Carvacrol-Induced Vacuole Dysfunction and Morphological Consequences in Nakaseomyces glabratus and Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Minimum Inhibitory Concentration (MIC)
2.3. Vacuolar Acidification
2.4. Membrane Integrity Assay
2.5. Germ Tube Formation Assay
2.6. Statistical Analysis
3. Results
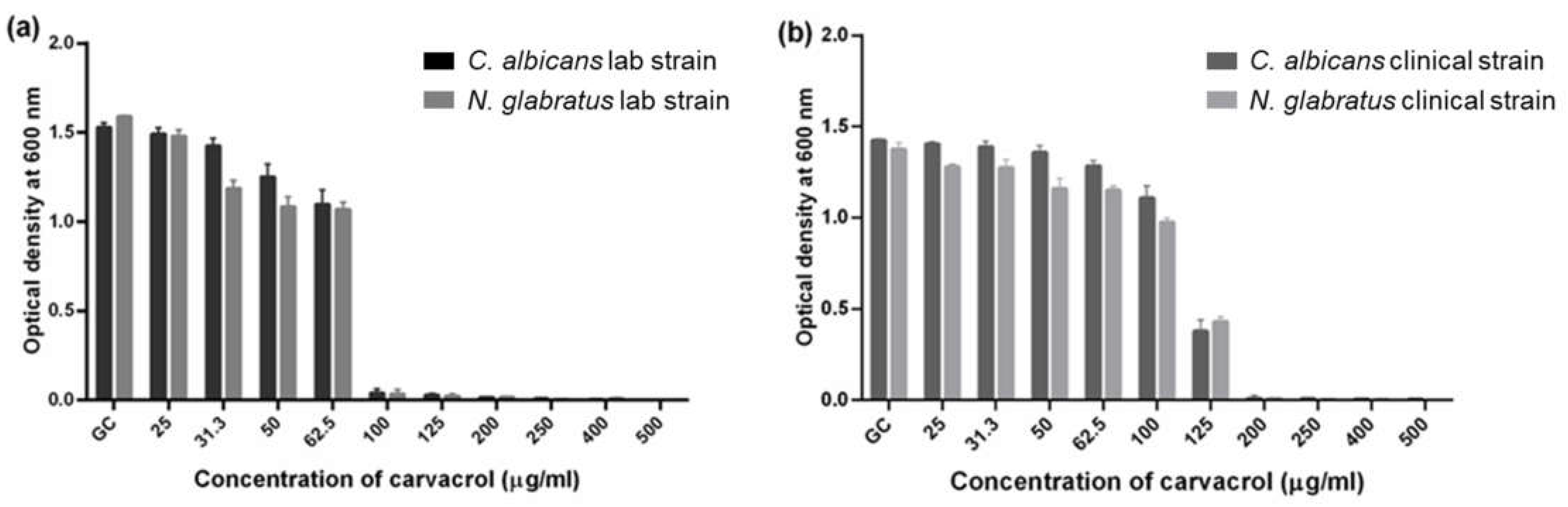
3.1. C. albicans and N. glabratus Growth Was Similarly Inhibited by Carvacrol
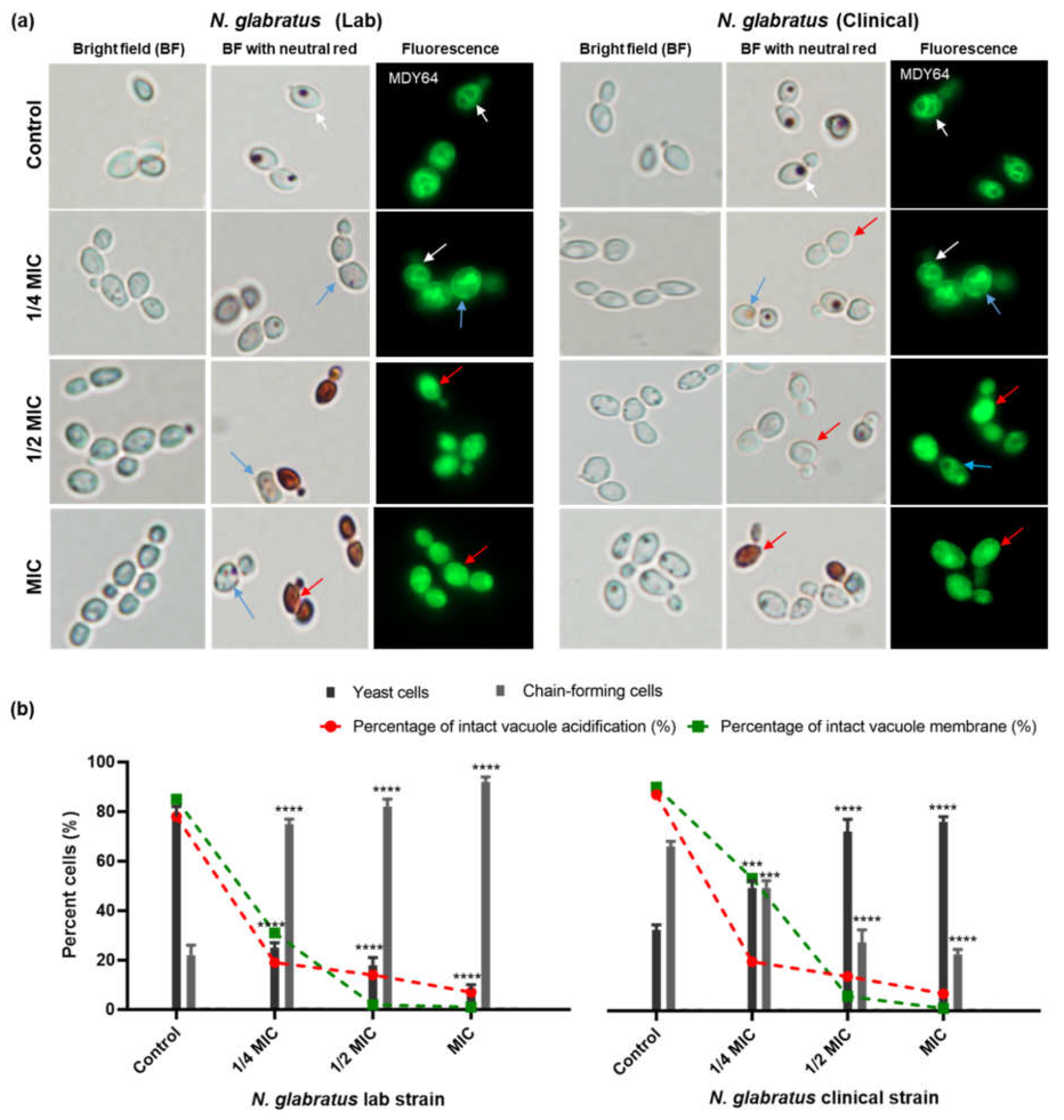
3.2. Carvacrol Disrupts Vacuole Acidification in C. albicans and N. glabratus
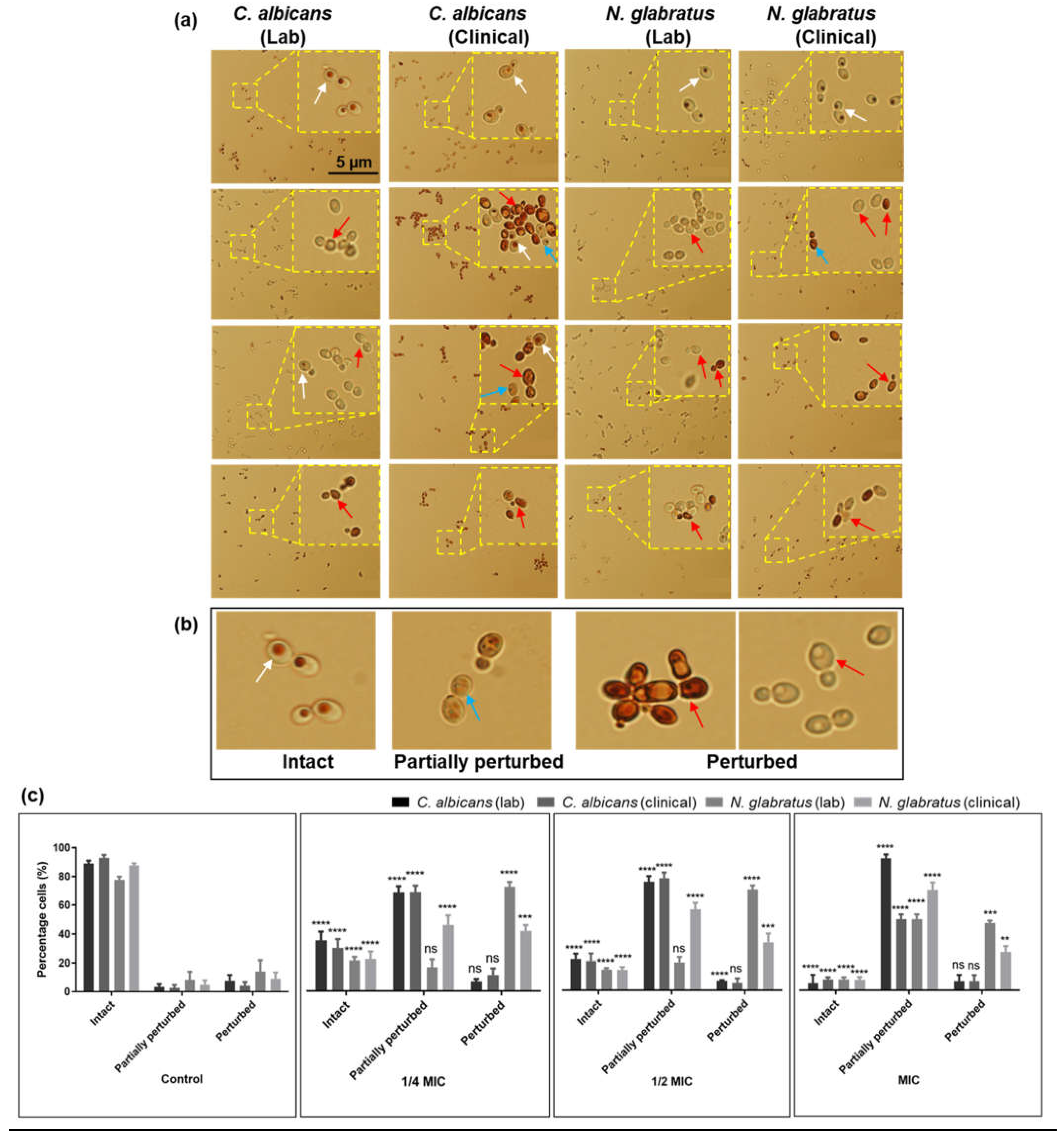
3.3. Carvacrol Disrupts C. albicans and N. glabratus Vacuolar Membrane Integrity
3.4. Carvacrol Disrupts Hyphal Formation in C. albicans
3.5. Carvacrol Induces Chain-Forming Cells in N. glabratus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, C.S.-Y.; Rosli, R.; Seow, H.F.; Chong, P.P. Candida and invasive candidiasis: Back to basics. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 21–31. [Google Scholar] [CrossRef]
- da Silva Dantas, A.; Lee, K.K.; Raziunaite, I.; Schaefer, K.; Wagener, J.; Yadav, B.; Gow, N.A. Cell biology of Candida albicans—Host interactions. Curr. Opin. Microbiol. 2016, 34, 111–118. [Google Scholar] [CrossRef]
- Rai, L.S.; Van Wijlick, L.; Bougnoux, M.; Bachellier-Bassi, S.; D’Enfert, C. Regulators of commensal and pathogenic life-styles of an opportunistic fungus—Candida albicans. Yeast 2021, 38, 243–250. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K. The Effect of Honokiol on Ergosterol Biosynthesis and Vacuole Function in Candida albicans. J. Microbiol. Biotechnol. 2020, 30, 1835–1842. [Google Scholar] [CrossRef]
- Santos, G.C.d.O.; Vasconcelos, C.C.; Lopes, A.J.O.; Cartágenes, M.D.S.S.; Filho, A.K.D.B.; Nascimento, F.R.F.D.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida albicans—Biology, molecular characterization, pathogenicity, and advances in diagnosis and control—An update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef]
