The Mite Steatonyssus periblepharus Is a Novel Potential Vector of the Bat Parasite Trypanosoma dionisii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Collection
2.2. Analysis of Ectoparasites
2.3. Light Microscopy, Morphometry and Statistical Analysis
2.4. DNA Isolation, PCR and Sequencing
2.5. Phylogenetic Analyses
3. Results
3.1. Bat Ectoparasites and Presence of Trypanosomes in Them
3.2. Molecular Identification of Trypanosomes and Phylogenetic Analysis
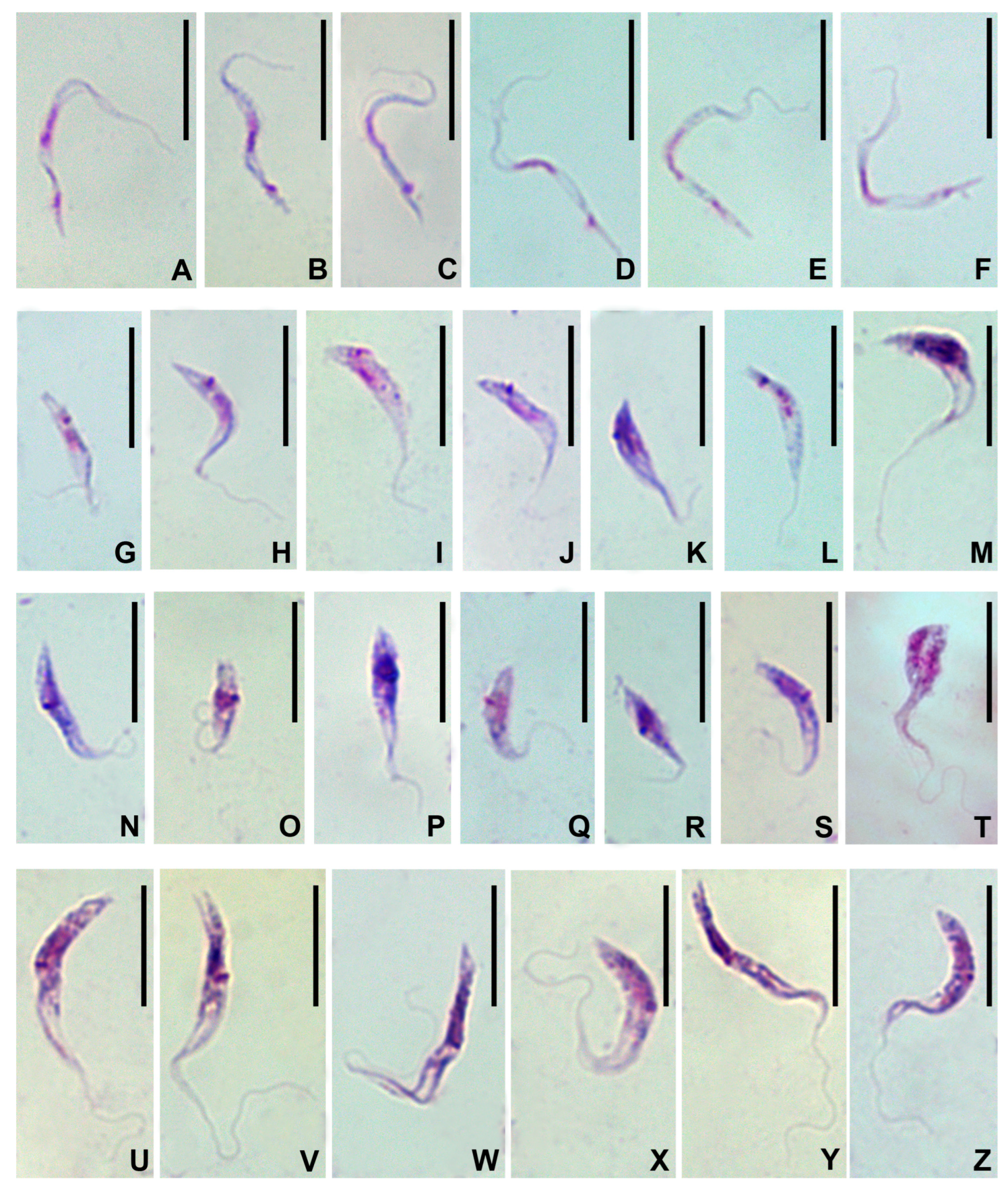
3.3. Morphology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.E.; Mittermeier, R.A. Handbook of the Mammals of the World; Lynx Edicions: Barcelona, Spain, 2019; Volume 9, p. 1008. [Google Scholar]
- Jansen, A.M.; Xavier, S.C.D.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasites Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Austen, J.M.; Barbosa, A.D. Diversity and epidemiology of bat trypanosomes: A one health perspective. Pathogens 2021, 10, 1148. [Google Scholar] [CrossRef]
- Podlipaev, S.A. Catalogue of World Fauna of Trypanosomatidae (Protozoa); Zoologicheskii Institut AN SSSR: Leningrad, Russia, 1990; Volume 144, p. 178. (In Russian) [Google Scholar]
- Marcili, A.; da Costa, A.P.; Soares, H.S.; Acosta Ida, C.; de Lima, J.T.; Minervino, A.H.; Melo, A.T.; Aguiar, D.M.; Pacheco, R.C.; Gennari, S.M. Isolation and phylogenetic relationships of bat trypanosomes from different biomes in Mato Grosso, Brazil. J. Parasitol. 2013, 99, 1071–1076. [Google Scholar] [CrossRef]
- Hodo, C.L.; Goodwin, C.C.; Mayes, B.C.; Mariscal, J.A.; Waldrup, K.A.; Hamer, S.A. Trypanosome species, including Trypanosoma cruzi, in sylvatic and peridomestic bats of Texas, USA. Acta Trop. 2016, 164, 259–266. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Teixeira, M.M.; Stevens, J.R. The evolution of Trypanosoma cruzi: The ’bat seeding’ hypothesis. Trends Parasitol. 2012, 28, 136–141. [Google Scholar] [CrossRef]
- Lima, L.; Silva, F.M.; Neves, L.; Attias, M.; Takata, C.S.; Campaner, M.; de Souza, W.; Hamilton, P.B.; Teixeira, M.M. Evolutionary insights from bat trypanosomes: Morphological, developmental and phylogenetic evidence of a new species, Trypanosoma (Schizotrypanum) erneyi sp. nov., in African bats closely related to Trypanosoma (Schizotrypanum) cruzi and allied species. Protist 2012, 163, 856–872. [Google Scholar] [CrossRef]
- Szpeiter, B.B.; Ferreira, J.; Assis, F.F.V.; Stelmachtchuk, F.N.; Peixoto, K.D.C.J.; Ajzenberg, D.; Minervino, A.H.H.; Gennari, S.M.; Marcili, A. Bat trypanosomes from Tapajós-Arapiuns extractive reserve in Brazilian Amazon. Rev. Bras. Parasitol. Vet. 2017, 26, 152–158. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Lima, L.; Xavier, S.; Herrera, H.M.; Rocha, F.L.; Roque, A.L.R.; Teixeira, M.M.G.; Jansen, A.M. Uncovering Trypanosoma spp. diversity of wild mammals by the use of DNA from blood clots. Int. J. Parasitol. Parasites Wildl. 2019, 8, 171–181. [Google Scholar] [CrossRef]
