Effect of Zinc Oxide and Copper Sulfate on Antibiotic Resistance Plasmid Transfer in Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
2.2. Heavy Metals and Antimicrobial Susceptibility Testing
2.3. Mating-Out Assays
2.4. Fluorescent Reactive Oxygen Species (ROS) Detection
2.5. mRNA Extraction and cDNA Synthesis
2.6. RT-qPCR
2.7. Statistical Analyses
3. Results
3.1. Increased PCF in Presence of Low Concentrations of Heavy Metals
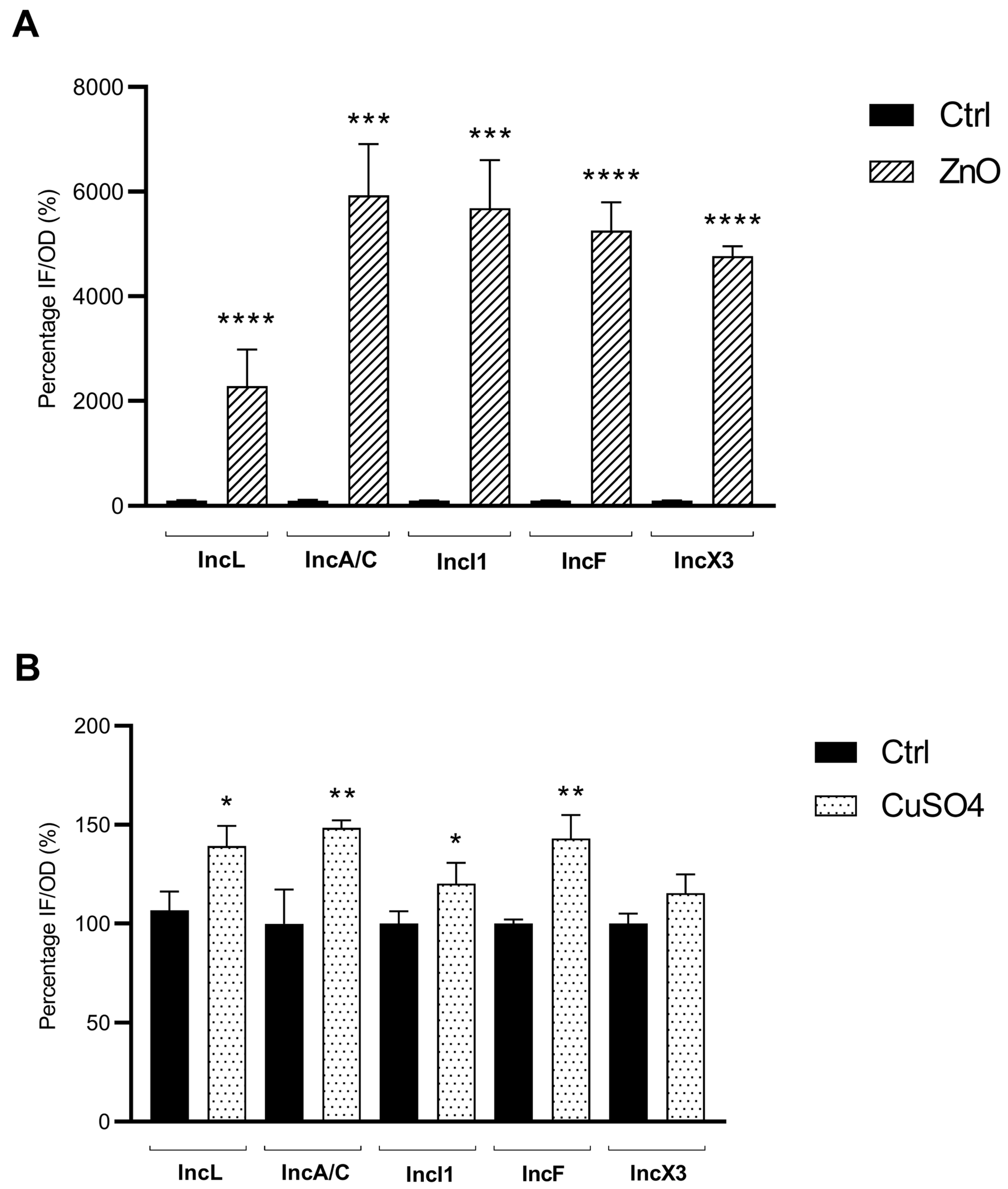
3.2. Sub-Inhibitory Concentrations of ZnO and CuSO4 Enhance Oxidative Stress Response in E. coli
3.3. Heavy Metals Sub-Lethal Dosage Treatment Does Not Alter SOS-Responsive Gene Expression for the Majority of the E. coli Strains
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 2 June 2023).