- Brunke, S.; Hube, B. Two unlike cousins: C andida albicans and C. glabrata infection strategies. Cell. Microbiol. 2013, 15, 701–708. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, R.; Luan, Z.; Ma, X. Risk of invasive candidiasis with prolonged duration of ICU stay: A systematic review and meta-analysis. BMJ Open 2020, 10, e036452. [Google Scholar] [CrossRef]
- Turner, S.A.; Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef]
- Kumar, K.; Askari, F.; Sahu, M.S.; Kaur, R. Candida glabrata: A lot more than meets the eye. Microorganisms 2019, 7, 39. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [CrossRef]
- Lv, Q.-Z.; Yan, L.; Jiang, Y.-Y. The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn. Virulence 2016, 7, 649–659. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Gamarra, S.; Garcia-Effron, G.; Park, S.; Perlin, D.S.; Rao, R. Requirement for Ergosterol in V-ATPase function underlies antifungal activity of azole Drugs. PLoS Pathog. 2010, 6, e1000939. [Google Scholar] [CrossRef]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef]
- Rajkowska, K.; Otlewska, A.; Kunicka-Styczyńska, A.; Krajewska, A. Candida albicans impairments induced by peppermint and clove oils at sub-inhibitory concentrations. Int. J. Mol. Sci. 2017, 18, 1307. [Google Scholar] [CrossRef]
- Shahina, Z.; El-Ganiny, A.M.; Minion, J.; Whiteway, M.; Sultana, T.; Dahms, T.E.S. Cinnamomum zeylanicum bark essential oil induces cell wall remodelling and spindle defects in Candida albicans. Fungal Biol. Biotechnol. 2018, 5, 1–16. [Google Scholar] [CrossRef]
- Shahina, Z.; Al Homsi, R.; Price, J.D.W.; Whiteway, M.; Sultana, T.; Dahms, T.E.S. Rosemary essential oil and its components 1,8-cineole and α-pinene induce ROS-dependent lethality and ROS-independent virulence inhibition in Candida albicans. PLoS ONE 2022, 17, e0277097. [Google Scholar] [CrossRef]
- Miranda-Cadena, K.; Marcos-Arias, C.; Mateo, E.; Aguirre-Urizar, J.M.; Quindós, G.; Eraso, E. In vitro activities of carvacrol, cinnamaldehyde and thymol against Candida biofilms. Biomed. Pharmacother. 2021, 143, 112218. [Google Scholar] [CrossRef]
- Stringaro, A.; Vavala, E.; Colone, M.; Pepi, F.; Mignogna, G.; Garzoli, S.; Cecchetti, S.; Ragno, R.; Angiolella, L. Effects of Mentha suaveolens Essential Oil Alone or in Combination with Other Drugs in Candida albicans. Altern. Med. 2014, 2014, 125904. [Google Scholar] [CrossRef]
