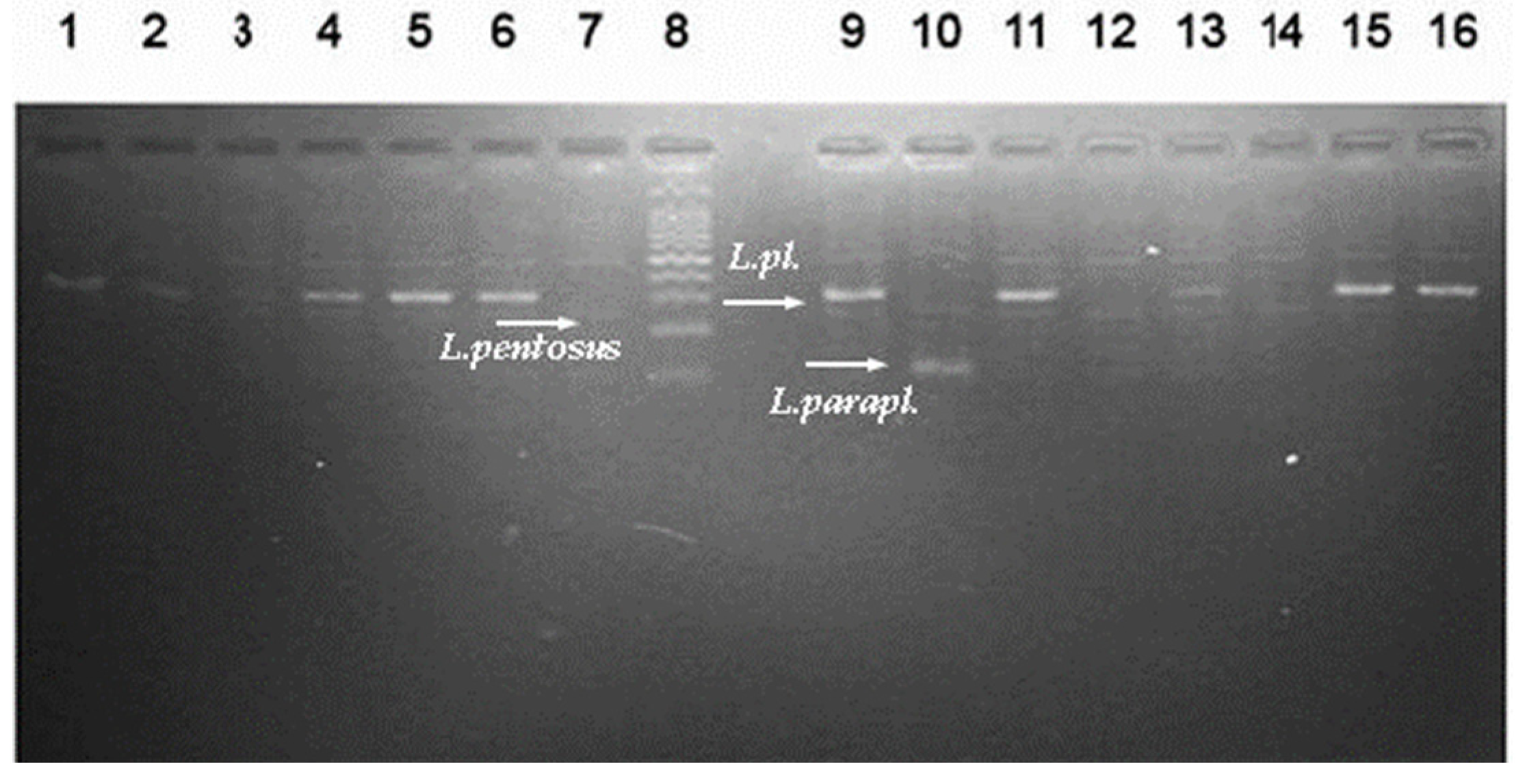
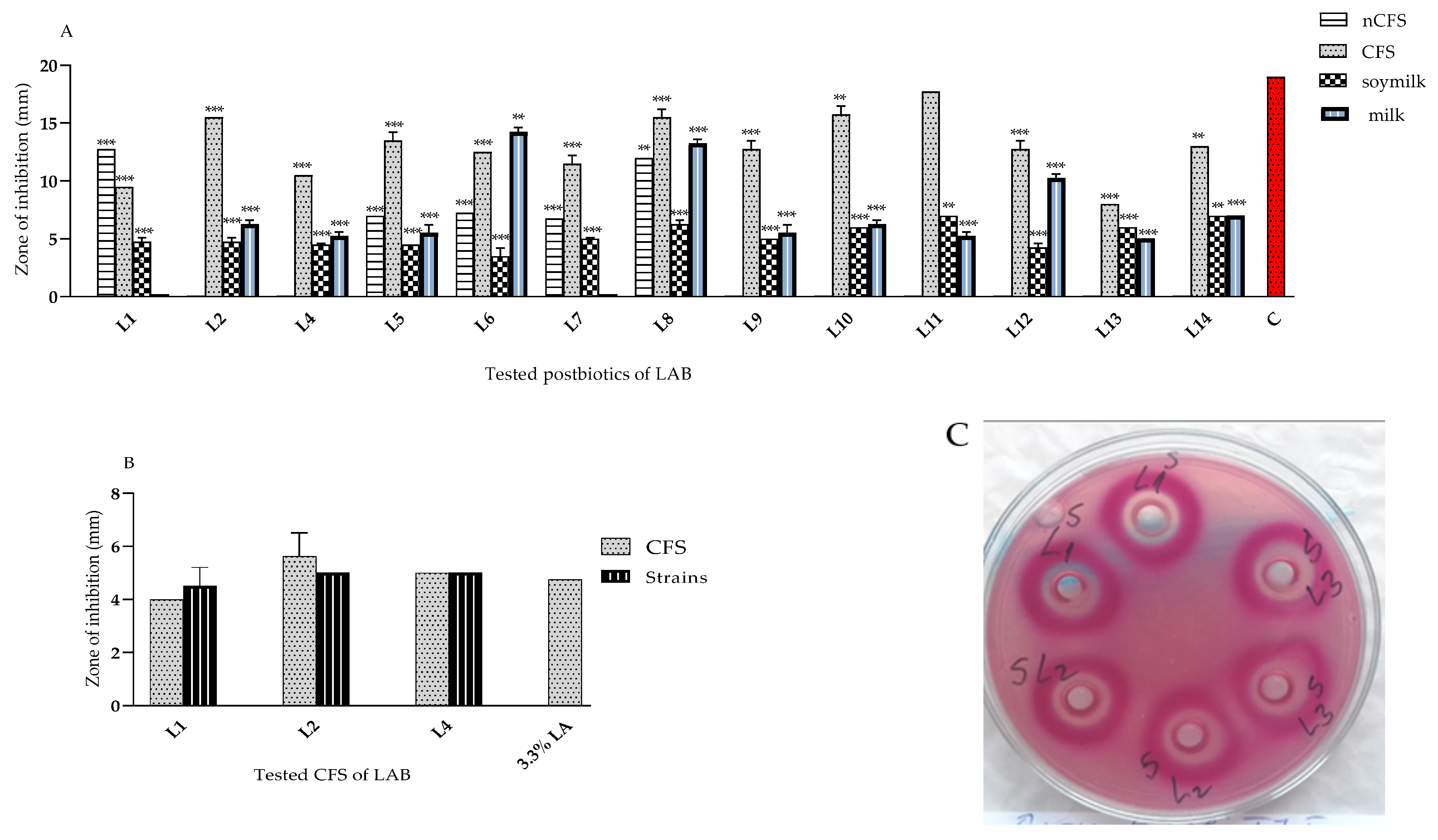
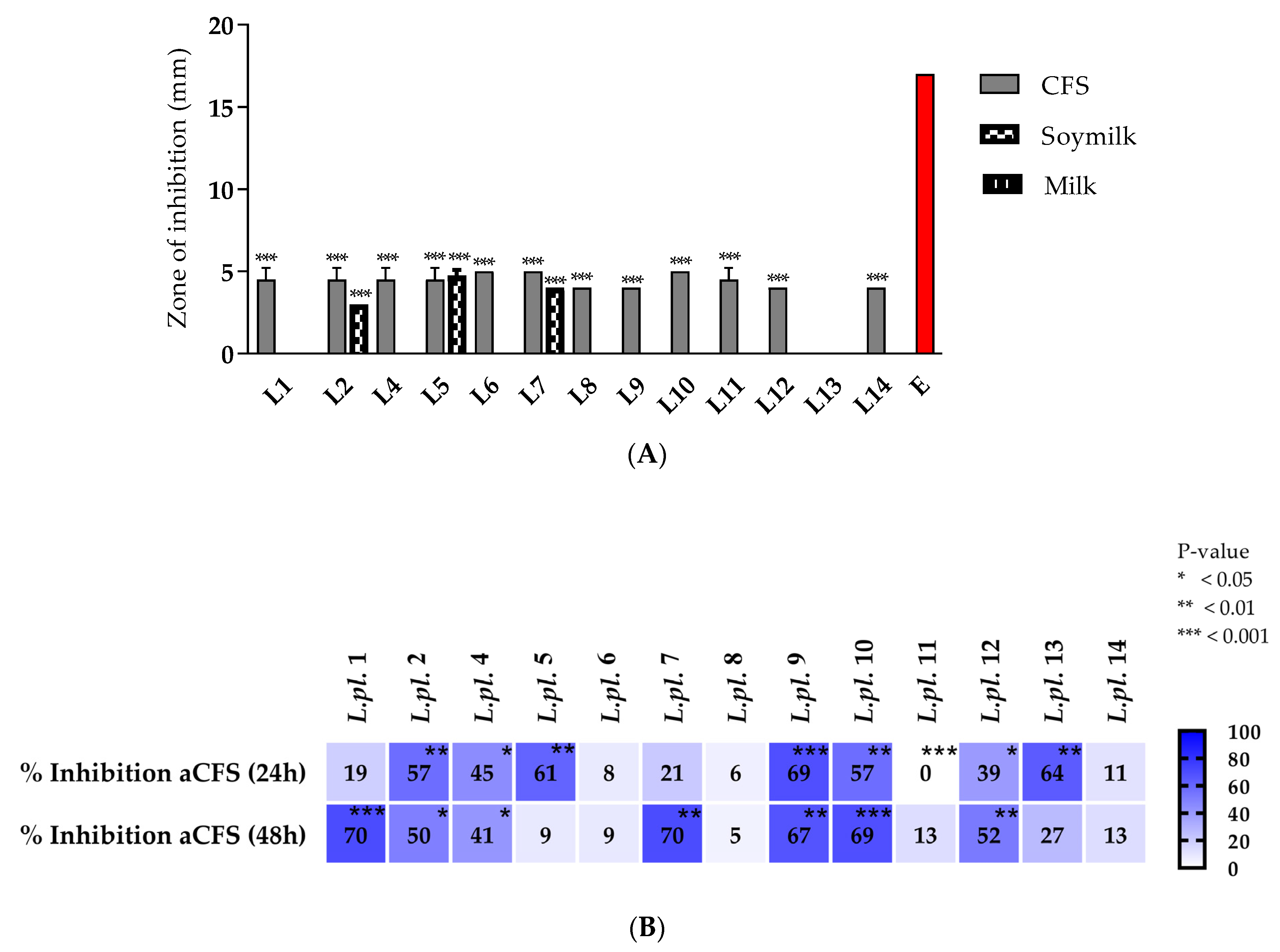
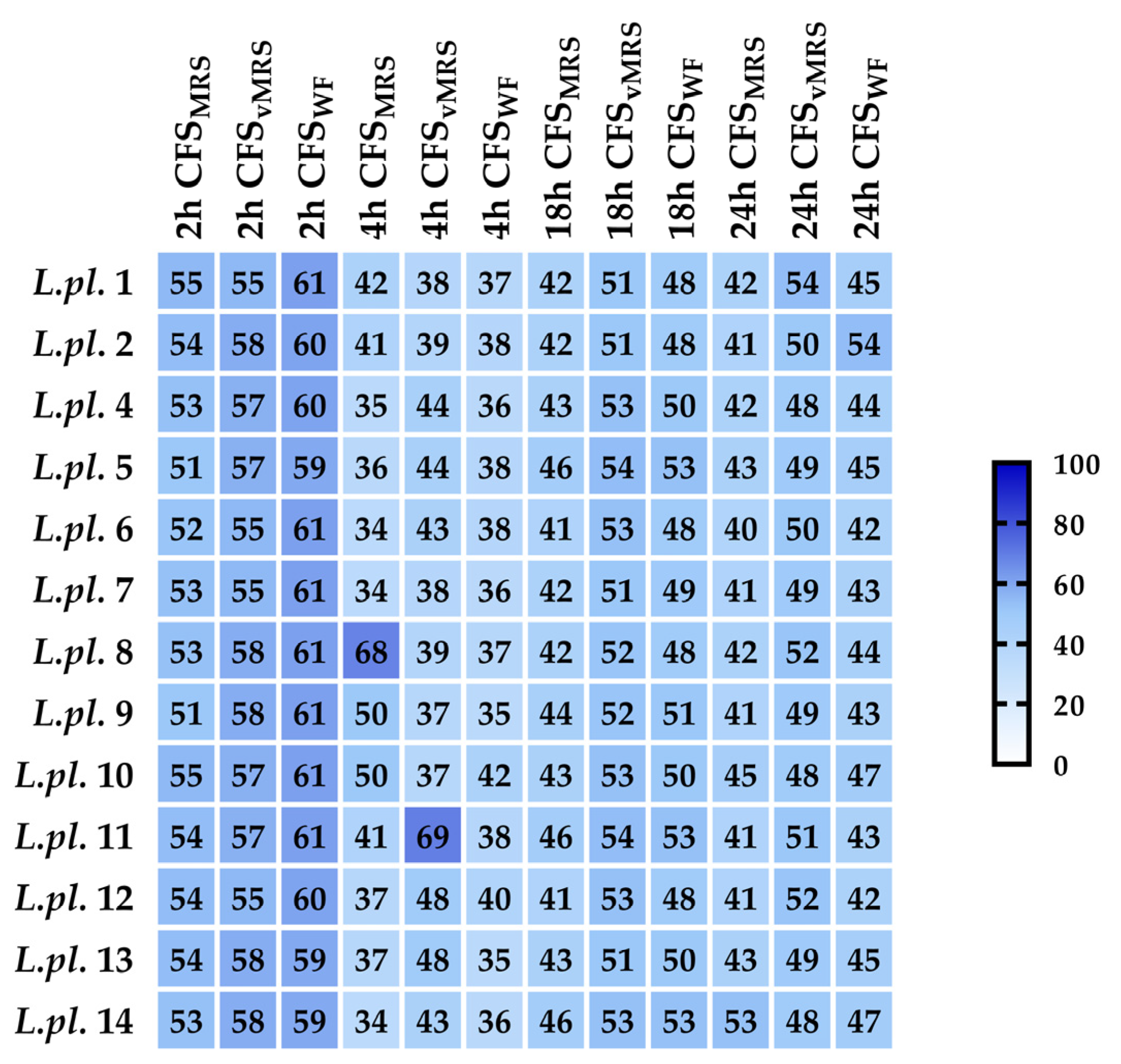


| No. | Test Microorganisms | Cultural Media |
|---|---|---|
| 1 | Staphylococcus aureus V00037 | BHI broth (Difco) and BHI agar |
| 2 | Escherichia coli HB101 (IMicB collection) | BHI broth (Difco) and BHI agar, MacConkey (Sigma, Germany) |
| 3 | Escherichia coli clinical strain TU5 | BHI broth (Difco) and BHI agar |
| 4 | Escherichia coli 420 | BHI broth (Difco) and BHI agar |
| 5 | Pseudomonas aeruginosa PAO1 International Reference Panel (De Soyza et al. 2013) [18] | Tryptic soy broth/agar (HiMedia) M63 (for biofilm assays) |