- World Organization for Animal Health (WOAH). The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials; World Organization for Animal Health: Paris, France, 2016; Available online: http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf%0Ahttp://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf%0Awww.oie.int/antimicrobial-resistance%0Ahttps://www.oie.int/file (accessed on 2 June 2023).
- Zhang, S.; Wang, Y.; Song, H.; Lu, J.; Yuan, Z.; Guo, J. Copper nanoparticles and copper ions promote horizontal transfer of plasmid-mediated multi-antibiotic resistance genes across bacterial genera. Environ. Int. 2019, 129, 478–487. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production; FAO: Rome, Italy, 2016; Available online: https://www.fao.org/3/i6209e/i6209e.pdf (accessed on 4 June 2023).
- Fraise, A.P. Biocide abuse and antimicrobial resistance—A cause for concern? J. Antimicrob. Chemother. 2002, 49, 11–12. [Google Scholar] [CrossRef]
- Qiu, Z.; Yu, Y.; Chen, Z.; Jin, M.; Yang, D.; Zhao, Z.; Wang, J.; Shen, Z.; Wang, X.; Qian, D.; et al. Nanoalumina promotes the horizontal transfer of multiresistance genes mediated by plasmids across genera. Proc. Natl. Acad. Sci. USA 2012, 109, 4944–4949. [Google Scholar] [CrossRef]
- Alonso, A.; Sanchez, P.; Martinez, J.L. Environmental selection of antibiotic resistance genes. Minireview. Environ. Microbiol. 2001, 3, 1–9. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Regional Office for Europe/European Centre for Disease Prevention and Control. In Antimicrobial Resistance Surveillance in Europe 2022—2020 Data; WHO: Copenhagen, Denmark, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-AMR-report-2022.pdf (accessed on 4 June 2023).
- Hayer, S.S.; Casanova-Higes, A.; Paladino, E.; Elnekave, E.; Nault, A.; Johnson, T.; Bender, J.; Perez, A.; Alvarez, J. Global Distribution of Extended Spectrum Cephalosporin and Carbapenem Resistance and Associated Resistance Markers in Escherichia coli of Swine Origin—A Systematic Review and Meta-Analysis. Front. Microbiol. 2022, 13, 853810. [Google Scholar] [CrossRef]
- Fischer, J.; Rodríguez, I.; Schmoger, S.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Escherichia coli producing VIM-1 carbapenemase isolated on a pig farm. J. Antimicrob. Chemother. 2012, 67, 1793–1795. [Google Scholar] [CrossRef]
- Girlich, D.; Poirel, L.; Carattoli, A.; Kempf, I.; Lartigue, M.-F.; Bertini, A.; Nordmann, P. Extended-Spectrum β-Lactamase CTX-M-1 in Escherichia coli Isolates from Healthy Poultry in France. Appl. Environ. Microbiol. 2007, 73, 4681–4685. [Google Scholar] [CrossRef]
- Cabal, A.; Rab, G.; Daza-Prieto, B.; Stöger, A.; Peischl, N.; Chakeri, A.; Mo, S.S.; Bock, H.; Fuchs, K.; Sucher, J.; et al. Characterizing Antimicrobial Resistance in Clinically Relevant Bacteria Isolated at the Human/Animal/Environment Interface Using Whole-Genome Sequencing in Austria. Int. J. Mol. Sci. 2022, 23, 11276. [Google Scholar] [CrossRef]
- De Vries, W.; Römkens, P.F.A.M.; Kros, J.; Voogd, J.C.; Schulte-Uebbing, L.F. Impacts of nutrients and heavy metals in European agriculture. In Current and Critical Inputs in Relation to Air, Soil and Water Quality; European Environment Agency: Vienna, Austria, 2022; Available online: https://www.eionet.europa.eu/etcs/etc-di/products/impacts-of-nutrients-and-heavy-metals-in-european-agriculture-current-and-critical-inputs-in-relation-to-air-soil-and-water-quality/@@download/file/D22%201821%20M1%20and%20M2%20Nutrients%20and%20heavy%20metals%20in%20soils%2001032022%20ETC-DI_30March.pdf (accessed on 10 June 2023).