- Chaillot, J.; Tebbji, F.; Remmal, A.; Boone, C.; Brown, G.W.; Bellaoui, M.; Sellam, A. The monoterpene carvacrol generates endoplasmic reticulum stress in the pathogenic fungus Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 4584–4592. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Yadegari, M.H.; Rajabibazl, M.; Ghaemi, E.A. Inhibitory effects of carvacrol on the expression of secreted aspartyl proteinases 1–3 in fluconazole-resistant Candida albicans isolates. Iran. J. Microbiol. 2016, 8, 401–409. [Google Scholar]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K.; et al. Carvacrol induces candida albicans apoptosis associated with Ca2+/Calcineurin Pathway. Front. Cell. Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.Q.; Muend, S.; Rao, R. Mechanism of Antifungal Activity of terpenoid phenols resembles calcium stress and inhibition of the tor pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef]
- Minematsu, A.; Miyazaki, T.; Shimamura, S.; Nishikawa, H.; Nakayama, H.; Takazono, T.; Saijo, T.; Yamamoto, K.; Imamura, Y.; Yanagihara, K.; et al. Vacuolar proton-translocating ATPase is required for antifungal resistance and virulence of Candida glabrata. PLoS ONE 2019, 14, e0210883. [Google Scholar] [CrossRef]
- Martínez-Muñoz, G.A.; Kane, P. Vacuolar and Plasma Membrane Proton Pumps Collaborate to Achieve Cytosolic pH Homeostasis in Yeast. J. Biol. Chem. 2008, 283, 20309–20319. [Google Scholar] [CrossRef]
- Rane, H.S.; Bernardo, S.M.; Hayek, S.R.; Binder, J.L.; Parra, K.J.; Lee, S.A. The Contribution of Candida albicans Vacuolar ATPase Subunit V1B, Encoded by VMA2, to Stress Response, Autophagy, and Virulence Is Independent of Environmental pH. Eukaryot. Cell 2014, 13, 1207–1221. [Google Scholar] [CrossRef]
- Formosa, C.; Schiavone, M.; Martin-Yken, H.; François, J.M.; Duval, R.E.; Dague, E. Nanoscale Effects of Caspofungin against Two Yeast Species, Saccharomyces cerevisiae and Candida albicans. Antimicrob. Agents Chemother. 2013, 57, 3498–3506. [Google Scholar] [CrossRef]