- Dario, M.A.; Rodrigues, M.S.; Barros, J.H.; Xavier, S.C.; D’Andrea, P.S.; Roque, A.L.; Jansen, A.M. Ecological scenario and Trypanosoma cruzi DTU characterization of a fatal acute Chagas disease case transmitted orally (Espirito Santo state, Brazil). Parasites Vectors 2016, 9, 477. [Google Scholar] [CrossRef]
- Hoare, C.A. The Trypanosomes of Mammals. A Zoological Monograph; Blackwell Scientific Publications: Oxford, UK, 1972; p. 768. [Google Scholar]
- Molyneux, D.H.; Stiles, J.K. Trypanosomatid—Vector interactions. Ann. Soc. Belg. Med. Trop. 1991, 71, 151–166. [Google Scholar]
- Gardner, R.A.; Molyneux, D.H. Schizotrypanum in British bats. Parasitology 1988, 97, 43–50. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Cruickshank, C.; Stevens, J.R.; Teixeira, M.M.G.; Mathews, F. Parasites reveal movement of bats between the New and Old Worlds. Mol. Phylogenet Evol. 2012, 63, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Austen, J.M.; Van Kampen, E.; Egan, S.L.; O’Dea, M.A.; Jackson, B.; Ryan, U.M.; Irwin, P.J.; Prada, D. First report of Trypanosoma dionisii (Trypanosomatidae) identified in Australia. Parasitology 2020, 147, 1801–1809. [Google Scholar] [CrossRef]
- Szentiványi, T.; Markotter, W.; Dietrich, M.; Clément, L.; Ançay, L.; Brun, L.; Genzoni, E.; Kearney, T.; Seamark, E.; Estók, P.; et al. Host conservation through their parasites: Molecular surveillance of vector-borne microorganisms in bats using ectoparasitic bat flies. Parasite 2020, 27, 72. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Ganyukova, A.I.; Frolov, A.O.; Malysheva, M.N.; Spodareva, V.V.; Yurchenko, V.; Kostygov, A.Y. A novel endosymbiont-containing trypanosomatid Phytomonas borealis sp. n. from the predatory bug Picromerus bidens (Heteroptera: Pentatomidae). Folia Parasitol. 2020, 67, 4. [Google Scholar] [CrossRef]
- Maslov, D.A.; Lukeš, J.; Jirků, M.; Simpson, L. Phylogeny of trypanosomes as inferred from the small and large subunit rRNAs: Implications for the evolution of parasitism in the trypanosomatid protozoa. Mol. Biochem. Parasitol. 1996, 75, 197–205. [Google Scholar] [CrossRef]
- Seward, E.A.; Votýpka, J.; Kment, P.; Lukeš, J.; Kelly, S. Description of Phytomonas oxycareni n. sp. from the salivary glands of Oxycarenus lavaterae. Protist 2017, 168, 71–79. [Google Scholar] [CrossRef]
