Assessing the Probiotic Effects of Pediococcus pentosaceus CACC616 in Weaned Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. P. pentosaceus Isolation and Identification
2.2. Tolerance to Artificial Gastrointestinal Conditions
2.3. Adhesion Ability to Intestinal Epithelial Cells
2.4. Hemolytic and Biogenic Amine (BA) Activities
2.5. Antibiotic Susceptibility (Minimal Inhibitory Concentration, MIC)
2.6. Cell Culture and Treatment
2.7. Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.8. Experimental Design, Diet, and Feeding
2.9. Chemical Analysis
2.10. Fecal Odorous Gas Analysis
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. mRNA Sequencing, Data Processing, and Metagenome Analysis
2.13. Statistical Analyses
3. Results
3.1. Potential for Survival in the Gastrointestinal Environment of P. pentosaceus CACC616
3.2. Safety Assessment of P. pentosaceus CACC616
3.3. Immune Modulation of P. pentosaceus CACC616 on LPS-Stimulated Inflammatory Cytokine Expression
3.4. Effect of Potential Probiotic Supplementation on Weaned Piglets
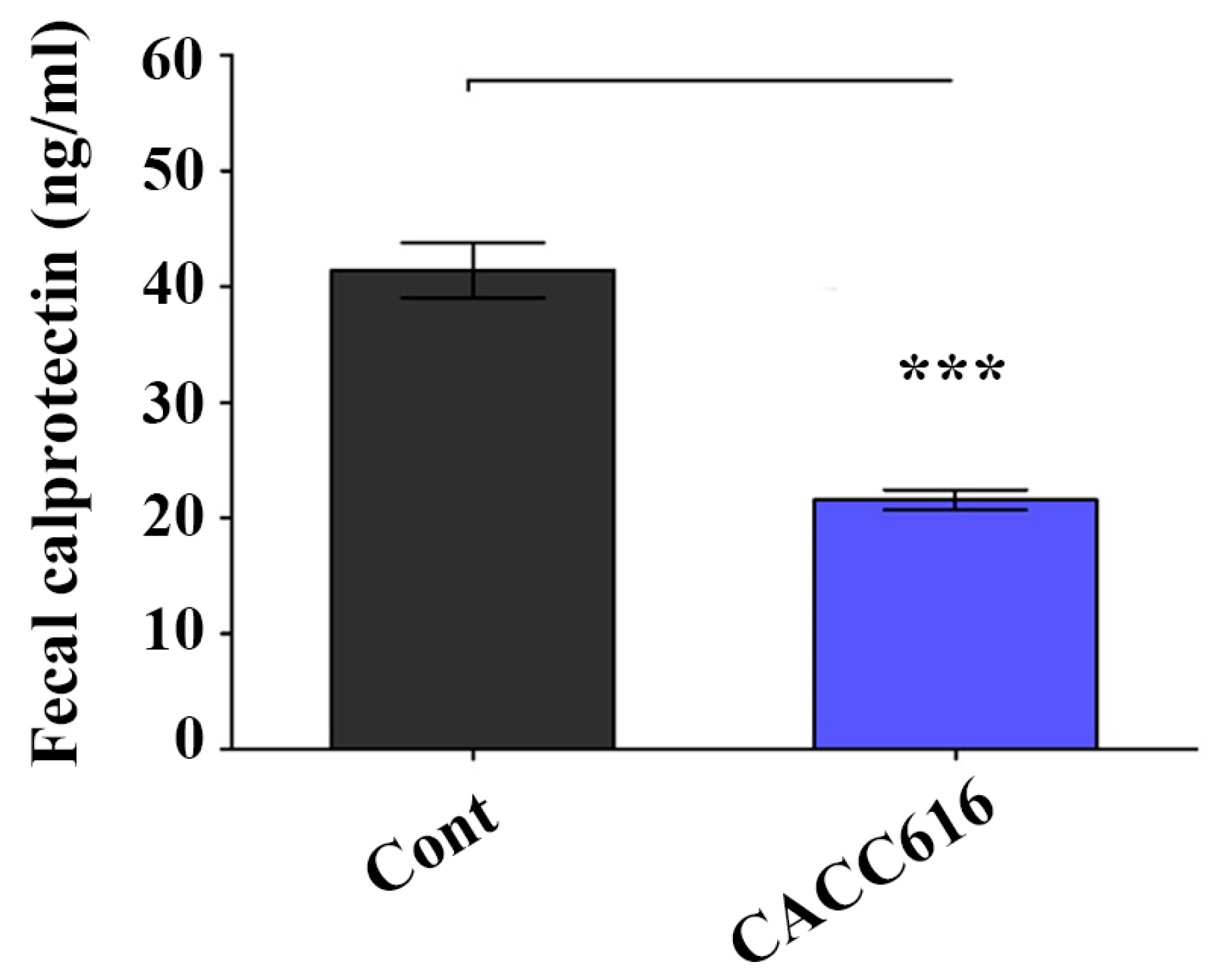
3.5. Effect of Potential Probiotic Supplementation on Fecal Noxious Gas Emissions
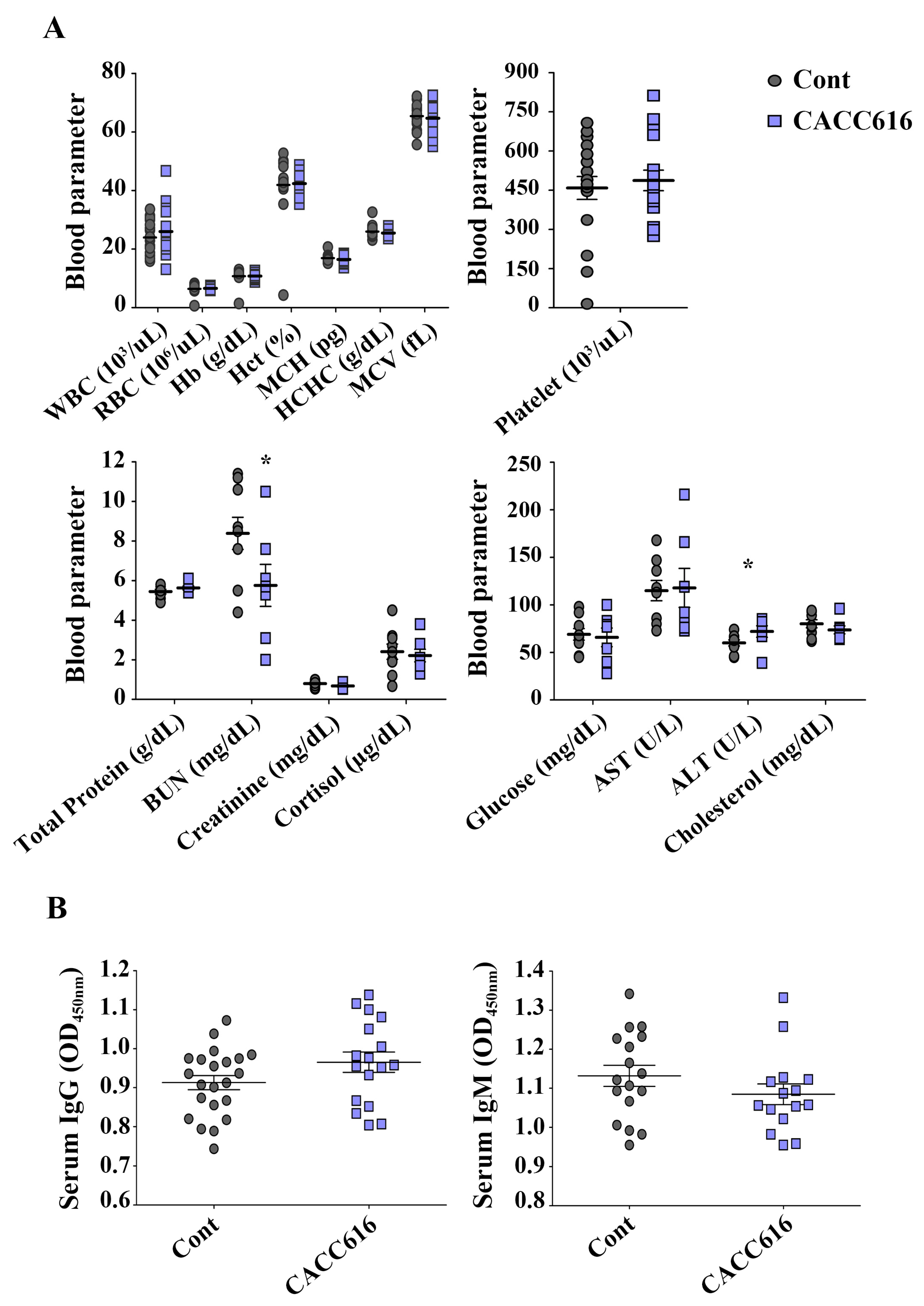
3.6. Effect of Potential Probiotic Supplementation on Hematological Parameters and Immune Modulation
3.7. Effect of Dietary CACC616 on the Fecal Microbiota Composition of Weaned Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Guo, Y.; Wen, Z.; Jiang, X.; Ma, X.; Han, X. Weaning stress perturbs gut microbiome and its metabolic profile in piglets. Sci. Rep. 2018, 8, 18068. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, J.; Zhou, H.; Wang, L.; Ding, S.; Wang, Y.; Li, A. Effects of microencapsulated Lactobacillus plantarum and fructooligosaccharide on growth performance, blood immune parameters, and intestinal morphology in weaned piglets. Food Agric. Immunol. 2018, 29, 84–94. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed]
- Łojewska, E.; Sakowicz, T. An alternative to antibiotics: Selected methods to combat zoonotic foodborne bacterial infections. Curr. Microbiol. 2021, 78, 4037–4049. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.N.; Laguna, J.S.; Millán, C.; Casabuena, O.; Gracia, M.I. Effects of a Bacillus-based probiotic and dietary energy content on the performance and nutrient digestibility of wean to finish pigs. Anim. Feed Sci. Technol. 2016, 221, 54–61. [Google Scholar] [CrossRef]