- Rex, J.H.; Alexander, B.D.; Andes, D.; Arthington-Skaggs, B.; Brown, S.D.; Chaturvedi, V. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. Clin. Lab. Stand. Inst. 2008, 28, 1–15. [Google Scholar]
- Corbacho, I.; Teixidó, F.; Velázquez, R.; Hernández, L.; Olivero, I. Yeast vacuole staining using quinacrine and neutral red. In Proceedings of the BioMicroWorld 2009, the 3rd International Conference on Environmental, Industrial and Applied Microbiology, Lisbon, Portugal, 2–4 December 2009; pp. 659–661. [Google Scholar] [CrossRef]
- Budzyńska, A.; Sadowska, B.; Więckowska-Szakiel, M.; Różalska, B. Enzymatic profile, adhesive and invasive properties of Candida albicans under the influence of selected plant essential oils. Acta Biochim. Pol. 2014, 61, 115–121. [Google Scholar] [CrossRef]
- Hayek, S.R.; Lee, S.A.; Parra, K.J. Advances in targeting the vacuolar proton-translocating ATPase (V-ATPase) for anti-fungal therapy. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.A.; Fonzi, W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.A.; Eberle, K.E.; Sturtevant, J.E.; Palmer, G.E. Role for Endosomal and Vacuolar GTPases in Candida albicans Pathogenesis. Infect. Immun. 2009, 77, 2343–2355. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Herman, P.K.; Emr, S.D. The fungal vacuole: Composition, function, and biogenesis. Microbiol. Rev. 1990, 54, 266–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Muend, S.; Rao, R. Dysregulation of Ion Homeostasis by Antifungal Agents. Front. Microbiol. 2012, 3, 133. [Google Scholar] [CrossRef]
- Veses, V.; Richards, A.; Gow, N.A.R. Vacuole inheritance regulates cell size and branching frequency of Candida albicans hyphae. Mol. Microbiol. 2009, 71, 505–519. [Google Scholar] [CrossRef]
- Yamada, N.; Murata, W.; Yamaguchi, Y.; Fujita, K.; Ogita, A.; Tanaka, T. Enhancing the fungicidal activity of amphotericin B via vacuole disruption by benzyl isothiocyanate, a cruciferous plant constituent. Lett. Appl. Microbiol. 2020, 72, 390–398. [Google Scholar] [CrossRef]
- Olsen, I. Attenuation of Candida albicans virulence with focus on disruption of its vacuole functions. J. Oral Microbiol. 2014, 6, 23898. [Google Scholar] [CrossRef]