- Gerasimov, E.S.; Kostygov, A.Y.; Yan, S.; Kolesnikov, A.A. From cryptogene to gene? ND8 editing domain reduction in insect trypanosomatids. Eur. J. Protistol. 2012, 48, 185–193. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Chistyakova, L.V.; Kostygov, A.Y.; Kornilova, O.A.; Yurchenko, V. Reisolation and redescription of Balantidium duodeni Stein, 1867 (Litostomatea, Trichostomatia). Parasitol. Res. 2014, 113, 4207–4215. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Garcia, H.A.; Blanco, P.A.; Rodrigues, A.C.; Rodrigues, C.M.F.; Takata, C.S.A.; Campaner, M.; Camargo, E.P.; Teixeira, M.M.G. Pan-American Trypanosoma (Megatrypanum) trinaperronei n. sp. in the white-tailed deer Odocoileus virginianus Zimmermann and its deer ked Lipoptena mazamae Rondani, 1878: Morphological, developmental and phylogeographical characterisation. Parasites Vectors 2020, 13, 308. [Google Scholar] [CrossRef]
- Lima, L.; Espinosa-Alvarez, O.; Ortiz, P.A.; Trejo-Varon, J.A.; Carranza, J.C.; Pinto, C.M.; Serrano, M.G.; Buck, G.A.; Camargo, E.P.; Teixeira, M.M. Genetic diversity of Trypanosoma cruzi in bats, and multilocus phylogenetic and phylogeographical analyses supporting Tcbat as an independent DTU (discrete typing unit). Acta Trop. 2015, 151, 166–177. [Google Scholar] [CrossRef]
- Akhoundi, M.; Sereno, D.; Durand, R.; Mirzaei, A.; Bruel, C.; Delaunay, P.; Marty, P.; Izri, A. Bed bugs (Hemiptera, Cimicidae): Overview of classification, evolution and dispersion. Int. J. Environ. Res. Public Health 2020, 17, 4576. [Google Scholar] [CrossRef]
- Brotánková, A.; Fialová, M.; Čepička, I.; Brzoňová, J.; Svobodová, M. Trypanosomes of the Trypanosoma theileri group: Phylogeny and new potential vectors. Microorganisms 2022, 10, 294. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Frolov, A.O.; Malysheva, M.N.; Ganyukova, A.I.; Drachko, D.; Yurchenko, V.; Agasoi, V.V. Development of two species of the Trypanosoma theileri complex in tabanids. Parasites Vectors 2022, 15, 95. [Google Scholar] [CrossRef]
- Calzolari, M.; Rugna, G.; Clementi, E.; Carra, E.; Pinna, M.; Bergamini, F.; Fabbi, M.; Dottori, M.; Sacchi, L.; Votýpka, J. Isolation of a trypanosome related to Trypanosoma theileri (Kinetoplastea: Trypanosomatidae) from Phlebotomus perfiliewi (Diptera: Psychodidae). Biomed. Res. Int. 2018, 2018, 2597074. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.M.; Lisboa, C.V.; Dario, M.A.; Novaes, R.L.M.; Tiepolo, L.M.; Moratelli, R.; Jansen, A.M. Old methods, new insights: Reviewing concepts on the ecology of trypanosomatids and Bodo sp. by improving conventional diagnostic tools. Pathogens 2023, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.N.L.; Sampaio Júnior, F.D.; Costa, S.M.; Farias, D.M.; Moura, M.A.O.; Bezerra Júnior, P.S.; Góes-Cavalcante, G.; Scofield, A. First report of natural infection by Trypanosoma cruzi in secretions of the scent glands and myocardium of Philander opossum (Marsupialia: Didelphidae): Parasitological and clinicopathological findings. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100463. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana, M., Jr.; Marcili, A.; Lima, L.; da Silva, F.M.; Junqueira, A.C.; Veludo, H.H.; Viola, L.B.; Campaner, M.; Nunes, V.L.; Paiva, F.; et al. Phylogeographical, ecological and biological patterns shown by nuclear (ssrRNA and gGAPDH) and mitochondrial (Cyt b) genes of trypanosomes of the subgenus Schizotrypanum parasitic in Brazilian bats. Int. J. Parasitol. 2010, 40, 345–355. [Google Scholar] [CrossRef]