- Wang, S.; Yao, B.; Gao, H.; Zang, J.; Tao, S.; Zhang, S.; Wang, J. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs. BMC Vet. Res. 2019, 15, 239. [Google Scholar] [CrossRef]
- Kook, S.Y.; Chung, E.C.; Lee, Y.; Lee, D.W.; Kim, S. Isolation and characterization of five novel probiotic strains from Korean infant and children faeces. PLoS ONE 2019, 14, e0223913. [Google Scholar] [CrossRef]
- Elbanna, K.; El Hadad, S.; Assaeedi, A.; Aldahlawi, A.; Khider, M.; Alhebshi, A. In vitro and in vivo evidences for innate immune stimulators lactic acid bacterial starters isolated from fermented camel dairy products. Sci. Rep. 2018, 8, 12553. [Google Scholar] [CrossRef]
- Nomoto, K. Prevention of infections by probiotics. J. Biosci. Bioeng. 2005, 100, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The use of lactic acid bacteria as a probiotic in swine diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhong, J. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Maintenance of gut microbiome stability for optimum intestinal health in pigs—A review. J. Anim. Sci. Biotechnol. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, F.; Niyitanga, E.; Chai, X.; Wang, S.; Liu, Y. The odor release regularity of livestock and poultry manure and the screening of deodorizing strains. Microorganisms 2021, 9, 2488. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Q.; Chen, Y.; Zheng, X.; Wang, C.; Qi, Y.; Yin, Y. Antioxidant potential of Pediococcus pentosaceus strains from the sow milk bacterial collection in weaned piglets. Microbiome 2022, 10, 83. [Google Scholar] [CrossRef]
- Song, D.; Lee, J.; Kim, K.; Oh, H.; An, J.; Chang, S.; Cho, J. Effects of dietary supplementation of Pediococcus pentosaceus strains from kimchi in weaned piglet challenged with Escherichia coli and Salmonella enterica. J. Anim. Sci. Technol. 2023, 65, 611. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Patel, B.H.M.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017, 195, 74–79. [Google Scholar] [CrossRef]
- Kim, J.A.; Bayo, J.; Cha, J.; Choi, Y.J.; Jung, M.Y.; Kim, D.H.; Kim, Y. Investigating the probiotic characteristics of four microbial strains with potential application in feed industry. PLoS ONE 2019, 14, e0218922. [Google Scholar] [CrossRef]