- European Medicines Agency (EMA). Questions and Answers on Veterinary Medicinal Products Containing Zinc Oxide to be Administered Orally to Food-Producing Species; European Medicines Agency (EMA): London, UK, 2017; Available online: https://www.ema.europa.eu/en/documents/referral/zinc-oxide-article-35-referral-questions-answers-veterinary-medicinal-products-containing-zinc-oxide_en.pdf (accessed on 12 June 2023).
- Azizi-Lalabadi, M.; Rafiei, L.; Divband, B.; Ehsani, A. Active packaging for Salmon stored at refrigerator with Polypropylene nanocomposites containing 4A zeolite, ZnO nanoparticles, and green tea extract. Food Sci. Nutr. 2020, 8, 6445–6456. [Google Scholar] [CrossRef]
- Hill, G.M.; Mahan, D.C.; Carter, S.D.; Cromwell, G.L.; Ewan, R.C.; Harrold, R.L.; Lewis, A.J.; Miller, P.S.; Shurson, G.C.; Veum, T.L. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J. Anim. Sci. 2001, 79, 934–941. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). News and Events. In Committee for Medicinal Products for Veterinary Use (CVMP) Meeting; European Medicines Agency (EMA): London, UK, 2016; Available online: https://www.ema.europa.eu/en/documents/press-release/committee-medicinal-products-veterinary-use-cvmp-meeting-6-8-december-2016_en.pdf (accessed on 2 June 2023).
- European Medicines Evaluation Agency (EMEA)—Veterinary Medicines Evaluation Unit. Copper Chloride, Copper Gluconate, Copper Heptanoate, Copper Oxide, Copper Methionate, Copper Sulphate and Dicopper Oxide—Summary Report; European Medicines Evaluation Agency (EMEA): London, UK, 1998; Available online: https://www.ema.europa.eu/en/documents/mrl-report/copper-chloride-copper-gluconate-copper-heptanoate-copper-oxide-copper-methionate-copper-sulphate_en.pdf (accessed on 2 June 2023).