- Zhang, Y.; Rao, R. Beyond ergosterol: Linking pH to antifungal mechanisms. Virulence 2010, 1, 551–554. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Lima, I.O.; Pereira, F.d.O.; de Oliveira, W.A.; Lima, E.d.O.; Menezes, E.A.; Cunha, F.A.; Diniz, M.d.F.F.M. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Doke, S.K.; Raut, J.S.; Dhawale, S.; Karuppayil, S.M. Sensitization of Candida albicans biofilms to fluconazole by terpenoids of plant origin. J. Gen. Appl. Microbiol. 2014, 60, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Virulence of Clinical Candida Isolates. Pathogens 2021, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Nowicka-Krawczyk, P.; Kunicka-Styczyńska, A. Effect of Clove and Thyme Essential Oils on Candida Biofilm Formation and the Oil Distribution in Yeast Cells. Molecules 2019, 24, 1954. [Google Scholar] [CrossRef] [PubMed]
- Borjihan, H.; Ogita, A.; Fujita, K.-I.; Hirasawa, E.; Tanaka, T. The vacuole-targeting fungicidal activity of amphotericin B against the pathogenic fungus Candida albicans and its enhancement by allicin. J. Antibiot. 2009, 62, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Delaney, C.; Short, B.; Butcher, M.C.; McKloud, E.; Williams, C.; Kean, R.; Ramage, G. Candida auris Phenotypic Heterogeneity Determines Pathogenicity In Vitro. Americ. Soc. Microbiol. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Shahina, Z.; Ndlovu, E.; Persaud, O.; Sultana, T.; Dahms, T.E.S. Candida albicans Reactive Oxygen Species (ROS)-Dependent Lethality and ROS-Independent Hyphal and Biofilm Inhibition by Eugenol and Citral. Microbiol. Spectr. 2022, 10, e0318322. [Google Scholar] [CrossRef]
- Lv, Q.; Yan, L.; Jiang, Y. The Importance of Vacuolar Ion Homeostasis and Trafficking in Hyphal Development and Virulence in Candida albicans. Front. Microbiol. 2021, 12, 779176. [Google Scholar] [CrossRef]
- Patenaude, C.; Zhang, Y.; Cormack, B.; Köhler, J.; Rao, R. Essential Role for Vacuolar Acidification in Candida albicans Virulence. J. Biol. Chem. 2013, 288, 26256–26264. [Google Scholar] [CrossRef]