- El-Sayed, A.I.; El-Borai, A.M.; Akl, S.H.; El-Aassar, S.A.; Abdel-Latif, M.S. Identification of Lactobacillus strains from human mother milk and cottage cheese revealed potential probiotic properties with enzymatic activity. Sci. Rep. 2022, 12, 22522. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Amore, G.; Beloeil, P.A.; Garcia Fierro, R.; Guerra, B.; Papanikolaou, A.; Rizzi, V.; Stoicescu, A.V. Manual for Reporting 2022 Antimicrobial Resistance Data within the Framework of Directive 2003/99/EC and Decision 2020/1729/EU; EFSA Supporting Publications: Parma, Italy, 2023; Volume 20, p. EN-7826. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2−ΔΔCT method. Method 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of the Official Analytical Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Zhang, S.; Cai, L.; Koziel, J.A.; Hoff, S.J.; Schmidt, D.R.; Clanton, C.J.; Heber, A.J. Field air sampling and simultaneous chemical and sensory analysis of livestock odorants with sorbent tubes and GC–MS/olfactometry. Sens. Actuators B. Chem. 2010, 146, 427–432. [Google Scholar] [CrossRef]
- Boeckman, J.X.; Sprayberry, S.; Korn, A.M.; Suchodolski, J.S.; Paulk, C.; Genovese, K.; Gill, J.J. Effect of chronic and acute enterotoxigenic E. coli challenge on growth performance, intestinal inflammation, microbiome, and metabolome of weaned piglets. Sci. Rep. 2022, 12, 5024. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.O.; Ascoli, C.A. Immunometric double-antibody sandwich enzyme-linked immunosorbent assay. Cold Spring Harb. Protoc. 2017, 2017, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.T.J.; Moonen-Leusen, H.W.M.; Van der Heijden, P.J.; Bokhout, B.A. The use of a double antibody sandwich ELISA and monoclonal antibodies for the assessment of porcine IgM, IgG and IgA concentrations. Vet. Immunol. Immunopathol. 1995, 44, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Eldahshoury, M.K.; Hurley, I.P. Direct sandwich ELISA to detect the adulteration of human breast milk by cow milk. J. Dairy Sci. 2023, 106, 5908–5915. [Google Scholar] [CrossRef]
- Walke, J.M.; Crowthe, J.R. The ELISA Guidebook; Humana Press: Totowa, NJ, USA, 2009; ISBN 978-1-60327-253-7. [Google Scholar]
- Bugenyi, A.W.; Lee, M.R.; Choi, Y.J.; Song, K.D.; Lee, H.K.; Son, Y.O.; Heo, J. Oropharyngeal, proximal colonic, and vaginal microbiomes of healthy Korean native black pig gilts. BMC Microbiol. 2023, 23, 3. [Google Scholar] [CrossRef]