- Ma, J.; Zhou, W.; Wu, J.; Liu, X.; Lin, J.; Ji, X.; Lin, H.; Wang, J.; Jiang, H.; Zhou, Q.; et al. Large-Scale Studies on Antimicrobial Resistance and Molecular Characterization of Escherichia coli from Food Animals in Developed Areas of Eastern China. Microbiol. Spectr. 2022, 10, e0201522. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug administration (FDA)—Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001; pp. 162–196. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Larsen, J.; Andersen, V.D.; Lester, C.H.; Skytte, T.S.S.; Hansen, F.; Olsen, S.S.; Mordhorst, H.; Skov, R.L.; Aarestrup, F.M.; et al. Characterization of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli obtained from Danish pigs, pig farmers and their families from farms with high or no consumption of third- or fourth-generation cephalosporins. J. Antimicrob. Chemother. 2014, 69, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P. Antibiotic resistance: Are we all doomed? Intern. Med. J. 2015, 45, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A. Resistance Plasmid Families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, A.J.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Zhang, W.-H.; Ren, S.-Q.; Yang, L.; Lü, D.-H.; Zeng, Z.-L.; Liu, Y.-H.; Jiang, H.-X. IncA/C Plasmid-Mediated Spread of CMY-2 in Multidrug-Resistant Escherichia coli from Food Animals in China. PLoS ONE 2014, 9, e96738. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Fortini, D.; García-Fernández, A. Contemporary IncI1 plasmids involved in the transmission and spread of antimicrobial resistance in Enterobacteriaceae. Plasmid 2021, 118, 102392. [Google Scholar] [CrossRef]
- Randall, L.P.; Clouting, C.; Horton, R.A.; Coldham, N.G.; Wu, G.; Clifton-Hadley, F.A.; Davies, R.H.; Teale, C.J. Prevalence of Escherichia coli carrying extended-spectrum -lactamases (CTX-M and TEM-52) from broiler chickens and turkeys in Great Britain between 2006 and 2009. J. Antimicrob. Chemother. 2011, 66, 86–95. [Google Scholar] [CrossRef]
- Ho, P.-L.; Wang, Y.; Liu, M.C.-J.; Lai, E.L.-Y.; Law, P.Y.-T.; Cao, H.; Chow, K.-H. IncX3 Epidemic Plasmid Carrying bla NDM-5 in Escherichia coli from Swine in Multiple Geographic Areas in China. Antimicrob. Agents Chemother. 2018, 62, e02295-17. [Google Scholar] [CrossRef]
- Pulss, S.; Stolle, I.; Stamm, I.; Leidner, U.; Heydel, C.; Semmler, T.; Prenger-Berninghoff, E.; Ewers, C. Multispecies and Clonal Dissemination of OXA-48 Carbapenemase in Enterobacteriaceae from Companion Animals in Germany, 2009–2016. Front. Microbiol. 2018, 9, 1265. [Google Scholar] [CrossRef]
- Liu, X.; Thungrat, K.; Boothe, D.M. Occurrence of OXA-48 Carbapenemase and Other β-Lactamase Genes in ESBL-Producing Multidrug Resistant Escherichia coli from Dogs and Cats in the United States, 2009–2013. Front. Microbiol. 2016, 7, 1057. [Google Scholar] [CrossRef]
- Irrgang, A.; Pauly, N.; Tenhagen, B.-A.; Grobbel, M.; Kaesbohrer, A.; Hammerl, J.A. Spill-Over from Public Health? First Detection of an OXA-48-Producing Escherichia coli in a German Pig Farm. Microorganisms 2020, 8, 855. [Google Scholar] [CrossRef]
- Zhuang, Z.; Lv, L.; Lu, J.; Lin, J.; Liu, J.-H. Emergence of Klebsiella pneumoniae and Enterobacter cloacae producing OXA-48 carbapenemases from retail meats in China, 2018. J. Antimicrob. Chemother. 2019, 74, 3632–3634. [Google Scholar] [CrossRef]