| 6 | Chromobacterium violaceum strain DSMZ 30191 | Tryptic soy broth/agar (HiMedia and Oxoid) |
| Strain | Molecular Identification of Isolates | MALDI-TOF MS Analysis | |||
|---|---|---|---|---|---|
| BLAST-16S rDNA Sequence—Organism (Best Match) | Similarity (%) | Species/NCBI Accession No | Organism (Best Match) | Score Value | |
| L8 | L. pentosus 124-2 | 99.79 | L. plantarum/ OR528606 | L. plantarum | 2.14 |
| L. plantarum CIP 103151; | 99.79 | ||||
| L9 | L. plantarum JCM 1149 | 100 | L. plantarum/ OR528607 | L. plantarum | 2.00 |
| L. pentosus 124-2 | 100 | ||||
| L. paraplantarum DSM 10667 | 100 | ||||
| L10 | L. plantarum JCM 1149; | 100 | L. plantarum/ OR528608 | L. plantarum | 2.07 |
| L. pentosus 124-2 | 100 | ||||
| L13 | L. plantarum JCM 1149 | 100 | L. plantarum/ OR528609 | L. plantarum | 2.16 |
| L. pentosus 124-2 | 100 | ||||
| L. paraplantarum DSM 10667 | 100 | ||||
| CFS from LAB | VFA Component, g/L | Total VFAs, g/L | ||||||
|---|---|---|---|---|---|---|---|---|
| Acetate | Propionate | i-Butyrate | Butyrate | i-Valerate | Valerate | Caproate | ||
| L. plantarum L1 | 8.23 | 0.11 | 0.00 | 0.07 | 0.06 | 0.10 | 0.07 | 8.64 |
| L. plantarum L2 | 8.65 | 0.13 | 0.00 | 0.07 | 0.06 | 0.18 | 0.06 | 9.15 |
| L. plantarum L4 | 9.03 | 0.22 | 0.00 | 0.00 | 0.07 | 0.11 | 0.09 | 9.52 |
| L. plantarum L5 | 16.97 | 0.59 | 0.00 | 0,10 | 0.34 | 0.22 | 0.18 | 18.40 |