- Alves, F.M.; Rangel, D.A.; Vilar, E.M.; Pavan, M.G.; Moratelli, R.; Roque, A.L.R.; Jansen, A.M. Trypanosoma spp. Neobats: Insights about those poorly known trypanosomatids. Int. J. Parasitol. Parasites Wildl. 2021, 16, 145–152. [Google Scholar] [CrossRef]
- Acosta, I.C.L.; da Costa, A.P.; Gennari, S.M.; Marcili, A. Survey of Trypanosoma and Leishmania in wild and domestic animals in an Atlantic rainforest fragment and surroundings in the state of Espirito Santo, Brazil. J. Med. Entomol. 2014, 51, 686–693. [Google Scholar] [CrossRef]
- Maia da Silva, F.; Marcili, A.; Lima, L.; Cavazzana, M., Jr.; Ortiz, P.A.; Campaner, M.; Takeda, G.F.; Paiva, F.; Nunes, V.L.; Camargo, E.P.; et al. Trypanosoma rangeli isolates of bats from Central Brazil: Genotyping and phylogenetic analysis enable description of a new lineage using spliced-leader gene sequences. Acta Trop. 2009, 109, 199–207. [Google Scholar] [CrossRef]
- dos Santos, F.C.B.; Lisboa, C.V.; Xavier, S.C.C.; Dario, M.A.; Verde, R.S.; Calouro, A.M.; Roque, A.L.R.; Jansen, A.M. Trypanosoma sp. diversity in Amazonian bats (Chiroptera; Mammalia) from Acre State, Brazil. Parasitology 2018, 145, 828–837. [Google Scholar] [CrossRef]
- Dario, M.A.; Lisboa, C.V.; Costa, L.M.; Moratelli, R.; Nascimento, M.P.; Costa, L.P.; Leite, Y.L.R.; Llewellyn, M.S.; Xavier, S.; Roque, A.L.R.; et al. High Trypanosoma spp. diversity is maintained by bats and triatomines in Espirito Santo state, Brazil. PLoS ONE 2017, 12, e0188412. [Google Scholar] [CrossRef]
- Dario, M.A.; Moratelli, R.; Schwabl, P.; Jansen, A.M.; Llewellyn, M.S. Small subunit ribosomal metabarcoding reveals extraordinary trypanosomatid diversity in Brazilian bats. PLoS Negl. Trop. Dis. 2017, 11, e0005790. [Google Scholar] [CrossRef]
- Orlova, M.; Stanyukovich, M.; Orlov, O. Gamasid Mites (Mesostigmata: Gamasina) Parasitizing Bats (Chiroptera: Rhinolophidae, Vespertilionidae, Molossidae) of Palaearctic Boreal Zone (Russia and Adjacent Countries); Publishing House of Tomsk State University: Tomsk, Russia, 2015. [Google Scholar]
- Orlova, M.; Chistiakov, D.; Orlov, O.; Kruger, F.; Kshnyasev, I. Ectoparasite fauna of pond bat Myotis dasycneme (Boie, 1825), (Chiroptera, Vespertilionidae) in Northern Eurasia. Biol. Commun. 2014, 1, 24–38. (In Russian) [Google Scholar]
- Marinkelle, C.J. Developmental stages of Trypanosoma cruzi-like flagellates in Cavernicola pilosa. Rev. Biol. Trop. 1982, 30, 107–111. [Google Scholar] [PubMed]
- Goncalves, C.S.; Avila, A.R.; de Souza, W.; Motta, M.C.M.; Cavalcanti, D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasites Vectors 2018, 11, 83. [Google Scholar] [CrossRef]