- Muktan, B.; Shrestha, U.T.; Dhungel, B.; Mishra, B.C.; Shrestha, N.; Adhikari, N.; Banjara, M.R.; Adhikari, B.; Rijal, K.R.; Ghimire, P. Plasmid mediated colistin resistant mcr-1 and co-existence of OXA-48 among Escherichia coli from clinical and poultry isolates: First report from Nepal. Gut Pathog. 2020, 12, 44. [Google Scholar] [CrossRef]
- Shun-Mei, E.; Zeng, J.-M.; Yuan, H.; Lu, Y.; Cai, R.-X.; Chen, C. Sub-inhibitory concentrations of fluoroquinolones increase conjugation frequency. Microb. Pathog. 2018, 114, 57–62. [Google Scholar] [CrossRef]
- Liu, X.; Tang, J.; Song, B.; Zhen, M.; Wang, L.; Giesy, J.P. Exposure to Al2O3 nanoparticles facilitates conjugative transfer of antibiotic resistance genes from Escherichia coli to Streptomyces. Nanotoxicology 2019, 13, 1422–1436. [Google Scholar] [CrossRef]
- Pribis, J.P.; García-Villada, L.; Zhai, Y.; Lewin-Epstein, O.; Wang, A.Z.; Liu, J.; Xia, J.; Mei, Q.; Fitzgerald, D.M.; Bos, J.; et al. Gamblers: An Antibiotic-Induced Evolvable Cell Subpopulation Differentiated by Reactive-Oxygen-Induced General Stress Response. Mol. Cell 2019, 74, 785–800.e7. [Google Scholar] [CrossRef]
- Sutton, M.D.; Smith, B.T.; Godoy, V.G.; Walker, G.C. The SOS Response: Recent insights into umuDC-dependent mutagenesis and DNA damage tolerance. Annu. Rev. Genet. 2000, 34, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Singleton, S.F. Inhibition of the Escherichia coli RecA protein: Zinc(II), copper(II) and mercury(II) trap RecA as inactive aggregates. J. Inorg. Biochem. 2004, 98, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.E.; Escobar, J.F.; Bair, K.L.; Sutton, M.D.; Crane, J.K. Zinc blocks SOS-induced antibiotic resistance via inhibition of RecA in Escherichia coli. PLoS ONE 2017, 12, e0178303. [Google Scholar] [CrossRef]
- Palm, M.; Fransson, A.; Hultén, J.; Stenman, K.B.; Allouche, A.; Chiang, O.E.; Constandse, M.L.; van Dijk, K.J.; Icli, S.; Klimesova, B.; et al. The Effect of Heavy Metals on Conjugation Efficiency of an F-Plasmid in Escherichia coli. Antibiotics 2022, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Ekhlas, D.; Soro, A.B.; Leonard, F.C.; Manzanilla, E.G.; Burgess, C.M. Examining the impact of zinc on horizontal gene transfer in Enterobacterales. Sci. Rep. 2022, 12, 20503. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Poirel, L.; Nordmann, P. Derepressed Transfer Properties Leading to the Efficient Spread of the Plasmid Encoding Carbapenemase OXA-48. Antimicrob. Agents Chemother. 2014, 58, 467–471. [Google Scholar] [CrossRef]
- Fournier, C.; de Sousa, M.A.; Escriva, B.F.; Sales, L.; Nordmann, P.; Poirel, L. Epidemiology of extended-spectrum β-lactamase-producing Enterobacteriaceae among healthcare students, at the Portuguese Red Cross Health School of Lisbon, Portugal. J. Glob. Antimicrob. Resist. 2020, 22, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Sadek, M.; Yao, Y.; Imirzalioglu, C.; Stephan, R.; Poirel, L.; Nordmann, P. Cross-Border Emergence of Escherichia coli Producing the Carbapenemase NDM-5 in Switzerland and Germany. J. Clin. Microbiol. 2021, 59, e02238-20. [Google Scholar] [CrossRef]
- Yanisch-Perron, C.; Vieira, J.; Messing, J. Improved M13 phage cloning vectors and host strains: Nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene 1985, 33, 103–119. [Google Scholar] [CrossRef]
- Veterinary committee on Antimicrobial Susceptibility Testing (VetCAST) from, EUCAST. Available Breakpoints of Antimicrobials for Veterinary Use, 1st ed.; EUCAST: Copenhagen, Denmark, 2015. [Google Scholar]
- Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. In VET01S, 5th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020.