| L. plantarum L5 * | 10.75 | 0.29 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 11.15 |
| L. plantarum L6 | 8.70 | 0.24 | 0.00 | 0.00 | 0.07 | 0.10 | 0.00 | 9.11 |
| L. plantarum L7 | 8.90 | 0.18 | 0.00 | 0.06 | 0.06 | 0.28 | 0.00 | 9.26 |
| L. plantarum L8 | 9.50 | 0.11 | 0.00 | 0.06 | 0.06 | 0,00 | 0.06 | 9.79 |
| C-MRS | 7.07 | 0.10 | 0.00 | 0.09 | 0.08 | 0.09 | 0.00 | 7.44 |
| C-Hiveg MRS * | 4.45 | 0.10 | 0.00 | 0.00 | 0.08 | 0.00 | 0.15 | 4.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobreva, L.; Borisova, D.; Paunova-Krasteva, T.; Dimitrova, P.D.; Hubenov, V.; Atanasova, N.; Ivanov, I.; Danova, S. From Traditional Dairy Product “Katak” to Beneficial Lactiplantibacillus plantarum Strains. Microorganisms 2023, 11, 2847. https://doi.org/10.3390/microorganisms11122847
Dobreva L, Borisova D, Paunova-Krasteva T, Dimitrova PD, Hubenov V, Atanasova N, Ivanov I, Danova S. From Traditional Dairy Product “Katak” to Beneficial Lactiplantibacillus plantarum Strains. Microorganisms. 2023; 11(12):2847. https://doi.org/10.3390/microorganisms11122847
Chicago/Turabian StyleDobreva, Lili, Dayana Borisova, Tsvetelina Paunova-Krasteva, Petya D. Dimitrova, Venelin Hubenov, Nikoleta Atanasova, Ivan Ivanov, and Svetla Danova. 2023. "From Traditional Dairy Product “Katak” to Beneficial Lactiplantibacillus plantarum Strains" Microorganisms 11, no. 12: 2847. https://doi.org/10.3390/microorganisms11122847