- Radziwill-Bienkowska, J.M.; Robert, V.; Drabot, K.; Chain, F.; Cherbuy, C.; Langella, P.; Kowalczyk, M. Contribution of plasmid-encoded peptidase S8 (PrtP) to adhesion and transit in the gut of Lactococcus lactis IBB477 strain. Appl. Microbiol. Biotechnol. 2017, 101, 5709–5721. [Google Scholar] [CrossRef]
- Kruse, R.; Essén-Gustavsson, B.; Fossum, C.; Jensen-Waern, M. Blood concentrations of the cytokines IL-1beta, IL-6, IL-10, TNF-alpha and IFN-gamma during experimentally induced swine dysentery. Acta Vet. Scand. 2008, 50, 1–7. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, N.K.; Paik, H.D. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food. Sci. Biotechnol. 2019, 28, 1521–1528. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Liao, C.; Jia, Y.; Li, J.; Shang, K.; Ding, K. Probiotic properties of chicken-derived highly adherent lactic acid bacteria and inhibition of enteropathogenic bacteria in Caco-2 cells. Microorganisms 2022, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, K.S.; Lee, J.; Lee, K.S.; Park, S.Y. Weissella koreensis and Pediococcus pentosaceus bacterial ghosts induce inflammatory responses as immunostimulants. Biochem. Biophys. Res. Commun. 2023, 676, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Pan, C.; Chen, X.; Jing, M.; Xie, J.; Gao, Y.; Li, P. Probiotic lactic acid bacteria alleviate pediatric IBD and remodel gut microbiota by modulating macrophage polarization and suppressing epithelial apoptosis. Front. Microbiol. 2023, 14, 1168924. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, R.; Oh, J.K.; Song, J.H.; Kang, D.K. Gut microbiome-produced metabolites in pigs: A review on their biological functions and the influence of probiotics. J. Anim. Sci. Technol. 2022, 64, 671. [Google Scholar] [CrossRef]
- Maltecca, C.; Dunn, R.; He, Y.; McNulty, N.P.; Schillebeeckx, C.; Schwab, C.; Tiezzi, F. Microbial composition differs between production systems and is associated with growth performance and carcass quality in pigs. Anim. Microbiome 2021, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, G.E.; Metzler-Zebeli, B.U.; Lawlor, P.G. Impact of intestinal microbiota on growth and feed efficiency in pigs: A review. Microorganisms 2020, 8, 1886. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, O.; Park, S. Effect of dietary protein levels on composition of odorous compounds and bacterial ecology in pig manure. Asian-Australas J. Anim. Sci. 2015, 28, 1362. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, W.; Shang, W.; Du, H.; Li, G.; Yao, W. How host gender affects the bacterial community in pig feces and its correlation to skatole production. Ann. Microbiol. 2015, 65, 2379–2386. [Google Scholar] [CrossRef]