- de la Rosa, J.M.O.; Nordmann, P.; Poirel, L. Antioxidant Molecules as a Source of Mitigation of Antibiotic Resistance Gene Dissemination. Antimicrob. Agents Chemother. 2021, 65, e02658-20. [Google Scholar] [CrossRef]
- Castro-Alférez, M.; Polo-López, M.I.; Fernández-Ibáñez, P. Intracellular mechanisms of solar water disinfection. Sci. Rep. 2016, 6, 38145. [Google Scholar] [CrossRef]
- Møller, T.S.B.; Liu, G.; Boysen, A.; Thomsen, L.E.; Lüthje, F.L.; Mortensen, S.; Møller-Jensen, J.; Olsen, J.E. Treatment with Cefotaxime Affects Expression of Conjugation Associated Proteins and Conjugation Transfer Frequency of an IncI1 Plasmid in Escherichia coli. Front. Microbiol. 2017, 8, 2365. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45e–445. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.; Perreten, V.; Poirel, L.; Nordmann, P. Molecular analysis of OXA-48-producing Escherichia coli in Switzerland from 2019 to 2020. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.M.; Menezes, J.; Marques, C.; Pomba, C.F. Companion Animals—An Overlooked and Misdiagnosed Reservoir of Carbapenem Resistance. Antibiotics 2022, 11, 533. [Google Scholar] [CrossRef]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, S.V.; RajeshKumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc Oxide Protects Cultured Enterocytes from the Damage Induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Ou, J.T. Effect of Zn 2+ on Bacterial Conjugation: Increase in Ability of F − Cells to Form Mating Pairs. J. Bacteriol. 1973, 115, 648–654. [Google Scholar] [CrossRef]
- Peng, S.; Herrero-Fresno, A.; Olsen, J.E.; Dalsgaard, A. Influence of zinc on CTX-M-1 β-lactamase expression in Escherichia coli. J. Glob. Antimicrob. Resist. 2020, 22, 613–619. [Google Scholar] [CrossRef]
- Klümper, U.; Dechesne, A.; Riber, L.; Brandt, K.K.; Gülay, A.; Sørensen, S.J.; Smets, B.F. Metal stressors consistently modulate bacterial conjugal plasmid uptake potential in a phylogenetically conserved manner. ISME J. 2017, 11, 152–165. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.; Hou, Z.; Wang, L.; Ma, D.; Yang, G.; Guo, S.; Luo, J.; Qi, L.; Luo, Y. Heavy metal copper accelerates the conjugative transfer of antibiotic resistance genes in freshwater microcosms. Sci. Total Environ. 2020, 717, 137055. [Google Scholar] [CrossRef] [PubMed]
- Buberg, M.L.; Witsø, I.L.; L’Abée-Lund, T.M.; Wasteson, Y. Zinc and Copper Reduce Conjugative Transfer of Resistance Plasmids from Extended-Spectrum Beta-Lactamase-Producing Escherichia coli. Microb. Drug Resist. 2020, 26, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Gennaro, M.L. Plasmids. In Encyclopedia of Genetics; Elsevier: Amsterdam, The Netherlands; Academic Press: New York, NY, USA, 2001; pp. 1485–1490. ISBN 9780122270802. [Google Scholar] [CrossRef]
- Giese, K.C.; Michalowski, C.B.; Little, J.W. RecA-Dependent Cleavage of LexA Dimers. J. Mol. Biol. 2008, 377, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Katayama, T. The Escherichia coli Cryptic Prophage Protein YfdR Binds to DnaA and Initiation of Chromosomal Replication Is Inhibited by Overexpression of the Gene Cluster yfdQ-yfdR-yfdS-yfdT. Front. Microbiol. 2016, 7, 239. [Google Scholar] [CrossRef]