- Palmer, G.E. Vacuolar trafficking and Candida albicans pathogenesis. Commun. Integr. Biol. 2011, 4, 240–242. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Gooday, G.W. Vacuolation, Branch Production and Linear Growth of Germ Tubes of Candida albicans. Microbiology 1982, 128, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, M.; Oyardi, O.; Kirinti, A. Oregano essential oil inhibits Candida spp. biofilms. Z. Naturforschung C 2021, 76, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.E. Endosomal and AP-3-Dependent Vacuolar Trafficking Routes Make Additive Contributions to Candida albicans Hyphal Growth and Pathogenesis. Eukaryot. Cell 2010, 9, 1755–1765. [Google Scholar] [CrossRef]
- Nishikawa, H.; Miyazaki, T.; Nakayama, H.; Minematsu, A.; Yamauchi, S.; Yamashita, K.; Takazono, T.; Shimamura, S.; Nakamura, S.; Izumikawa, K.; et al. Roles of vacuolar H+-ATPase in the oxidative stress response of Candida glabrata. FEMS Yeast Res. 2016, 16, fow054. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuna, E.; Ndlovu, E.; Molaeitabari, A.; Shahina, Z.; Dahms, T.E.S. Carvacrol-Induced Vacuole Dysfunction and Morphological Consequences in Nakaseomyces glabratus and Candida albicans. Microorganisms 2023, 11, 2915. https://doi.org/10.3390/microorganisms11122915
Acuna E, Ndlovu E, Molaeitabari A, Shahina Z, Dahms TES. Carvacrol-Induced Vacuole Dysfunction and Morphological Consequences in Nakaseomyces glabratus and Candida albicans. Microorganisms. 2023; 11(12):2915. https://doi.org/10.3390/microorganisms11122915
Chicago/Turabian StyleAcuna, Eliz, Easter Ndlovu, Ali Molaeitabari, Zinnat Shahina, and Tanya Elizabeth Susan Dahms. 2023. "Carvacrol-Induced Vacuole Dysfunction and Morphological Consequences in Nakaseomyces glabratus and Candida albicans" Microorganisms 11, no. 12: 2915. https://doi.org/10.3390/microorganisms11122915
APA StyleAcuna, E., Ndlovu, E., Molaeitabari, A., Shahina, Z., & Dahms, T. E. S. (2023). Carvacrol-Induced Vacuole Dysfunction and Morphological Consequences in Nakaseomyces glabratus and Candida albicans. Microorganisms, 11(12), 2915. https://doi.org/10.3390/microorganisms11122915