- Burrough, E.R.; Arruda, B.L.; Plummer, P.J. Comparison of the luminal and mucosa-associated microbiota in the colon of pigs with and without swine dysentery. Front. Vet. Sci. 2017, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Misiukiewicz, A.; Gao, M.; Filipiak, W.; Cieslak, A.; Patra, A.K.; Szumacher-Strabel, M. Methanogens and methane production in the digestive systems of nonruminant farm animals. Animal 2021, 15, 100060. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, X.; Li, Y.; Chen, M.; Xue, L.; Wang, J.; Wu, Q. Pediococcus pentosaceus IM96 exerts protective effects against enterohemorrhagic Escherichia coli O157: H7 infection in vivo. Foods 2021, 10, 2945. [Google Scholar] [CrossRef]



| Antibiotic | MIC (µg/mL) 1 |
|---|---|
| Ampicillin | ≥3.0 |
| Vancomycin | 0.0 |
| Gentamicin | ≥32.0 |
| Kanamycin | 0.0 |
| Streptomycin | ≥384.0 |
| Erythromycin | 1.0 |
| Clindamycin | ≥0.094 |
| Tetracycline | ≥24.0 |
| Chloramphenicol | ≥6.0 |
| BA *-Producing Ability (ppm) | Total Concentration (ppm) | ||||
|---|---|---|---|---|---|
| Putrescine | Cadaverine | Histamine | Spermidine | Spermine | |
| 5.19 | 2.59 | ND | 11.5 | 5.45 | 24.73 |
| Items (0–26 d) | Control | Pediococcus pentosaceus CACC616 |
|---|---|---|
| Initial BW (kg) | 7.7 ± 0.3 | 7.3 ± 0.3 |
| Final BW (kg) | 16.9 ± 0.6 | 16.5 ± 0.6 |
| ADG (g) | 354 ± 13 | 354 ± 12 |
| ADFI (g) | 587 ± 26 | 558 ±24 |
| FCR | 1.70 ± 0.07 | 1.63 ± 0.06 |
| Items | Control | Pediococcus pentosaceus CACC616 |
|---|---|---|
| VFAs | ||
| Acetic acid | 3727.5 ± 689.0 a | 3190.0 ± 754.0 a |
| Propionic acid | 14,978.6 ± 668.6 a | 15,119.5 ± 2205.5 a |
| Isobutyric acid | 2212.2 ± 96.9 a | 1994.0 ± 679.0 a |
| Butyric acid | 8759.0 ± 255.2 a | 4102.5 ± 748.5 b* |
| Isovaleric acid | 2391.6 ± 83.8 a | 1429.7 ± 259.4 a |
| Valeric acid | 5096.0 ± 80.8 a | 2641.7 ± 567.9 b** |
| VOCs | ||
| Phenol | 3.9 ± 0.1 a | 10.0 ± 0.6 b** |
| p-cresol | 526.7 ± 21.8 a | 281.0 ± 59.0 b* |
| Indole | 1.4 ± 1.4 a | 0.3 ± 0.0 a |
| Skatole | 52.0 ± 6.0 a | 21.9 ± 5.3 b* |