- Baharoglu, Z.; Bikard, D.; Mazel, D. Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation. PLOS Genet. 2010, 6, e1001165. [Google Scholar] [CrossRef]
- Bagdasarian, M.; Bailone, A.; Angulo, J.F.; Scholz, P.; Bagdasarian, M.; Devoret, R. PsiB, an anti-SOS protein, is transiently expressed by the F sex factor during its transmission to an Escherichia coli K-12 recipient. Mol. Microbiol. 1992, 6, 885–893. [Google Scholar] [CrossRef]
| Control | ½ MIC | ||||
|---|---|---|---|---|---|
| Plasmids | MIC (µg/mL) | CF | FC | CF | FC |
| IncL | 1024 | 1.19 × 100 ± 2.65 × 10−2 | 1 | 1.38 × 101 ± 4.54 × 100 | 13.8 ** |
| IncA/C | 256 | 1.00 × 100 ± 5.30 × 10−1 | 1 | 9.93 × 100 ± 4.54 × 100 | 9.9 * |
| IncI1 | 1024 | 9.99 × 10−1 ± 7.98 × 10−1 | 1 | 3.77 × 100 ± 2.19 × 100 | 3.8 |
| IncF | 256 | 6.20 × 10−1 ± 1.07 × 100 | 1 | 1.54 × 10−1 ± 1.44 × 10−1 | 0.12 |
| IncX3 | 256 | 9.99 × 10−1 ± 3.83 × 10−1 | 1 | 1.84 × 101 ± 1.03 × 101 | 18.4 * |
| Control | ½ MIC | ||||
|---|---|---|---|---|---|
| Plasmids | MIC (µg/mL) | CF | FC | CF | FC |
| IncL | 1.024 | 1.00 × 100 ± 6.78 × 10−1 | 1 | 1.69 × 101 ± 9.86 × 100 | 17.0 * |
| IncA/C | 1.024 | 1.00 × 100 ± 1.28 × 100 | 1 | 1.32 × 100 ± 1.40 × 100 | 1.3 |
| IncI1 | 1.024 | 1.00 × 100 ± 1.12 × 100 | 1 | 1.28 × 100 ± 6.78 × 10−1 | 1.3 |
| IncF | 1.024 | 9.99 × 10−1 ± 2.27 × 10−1 | 1 | 1.31 × 100 ± 1.12 × 100 | 1.3 |
| IncX3 | 1.024 | 9.99 × 10−1 ± 3.83 × 10−1 | 1 | 5.12 × 10−1 ± 4.49 × 10−1 | 0.5 |
| 2−ΔΔCT | ||||
|---|---|---|---|---|
| Gene | Isolate | C | Zinc Oxide | Copper Sulphate |
| recA | N502-IncL | 1 | 0.10 ± 0.04 | 2.31 ± 2.09 |
| R2672-IncA/C | 1 | 3.49 ± 1.96 | 0.75 ± 0.10 | |
| R975-IncI1 | 1 | 7.53 ± 5.15 | 0.64 ± 0.26 | |
| R5059-IncF | 1 | 29.08 ± 20.46 | 0.50 ± 0.25 | |
| R5998-IncX3 | 1 | 38.12 ± 8.73 ** | 0.37 ± 0.10 | |
| sfiA | N502-IncL | 1 | 0.03 ± 0.01 | 0.54 ± 0.44 |
| R2672-IncA/C | 1 | 27.41 ± 30.75 | 0.40 ± 0.09 | |
| R975-IncI1 | 1 | 26.45 ± 17.95 | 0.20 ± 0.06 | |
| R5059-IncF | 1 | 6.05 ± 3.75 | 0.14 ± 0.08 | |
| R5998-IncX3 | 1 | 45.92 ± 13.40 ** | 1.27 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raro, O.H.F.; Poirel, L.; Nordmann, P. Effect of Zinc Oxide and Copper Sulfate on Antibiotic Resistance Plasmid Transfer in Escherichia coli. Microorganisms 2023, 11, 2880. https://doi.org/10.3390/microorganisms11122880
Raro OHF, Poirel L, Nordmann P. Effect of Zinc Oxide and Copper Sulfate on Antibiotic Resistance Plasmid Transfer in Escherichia coli. Microorganisms. 2023; 11(12):2880. https://doi.org/10.3390/microorganisms11122880
Chicago/Turabian StyleRaro, Otávio Hallal Ferreira, Laurent Poirel, and Patrice Nordmann. 2023. "Effect of Zinc Oxide and Copper Sulfate on Antibiotic Resistance Plasmid Transfer in Escherichia coli" Microorganisms 11, no. 12: 2880. https://doi.org/10.3390/microorganisms11122880
APA StyleRaro, O. H. F., Poirel, L., & Nordmann, P. (2023). Effect of Zinc Oxide and Copper Sulfate on Antibiotic Resistance Plasmid Transfer in Escherichia coli. Microorganisms, 11(12), 2880. https://doi.org/10.3390/microorganisms11122880