| Bat Hosts | Ectoparasites (Trypanosome-Infected/Total) | ||||
|---|---|---|---|---|---|
| ID | Species | Sex and Age | Steatonyssus periblepharus | Spinturnix sp. | Ischnopsyllus variabilis |
| 01 | Pipistrellus nathusii |  ad. ad. | - | - | 0/1 |
| 02 | Pipistrellus nathusii |  ad. ad. | - | - | 0/2 |
| 03 | Pipistrellus nathusii |  ad. ad. | - | - | 0/2 |
| 04 | Pipistrellus nathusii |  sad. sad. | 3/7 | - | - |
| 05 | Pipistrellus nathusii |  ad. ad. | 0/3 | - | 0/3 |
| 06 | Pipistrellus nathusii |  sad. sad. | 2/4 | - | 0/2 |
| 07 | Pipistrellus nathusii |  ad. ad. | 0/1 | - | 0/1 |
| 08 | Pipistrellus nathusii |  sad. sad. | 0/3 | - | 0/6 |
| 09 | Pipistrellus nathusii |  sad. sad. | 3/6 | - | - |
| 10 | Pipistrellus nathusii |  sad. sad. | 1/2 | - | 0/1 |
| 11 | Myotis daubentonii |  ad. ad. | 0/1 | 0/1 | - |
| Total: | 9/27 | 0/1 | 0/18 | ||
| Morphotype | N | Length | Width | Nucleus | N–A | K–A | Flagellum |
|---|---|---|---|---|---|---|---|
| Slender trypomastigotes | 35 | 22.0 ± 0.4 (15.9–27.0) | 0.8 ± 0.03 (0.5–1.2) | 3.6 ± 0.1 (2.6–4.9) | 12.0 ± 0.3 (5.5–15.1) | 18.8 ± 0.4 (14.7–23.0) | 2.3 ± 0.3 (0.0–5.9) |
| Stumpy trypomastigotes | 15 | 12.6 ± 0.5 (9.2–16.3) | 1.7 ± 0.1 (1.1–2.6) | 1.8 ± 0.1 (1.1–2.3) | 6.2 ± 0.3 (3.0–7.8) | 9.5 ± 0.5 (6.1–12.0) | 5.7 ± 0.7 (0.0–9.4) |
| Short epimastigotes | 32 | 10.2 ± 0.4 (4.1–14.2) | 1.7 ± 0.1 (1.0–2.5) | 1.8 ± 0.1 (0.8–2.4) | 5.3 ± 0.3 (1.8–9.1) | 5.4 ± 0.3 (2.3–8.0) | 6.2 ± 0.4 (3.1–12.3) |
| Long epimastigotes | 26 | 19.6 ± 0.6 (14.7–26.2) | 1.7 ± 0.1 (1.0–2.4) | 1.9 ± 0.1 (1.2–2.5) | 11.4 ± 0.5 (7.7–18.3) | 10.8 ± 0.5 (6.9–16.1) | 14.5 ± 0.7 (9.0–20.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malysheva, M.N.; Ganyukova, A.I.; Frolov, A.O.; Chistyakov, D.V.; Kostygov, A.Y. The Mite Steatonyssus periblepharus Is a Novel Potential Vector of the Bat Parasite Trypanosoma dionisii. Microorganisms 2023, 11, 2906. https://doi.org/10.3390/microorganisms11122906
Malysheva MN, Ganyukova AI, Frolov AO, Chistyakov DV, Kostygov AY. The Mite Steatonyssus periblepharus Is a Novel Potential Vector of the Bat Parasite Trypanosoma dionisii. Microorganisms. 2023; 11(12):2906. https://doi.org/10.3390/microorganisms11122906
Chicago/Turabian StyleMalysheva, Marina N., Anna I. Ganyukova, Alexander O. Frolov, Dmitriy V. Chistyakov, and Alexei Yu. Kostygov. 2023. "The Mite Steatonyssus periblepharus Is a Novel Potential Vector of the Bat Parasite Trypanosoma dionisii" Microorganisms 11, no. 12: 2906. https://doi.org/10.3390/microorganisms11122906
APA StyleMalysheva, M. N., Ganyukova, A. I., Frolov, A. O., Chistyakov, D. V., & Kostygov, A. Y. (2023). The Mite Steatonyssus periblepharus Is a Novel Potential Vector of the Bat Parasite Trypanosoma dionisii. Microorganisms, 11(12), 2906. https://doi.org/10.3390/microorganisms11122906