| Hydrogen sulfide | 1109 ± 594.2 a | 192.7 ± 24.3 a |
| Methyl mercaptan | 171.7 ± 12.4 a | 59.7 ± 15.4 b*** |
| Genus | D0 | D26 | ||
|---|---|---|---|---|
| Control | CACC616 | Control | CACC616 | |
| Psychrobacter | 39.10 | 44.15 | 0.33 | 0.01 |
| Unknown_Lachnospiraceae | 13.39 | 9.74 | 5.45 | 5.71 |
| Ruminococcaceae UCG | 12.28 | 14.13 | 14.11 | 14.01 |
| Prevotella | 6.25 | 2.95 | 16.58 | 14.98 |
| [Eubacterium] coprostanoligenes group | 4.34 | 3.45 | 2.68 | 1.74 |
| Uncultured_bacterium_Porphyromonadaceae | 4.06 | 3.82 | 6.82 | 5.71 |
| Unknown_Ruminococcaceae | 3.12 | 2.35 | 1.29 | 1.15 |
| Rikenellaceae RC9 gut group | 2.49 | 3.94 | 6.48 | 5.64 |
| Prevotellaceae NK3B31 group | 2.43 | 0.80 | 5.31 | 7.09 |
| Unknown_Prevotellaceae | 2.30 | 0.84 | 2.13 | 2.80 |
| Uncultured_bacterium_Muribaculaceae | 2.06 | 1.14 | 1.81 | 3.54 |
| Ruminococcaceae NK4A214 group | 1.81 | 2.06 | 3.30 | 3.24 |
| Phascolarctobacterium | 1.66 | 1.93 | 2.07 | 2.79 |
| Alloprevotella | 1.66 | 0.89 | 3.36 | 4.54 |
| Clostridium sensu stricto 1 | 1.47 | 1.08 | 3.27 | 4.36 |
| Lactobacillus | 0.72 | 1.17 | 9.14 | 9.43 |
| Escherichia–Shigella | 0.69 | 5.34 | 10.45 | 7.94 |
| Unknown_Veillonellaceae | 0.01 | 0.01 | 3.09 | 3.27 |
| Agathobacter | 0.00 | 0.00 | 2.18 | 1.91 |
| <Others (0.1%) | 0.16 | 0.20 | 0.15 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Song, J.; Park, M.A.; Jang, H.-J.; Son, S.; Kim, D.-H.; Kim, Y. Assessing the Probiotic Effects of Pediococcus pentosaceus CACC616 in Weaned Piglets. Microorganisms 2023, 11, 2890. https://doi.org/10.3390/microorganisms11122890
Park S, Song J, Park MA, Jang H-J, Son S, Kim D-H, Kim Y. Assessing the Probiotic Effects of Pediococcus pentosaceus CACC616 in Weaned Piglets. Microorganisms. 2023; 11(12):2890. https://doi.org/10.3390/microorganisms11122890
Chicago/Turabian StylePark, Soyeon, Jeongsup Song, Mi Ae Park, Hyun-Jun Jang, Seoyun Son, Dae-Hyuk Kim, and Yangseon Kim. 2023. "Assessing the Probiotic Effects of Pediococcus pentosaceus CACC616 in Weaned Piglets" Microorganisms 11, no. 12: 2890. https://doi.org/10.3390/microorganisms11122890
APA StylePark, S., Song, J., Park, M. A., Jang, H.-J., Son, S., Kim, D.-H., & Kim, Y. (2023). Assessing the Probiotic Effects of Pediococcus pentosaceus CACC616 in Weaned Piglets. Microorganisms, 11(12), 2890. https://doi.org/10.3390/microorganisms11122890






